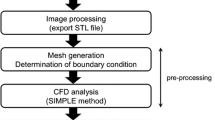
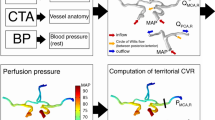
Abstract
The Circle of Willis (CoW) is a ring-like network of blood vessels that perfuses the brain. Flow in the collateral pathways that connect major arterial inputs in the CoW change dynamically in response to vessel narrowing or occlusion. Vasospasm is an involuntary constriction of blood vessels following subarachnoid hemorrhage (SAH), which can lead to stroke. This study investigated interactions between localization of vasospasm in the CoW, vasospasm severity, anatomical variations, and changes in collateral flow directions. Patient-specific computational fluid dynamics (CFD) simulations were created for 25 vasospasm patients. Computed tomographic angiography scans were segmented capturing the anatomical variation and stenosis due to vasospasm. Transcranial Doppler ultrasound measurements of velocity were used to define boundary conditions. Digital subtraction angiography was analyzed to determine the directions and magnitudes of collateral flows as well as vasospasm severity in each vessel. Percent changes in resistance and viscous dissipation were analyzed to quantify vasospasm severity and localization of vasospasm in a specific region of the CoW. Angiographic severity correlated well with percent changes in resistance and viscous dissipation across all cerebral vessels. Changes in flow direction were observed in collateral pathways of some patients with localized vasospasm, while no significant changes in flow direction were observed in others. CFD simulations can be leveraged to quantify the localization and severity of vasospasm in SAH patients. These factors as well as anatomical variation may lead to changes in collateral flow directions. Future work could relate localization and vasospasm severity to clinical outcomes like the development of infarct.








Similar content being viewed by others
References
Chillon, J. M., and G. L. Baumbach. Primer on Cerebrovascular Diseases. San Diego: Academic Press, pp. 51–54, 1997.
Barr, M. L., and J. A. Kiernan. The Human Nervous System: An Anatomical Viewpoint. Philadelphia: Lippincott Co., p. 451, 1993.
Ikram, A., M. A. Javaid, S. Ortega-Gutierrez, M. Selim, S. Kelangi, S. Muhammad, H. Anwar, M. T. Torbey, and A. A. Divani. Delayed cerebral ischemia after subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 30:1–9, 2021. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106064.
Findlay, J. M., J. Nisar, and T. Darsaut. Cerebral vasospasm: a review. Can. J. Neurol. Sci. 43:15–32, 2016. https://doi.org/10.1017/cjn.2015.288.
Fergusen, S., and R. L. Macdonald. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 60:658–667, 2007. https://doi.org/10.1227/01.NEU.0000255396.23280.31.
Bang, O. Y., J. L. Saver, B. H. Buck, J. R. Alger, S. Starkman, B. Ovbiagele, D. Kim, R. Jahan, G. R. Duckwiler, S. R. Yoon, F. Viñuela, and D. S. Liebeskind. Impact of collateral flow on tissue fate in acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry. 79:625–629, 2008. https://doi.org/10.1136/jnnp.2007.132100.
Bozzao, L., L. M. Fantozzi, S. Bastianello, A. Bozzao, and C. Fieschi. Early collateral blood supply and late parenchymal brain damage in patients with middle cerebral artery occlusion. Stroke. 20:735–740, 1989. https://doi.org/10.1161/01.STR.20.6.735.
Christoforidis, G. A., Y. Mohammad, D. Kehagias, B. Avutu, and A. P. Slivka. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. Am. J. Neuroradiol. 26:1789–1797, 2005.
Kim, J. J., N. J. Fischbein, Y. Lu, D. Pham, and W. P. Dillon. Regional angiographic grading system for collateral flow: correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke. 35:1340–1344, 2004. https://doi.org/10.1161/01.STR.0000126043.83777.3a.
Zareie, H., D. A. Quain, M. Parsons, K. J. Inder, P. McElduff, F. Miteff, N. J. Spratt, and C. Levi. The influence of anterior cerebral artery flow diversion measured by transcranial Doppler on acute infarct volume and clinical outcome in anterior circulation stroke. Int. J. Stroke. 8:228–234, 2012. https://doi.org/10.1111/J.1747-4949.2012.00801.X.
Bisschops, R. H. C., C. J. M. Klijn, L. J. Kappelle, A. C. Huffelen, and J. Grond. Collateral flow and ischemic brain lesions in patients with unilateral carotid artery occlusion. Neurology. 60:1435–1441, 2003. https://doi.org/10.1212/01.WNL.0000061616.96745.90.
Doblar, D. D., N. V. Plyushcheva, W. Jordan, and H. McDowell. Predicting the effect of carotid artery occlusion during carotid endarterectomy. Stroke. 29:2038–2042, 1998. https://doi.org/10.1161/01.STR.29.10.2038.
Everdingen, K. J., G. H. Visser, C. J. M. Klijn, L. J. Kappelle, and J. Der Grond. Role of collateral flow on cerebral hemodynamics in patients with unilateral internal carotid artery occlusion. Ann. Neurol. 44:167–176, 1998. https://doi.org/10.1002/ANA.410440206.
Hendrikse, J., M. J. Hartkamp, B. Hillen, W. P. T. M. Mali, and J. V. D. Grond. Collateral ability of the Circle of Willis in patients with unilateral internal carotid artery occlusion. Stroke. 32:2768–2773, 2001. https://doi.org/10.1161/HS1201.099892.
Muller, M., M. Hermes, H. Bruckmann, and K. Schimrigk. Transcranial Doppler ultrasound in the evaluation of collateral blood flow in patients with internal carotid artery occlusion: correlation with cerebral angiography. Am. J. Neuroradiol. 16:195–202, 1995.
Reinhard, M., T. Müller, B. Guschlbauer, J. Timmer, and A. Hetzel. Dynamic cerebral autoregulation and collateral flow patterns in patients with severe carotid stenosis or occlusion. Ultrasound Med. Biol. 29:1105–1113, 2003. https://doi.org/10.1016/S0301-5629(03)00954-2.
Ringelstein, E. B., C. Weiller, M. Weckesser, and S. Weckesser. Cerebral vasomotor reactivity is significantly reduced in low-flow as compared to thromboembolic infarctions: the key role of the Circle of Willis. J. Neurol. Sci. 121:103–109, 1994. https://doi.org/10.1016/0022-510X(94)90163-5.
Lindegaard, K.F., S. J. Bakke, P. Grolimund, R. Aaslid, P. Huber, and H. Nornes. Assessment of intracranial hemodynamics in carotid artery disease by transcranial Doppler ultrasound. J. Neurosurg. 63:890–898, 1985. https://doi.org/10.3171/JNS.1985.63.6.0890.
Rutgers, D. R., C. J. M. Klijn, L. J. Kappelle, A. C. Huffelen, and J. Grond. A longitudinal study of collateral flow patterns in the Circle of Willis and the ophthalmic artery in patients with a symptomatic internal carotid artery occlusion. Stroke. 31:1913–1920, 2000. https://doi.org/10.1161/01.STR.31.8.1913.
Schomer, D. F., M. P. Marks, G. K. Steinberg, I. M. Johnstone, D. B. Boothroyd, M. R. Ross, N. J. Pelc, and D. R. Enzmann. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N. Engl. J. Med. 330:1565–1570, 1994. https://doi.org/10.1056/NEJM199406023302204.
Wilterdink, J. L., E. Feldmann, K. L. Furie, M. Bragoni, and J. G. Benavides. Transcranial Doppler ultrasound battery reliably identifies severe internal carotid artery stenosis. Stroke. 28:133–136, 1997. https://doi.org/10.1161/01.STR.28.1.133.
Klötzsch, C., O. Popescu, and P. Berlit. Assessment of the posterior communicating artery by transcranial color-coded duplex sonography. Stroke. 27:486–489, 1996. https://doi.org/10.1161/01.STR.27.3.486.
Moftakhar, P., D. L. Cooke, H. J. Fullerton, N. U. Ko, M. R. Amans, J. A. Narvid, C. F. Dowd, R. T. Higashida, V. V. Halbach, and S. W. Hetts. Extent of collateralization predicting symptomatic cerebral vasospasm among pediatric patients: correlations among angiography, transcranial Doppler ultrasonography, and clinical findings. J. Neurosurg. Pediatr. 15:282–290, 2015. https://doi.org/10.3171/2014.9.PEDS14313.
Al-Mufti, F., J. Witsch, N. Manning, M. Crimmins, K. Amuluru, S. Agarwal, S. Park, J. Z. Willey, H. Kamel, E. S. Connolly, P. M. Meyers, and J. Claassen. Severity of cerebral vasospasm associated with development of collaterals following aneurysmal subarachnoid hemorrhage. J. NeuroInterv. Surg. 10:638–643, 2018. https://doi.org/10.1136/NEURINTSURG-2017-013410.
Topcu, A., A. Ozkul, A. Yilmaz, H. J. Yi, D. S. Shin, and B. Kim. The impact of collateral status on cerebral vasospasm and delayed cerebral ischemia in subarachnoid hemorrhage. J. Cerebrovasc. Endovasc. Neurosurg. 2023. https://doi.org/10.7461/JCEN.2023.E2022.11.005.
Grinberg, L., E. Cheever, T. Anor, J. R. Madsen, and G. E. Karniadakis. Modeling blood flow circulation in intracranial arterial networks: a comparative 3D/1D simulation study. Ann. Biomed. Eng. 39:297–309, 2011. https://doi.org/10.1007/s10439-010-0132-1.
Cebral, J. R., M. A. Castro, O. Soto, R. Löhner, and N. Alperin. Blood-flow models of the Circle of Willis from magnetic resonance data. J. Eng. Math. 47:369–386, 2003. https://doi.org/10.1023/B:ENGI.0000007977.02652.02.
Gaidzik, F., S. Pathiraja, S. Saalfeld, D. Stucht, O. Speck, D. Thévenin, and G. Janiga. Hemodynamic data assimilation in a subject-specific Circle of Willis geometry. Clin. Neuroradiol. 31:643–651, 2021. https://doi.org/10.1007/S00062-020-00959-2/FIGURES/3.
Perinajová, R., P. Ooij, and S. Kenjereš. On the identification of hypoxic regions in subject-specific cerebral vasculature by combined CFD/MRI. R. Soc. Open Sci. 2023. https://doi.org/10.1098/RSOS.220645/.
Berg, P., D. Stucht, G. A. Janiga, O. Beuing, O. Speck, and D. Thevenin. Cerebral blood flow in a healthy Circle of Willis and two intracranial aneurysms: computational fluid dynamics versus four-dimensional phase-contrast magnetic resonance imaging. J. Biomed. Eng. 136:041003–19, 2014. https://doi.org/10.1115/1.4026108.
Zuleger, D. I., D. Poulikakos, A. Valavanis, and S. S. Kollias. Combining magnetic resonance measurements with numerical simulations—extracting blood flow physiology information relevant to the investigation of intracranial aneurysms in the Circle of Willis. Int. J. Heat Fluid Flow. 31:1032–1039, 2010. https://doi.org/10.1016/j.ijheatfluidflow.2010.07.003.
Jou, L. D., D. H. Lee, and M. E. Mawad. Cross-flow at the anterior communicating artery and its implication in cerebral aneurysm formation. J. Biomech. 43:2189–2195, 2010. https://doi.org/10.1016/j.jbiomech.2010.03.039.
Mukherjee, D., N. D. Jani, J. Narvid, and S. C. Shadden. The role of Circle of Willis anatomy variations in cardio-embolic stroke: a patient-specific simulation based study. Ann. Biomed. Eng. 46:1128–1145, 2018. https://doi.org/10.1007/S10439-018-2027-5/FIGURES/8.
Schollenberger, J., N. H. Osborne, L. Hernandez-Garcia, and C. A. Figueroa. A combined computational fluid dynamics and arterial spin labeling MRI modeling strategy to quantify patient-specific cerebral hemodynamics in cerebrovascular occlusive disease. Front. Bioeng. Biotechnol. 2021. https://doi.org/10.3389/FBIOE.2021.722445/FULL.
Liu, J., Z. Yan, Y. Pu, W.-S. Shiu, J. Wu, R. Chen, X. Leng, H. Qin, X. Liu, B. Jia, L. Song, Y. Wang, Z. Miao, Y. Wang, L. Liu, and X.C. Cai. Functional assessment of cerebral artery stenosis: a pilot study based on computational fluid dynamics. J. Cereb. Blood Flow Metab. 37:2567–2576, 2017. https://doi.org/10.1177/0271678X16671321.
Moore, S., T. David, J. G. Chase, J. Arnold, and J. Fink. 3D models of blood flow in the cerebral vasculature. J. Biomech. 39:1454–1463, 2006. https://doi.org/10.1016/j.jbiomech.2005.04.005.
Kim, C. S. Numerical simulation of auto-regulation and collateral circulation in the human brain. J. Mech. Sci. Technol. 21:525–535, 2007. https://doi.org/10.1007/BF02916315.
Holmgren, M., K. H. Støverud, L. Zarrinkoob, A. Wåhlin, J. Malm, and A. Eklund. Middle cerebral artery pressure laterality in patients with symptomatic ICA stenosis. PLoS ONE. 2021. https://doi.org/10.1371/journal.pone.0245337.
Holmgren, M., P. Holmlund, K. H. Støverud, L. Zarrinkoob, A. Wåhlin, J. Malm, and A. Eklund. Prediction of cerebral perfusion pressure during carotid surgery-a computational fluid dynamics approach. Clin. Biomech. 2022. https://doi.org/10.1016/j.clinbiomech.2022.105827.
Chittiboina, P., B. Guthikonda, C. Wollblad, and S. A. Conrad. A computational simulation of the effect of hemodilution on oxygen transport in middle cerebral artery vasospasm. J. Cereb. Blood Flow Metab. 31:2209–2217, 2011. https://doi.org/10.1038/jcbfm.2011.83.
Robinson, J. S., M. S. Walid, S. Hyun, R. O’Connell, C. Menard, and B. Bohleber. Computational modeling of HHH therapy and impact of blood pressure and hematocrit. World Neurosurg. 74:294–296, 2010. https://doi.org/10.1016/j.wneu.2010.02.059.
Shiba, M., F. Ishida, and K. Furukawa. Computational fluid dynamics for predicting delayed cerebral ischemia after subarachnoid hemorrhage. J. Neurol. Disord. Stroke. 5:1–4, 2017. https://doi.org/10.1007/s10877-018-0132-5.
Straccia, A., F. Chassagne, D. I. Bass, G. Barros, D. F. Leotta, F. Sheehan, D. Sharma, M. R. Levitt, and A. Aliseda. A novel patient-specific computational fluid dynamics study of the activation of primary collateral pathways in the Circle of Willis during vasospasm. J. Biomech. Eng. 2023. https://doi.org/10.1115/1.4055813.
Ryu, J., N. Ko, X. Hu, and S. C. Shadden. Numerical investigation of vasospasm detection by extracranial blood velocity ratios. Cerebrovasc. Dis. 43:214–222, 2017. https://doi.org/10.1159/000454992.
Ambarki, K., P. Hallberg, G. Jóhannesson, C. Lindén, L. Zarrinkoob, A. Wåhlin, R. Birgander, J. Malm, and A. Eklund. Blood flow of ophthalmic artery in healthy individuals determined by phase-contrast magnetic resonance imaging. Investig. Ophthalmol. Visual Sci. 54:2738–2745, 2013. https://doi.org/10.1167/iovs.13-11737.
Nagasawa, S., M. Kawanishi, Y. Tada, S. Kawabata, and T. Ohta. Intra-operative measurement of cortical arterial flow volumes in posterior circulation using doppler sonography. Neurol. Res. 22:194–196, 2000. https://doi.org/10.1080/01616412.2000.11741060.
Rayz, V. L., L. Boussel, L. Ge, J. R. Leach, A. J. Martin, M. T. Lawton, C. Mcculloch, and D. Saloner. Flow residence time and regions of intraluminal thrombus deposition in intracranial aneurysms. Ann. Biomed. Eng. 38:3058–3069, 2010. https://doi.org/10.1007/s10439-010-0065-8.
Sheth, S. A., N. Sanossian, Q. Hao, S. Starkman, L. K. Ali, D. Kim, N. R. Gonzalez, S. Tateshima, R. Jahan, G. R. Duckwiler, J. L. Saver, F. Vinuela, and D. S. Liebeskind. Collateral flow as causative of good outcomes in endovascular stroke therapy. J. NeuroInterv. Surg. 2014. https://doi.org/10.1136/neurintsurg-2014-011438.
Nathal, E., F. Lóopez-González, and C. Rios. Angiographic scale for evaluation of cerebral vasospasm. Acta Neurochirurgica, Supplementum. 2008. https://doi.org/10.1007/978-3-211-75718-5_43/COVER.
Otite, F., S. Mink, C. O. Tan, A. Puri, A. A. Zamani, A. Mehregan, S. Chou, S. Orzell, S. Purkayastha, R. Du, and F. A. Sorond. Impaired cerebral autoregulation is associated with vasospasm and delayed cerebral ischemia in subarachnoid hemorrhage. Stroke. 45:677–682, 2014. https://doi.org/10.1161/STROKEAHA.113.002630/-/DC1.
Acknowledgements
We would like to thank Shumaila Ahmed, Karen Velderrain-Lopez, and François Ramon for creating patient-specific segmentations, François Ramon for creating the PyTec scripts to calculate resistance, and Erin Espeland for generating CFD models from patient-specific data. We are grateful for Katie Paulson, Ameer Dharamshi, and Ethan Ashby, who provided guidance and insight into presenting the statistical results in an intuitive way.
Funding
This work was funded by the National Science Foundation Graduate Research Fellowship Program (NSF GRFP) AHRQ (Grant No. 1 R18 HS 026690; Funder ID: 10.13039/100000133), the Royalty Research Fund at the University of Washington, the CRBT Strategic Research Investment through the Seattle Children’s Research Institute, the Locke Trust through a gift to the Division of Cardiology of the University of Washington, the American Heart Association via a Postdoctoral fellowship (19POST34450082), and the National Institute of Health Grants R01NS105692 and R25NS079200. This work was facilitated through the use of advanced computational, storage, and networking infrastructure provided by the Hyak supercomputer system and funded by the Student Technology Fund at the University of Washington.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Associate Editor Stefan M. Duma oversaw review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendices
Appendix A
Definition of Antegrade Collateral Flow Directions
See Fig. 9.
Mesh Independence Study
The mesh independence study was performed by comparing the percent changes in resistance and dissipation between the baseline and vasospasm conditions across four mesh resolutions: a base size of 0.6 mm (3.5 million elements during baseline and 2.8 million elements during vasospasm), 0.35 mm (4.4 million elements during baseline and vasospasm), 0.2 mm (13 million elements during baseline and 10.5 million elements during vasospasm), and 0.15 mm (33 million elements during baseline and 22 million elements during vasospasm). The percent changes in resistance and dissipation were compared between each of the meshes. The differences in percent change in resistance between the coarsest and finest meshes were below 15 in vessels exhibiting an absolute change below 1000%, below 100 in vessels with an absolute change between 1000% and 1200%, and below 500 for one vessel with greater than 3000% absolute change. The differences in percent change in dissipation between the coarsest and finest meshes were below 50 in vessels exhibiting an absolute change below 800%, below 100 in vessels with an absolute change between 800% and 2000%, and below 100 for vessels with greater than 2000% absolute change. Given the relatively small differences between the percent changes in resistance and dissipation compared to their absolute values, the CFD results were considered to be insensitive to mesh resolution in the range of base sizes between 0.6 mm and 0.15 mm.
The base size of 0.55 mm was selected because a previous mesh study showed that the collateral flow rates were consistent between this sizing and the finer meshes [43].
Determination of CFD Metric Threshold for Discretizing Severity
See Fig. 10.
Comparison Between CFD Metrics with Angiographic Severity Across Patients
See Table 1.
Collateral Flow Directions
Diversity Statement
Recent work in several fields of science has identified a bias in citation practices such that papers from women and other minority scholars are undercited relative to the number of papers in the field. We recognize this bias and have worked diligently to ensure that we are referencing appropriate papers with fair gender and racial author inclusion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Straccia, A., Barbour, M.C., Chassagne, F. et al. Numerical Modeling of Flow in the Cerebral Vasculature: Understanding Changes in Collateral Flow Directions in the Circle of Willis for a Cohort of Vasospasm Patients Through Image-Based Computational Fluid Dynamics. Ann Biomed Eng (2024). https://doi.org/10.1007/s10439-024-03533-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-024-03533-w