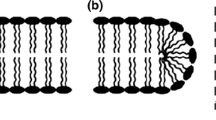
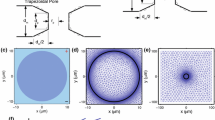
Abstract
Escherichia coli bacterium is a rod-shaped organism composed of a complex double membrane structure. Knowledge of electric field driven ion transport through both membranes and the evolution of their induced permeabilization has important applications in biomedical engineering, delivery of genes and antibacterial agents. However, few studies have been conducted on Gram-negative bacteria in this regard considering the contribution of all ion types. To address this gap in knowledge, we have developed a deterministic and stochastic Brownian dynamics model to simulate in 3D space the motion of ions through pores formed in the plasma membranes of E. coli cells during electroporation. The diffusion coefficient, mobility, and translation time of Ca2+, Mg2+, Na+, K+, and Cl− ions within the pore region are estimated from the numerical model. Calculations of pore’s conductance have been validated with experiments conducted at Gustave Roussy. From the simulations, it was found that the main driving force of ionic uptake during the pulse is the one due to the externally applied electric field. The results from this work provide a better understanding of ion transport during electroporation, aiding in the design of electrical pulses for maximizing ion throughput, primarily for application in cancer treatment.













Similar content being viewed by others
References
Abrahamson, A. A. Born–Mayer-type interatomic potential for neutral ground-state atoms with Z = 2 to Z = 105. Phys. Rev. 178:76–79, 1969.
Alegun, O., A. Pandeya, J. Cui, I. Ojo, and Y. Wei. Donnan potential across the outer membrane of Gram-negative bacteria and its effect on the permeability of antibiotics. Antibiotics (Basel). 10:701, 2021.
Basavaraju, M., and B. S. Gunashree. Escherichia coli: an overview of main characteristics. In: Escherichia coli—Old and New Insights. London: IntechOpen, 2023.
Bayer, M. E. Zones of membrane adhesion in the cryofixed envelope of Escherichia coli. J. Struct. Biol. 107:268–280, 1991.
Berendsen, H. J. Molecular dynamics simulations: the limits and beyond. In: Computational Molecular Dynamics: Challenges, Methods, Ideas. Lecture Notes in Computational Science and Engineering. Berlin: Springer, 1999.
Birdsall, C. K., and A. B. Langdon. Plasma Physics via Computer Simulations. New York: McGraw-Hill Book Company, 1985.
Bonzanni, M., S. L. Payne, M. Adelfio, D. L. Kaplan, M. Levin, and M. J. Oudin. Defined extracellular ionic solution to study and manipulate the cellular resting membrane potential. Biol. Open. 9(1):bio048553, 2020.
Catipovic, M. A., P. M. Tyler, J. G. Trapani, and A. R. Carter. Improving the quantification of Brownian motion. Am. J. Phys. 81:485–491, 2013.
Chen, J. C., and A. S. Kim. Brownian Dynamics, Molecular Dynamics, and Monte Carlo modeling of colloidal systems. Adv. Colloid Interface Sci. 112:159–173, 2004.
Chini, T. K., and D. Ghose. On the interaction potential in low energy ion scattering. Nucl. Instrum. Methods Phys. Res. B. 42:293–294, 1989.
Chung, S., T. W. Allen, M. Hoyles, and S. Kuyucak. Permeation of ions across the potassium channel: Brownian dynamics studies. Phys. Rev. E. 77:2117–2155, 1999.
Chung, S., M. Hoyles, T. Allen, and S. Kuyucak. Study of ionic currents across a model membrane channel using Brownian dynamics. Biophys. J. 75:793–809, 1998.
Corry, B., T. W. Allen, S. Kuyukak, and S.-H. Chung. Mechanisms of permeation and selectivity in calcium channels. Biophys. J. 80:195–214, 2001.
Cory, B., M. Hoyles, T. W. Allen, M. Walker, S. Kuyucak, and S. Chung. Reservoir boundaries in Brownian dynamics simulations of ion channels. Biophys. J. 82:1975–1984, 2002.
DeBruin, K. A., and W. Krassowska. Modeling electroporation in a single cell. I. Effects of field strength and rest potential. Biophys. J. 77:1213–1224, 1999.
Diem, M., and C. Oostenbrink. The effect of different cutoff schemes in molecular simulations of proteins. J. Comput. Chem. 41(32):2740–2749, 2020.
Einstein, A. On the movement of small particles suspended in a stationary liquid demanded by the molecular kinetic theory of heat (original in German). Ann. Phys. 17:549–560, 1905.
El-Hag, A., and S. H. Jayaram. Effect of biological cell size and shape on killing efficiency of pulsed electric field. In: 2008 IEEE International Conference on Dielectric Liquids, 2008.
Eynard, N., F. Rodriguez, J. Trotard, and J. Tessie. Electrooptics studies of Escherichia coli electropulsation: orientation, permeabilization, and gene transfer. Biophys. J. 75:2587–2596, 1998.
Gapsys, V., and B. Groot. On the importance of statistics in molecular simulations for thermodynamics, kinetics and simulation box size. eLife. 9:e57589, 2020.
Gothelf, A., L. M. Mir, and J. Gehl. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat. Rev. 29:371–387, 2003.
Graham, L. L., T. J. Beveridge, and N. Nanninga. Periplasmic space and the concept of the periplasm. Trends Biochem. Sci. 16:328–329, 1991.
Green, M. S. Markoff random processes and the statistical mechanics of time-dependent phenomena. II. Irreversible processes in fluids. J. Chem. Phys. 22:398–413, 1954.
Hastings, C. Approximations for Digital Computers. Princeton: Princeton University Press, 1955.
Ho, S. Y., and G. S. Mittal. Electroporation of cell membranes: a review. Crit. Rev. Biotechnol. 16:349–362, 1996.
Hoejholt, K. L., T. Muzic, S. D. Jensen, L. T. Dalgaard, M. Bilgin, J. Nylandsted, T. Heimburg, S. K. Frandsen, and J. Gehl. Calcium electroporation and electrochemotherapy for cancer treatment: importance of cell membrane composition investigated by lipidomics, calorimetry and in vitro efficacy. Sci. Rep. 9:4758, 2019.
Hoyles, M., S. Kuyucak, and S. Chung. Computer simulation of ion conductance in membrane channels. Phys. Rev. E. 58:3654–3661, 1998.
Hu, Q., and R. P. Joshi. Comparative evaluation of transmembrane ion transport due to monopolar and bipolar nanosecond, high-intensity electroporation pulses based on full three-dimensional analyses. J. Appl. Phys.122:034701, 2017.
Idalia, V.-M. N., and F. Bernardo. Escherichia coli as a model organism and its application in biotechnology. In: Escherichia coli—Recent Advances on Physiology, Pathogenesis and Biotechnological Applications. London: InTechOpen, 2017.
Im, W. S., S. Seefeld, and B. Roux. A grand canonical Monte Carlo-Brownian dynamics algorithm for simulating ion channels. Biophys. J. 79:788–801, 2000.
Joshi, R. P., Q. Hu, R. Aly, K. H. Schoenbach, and H. P. Hjalmarson. Self-consistent simulations of electroporation dynamics in biological cells subjected to ultrashort electrical pulses. Phys. Rev. E.64:011913, 2001.
Kestin, J., M. Sokolov, and W. Wakeham. Viscosity of liquid water in the range − 8 °C to 150 °C. J. Phys. Chem. Ref. Data. 7:941, 1978.
Kotnik, T., L. Rems, M. Tarek, and D. Miklavcic. Membrane electroporation and electropermeabilization: mechanisms and models. Annu. Rev. Biophys. 6:63–91, 2019.
Krassowska, W., and P. D. Filev. Modeling electroporation in a single cell. Biophys. J. 92:404–417, 2007.
Kubo, R. Statistical-mechanical theory of irreversible processes. I. General theory and simple applications to magnetic and conduction problems. J. Phys. Soc. Jpn. 12:570–586, 1957.
Lemons, D. S., and A. Gythiel. Paul Langevin’s 1908 paper “On the Theory of Brownian Motion” [“Sur la theorie du mouvement brownien,” C. R. Acad. Sci. (Paris) 145, 530–533 (1908)]. Am. J. Phys. 65:1079–1081, 1997.
Leontiadou, H., A. E. Mark, and S. J. Marrink. Ion transport across transmembrane pores. Biophys. J. 92:4209–4215, 2007.
Levitt, D. G. Modeling of ion channels. J. Gen. Physiol. 113:789–794, 1999.
Lide, D. R., and H. V. Kehiaian. CRC Handbook of Thermophysical and Thermochemical Data. Boca Raton: CRC Press, 1994.
Lo, C. J., M. C. Leake, T. Pilizota, and R. M. Berry. Nonequivalence of membrane voltage and ion-gradient as driving forces for the bacterial flagellar motor at low load. Biophys. J. 93:294–302, 2007.
Lomize, A. L., I. D. Pogozheva, and H. I. Mosberg. Anisotropic solvent model of the lipid bilayer. 1. Parameterization of long-range electrostatics and first solvation shell effects. J. Chem. Inf. Model. 51:918–929, 2011.
Lomize, A. L., I. D. Pogozheva, and H. I. Mosberg. Anisotropic solvent model of the lipid bilayer. 2. Energetics of insertion of small molecules, peptides, and proteins in membranes. J. Chem. Inf. Model. 51:930–946, 2011.
Miklavcic, D., and R. V. Davalos. Electrochemotherapy (ECT) and irreversible electroporation (IRE)—advanced techniques for treating deep-seated tumors based on electroporation. Biomed. Eng. Online.14:l1, 2015.
Milo, R., and R. Phillips. Cell Biology by the Numbers. New York: Garland Science, 2015.
Moran, J. L., N. N. Dingari, P. A. Garcia, and C. R. Buie. Numerical study of the effect of soft layer properties on bacterial electroporation. Bioelectrochemistry. 123:261–272, 2018.
Neu, J., and W. Krassowska. Modeling postshock evolution of large electropores. Phys. Rev. E.67:021915, 2003.
Neu, J., K. Smith, and W. Krassowska. Electrical energy required to form large conducting pores. Bioelectrochemistry. 60:107–114, 2003.
Papoulis, A. Probability, Random Variables, and Stochastic Processes. New York: McGraw Hill, 1984.
Piggot, T., D. Holdbrook, and S. Khalid. Electroporation of the E. coli and S. aureus membranes: Molecular Dynamics simulations of complex bacterial membranes. J. Phys. Chem. B. 115:13381–13388, 2011.
Pluhackova, K., and R. A. Böckmann. Biomembranes in atomistic and coarse-grained simulations. J. Phys. Condens. Matter.27:323103, 2015.
Pucihar, G., T. Kotnik, D. Miklavcic, and J. Tessie. Kinetics of transmembrane transport of small molecules into electropermeabilized cells. Biophys. J. 95:2837–2848, 2008.
Riley, M. Correlates of smallest sizes for microorganisms. In: Size Limits of Very Small Microorganisms: Proceedings of a Workshop, 1999.
Rosazza, C., S. Haberl Meglic, A. Zumbusch, M. Rols, and D. Miklavcic. Gene electrotransfer: a mechanistic perspective. Curr. Gene Ther. 16:98–129, 2016.
Sabah, N. H. Rectification in biological membranes. IEEE EMB Mag. 19(1):106–113, 2000.
Sandblom, J., P. George, and J. Galvanovskis. The frequency response of ion channel currents in a combined diffusion and barrier type model. J. Theor. Biol. 176:153–160, 1995.
Sansom, M., I. Shrivastaya, J. Bright, J. Tate, C. Capener, and P. Biggin. Potassium channels: structures, models, simulations. Biochim. Biophys. Acta Biomembr. 1565:11, 2002.
Saulis, G., and R. Saule. Size of the pores created by an electric pulse: microsecond vs millisecond pulses. Biochim. Biophys. Acta. 3032–3039:2012, 1818.
Shen, L. C., and J. A. Kong. Applied Electromagnetism. Boston: PWS Publishers, 1987.
Smythe, W. R. Static and Dynamic Electricity. New York: McGraw-Hill, 1968.
Spanggaard, I., M. Snoj, A. Cavalcanti, C. Bouquet, G. Sersa, C. Robert, M. Cemazar, E. Dam, B. Vasseur, P. Attali, L. M. Mir, and J. Gehl. Gene electrotransfer of plasmid antiangiogenic metargidin peptide (AMEP) in disseminated melanoma: safety and efficacy results of a phase I first-in-man study. Hum. Gene Ther. Clin. Dev. 24:99–107, 2013.
Swope, W. C., H. C. Andersen, P. H. Berens, and K. R. Wilson. A computer simulation method for the calculation of equilibrium constants for the formation of physical clusters of molecules: application to small water clusters. J. Chem. Phys. 76:648, 1982.
Sözer, E. B., S. Haldar, P. S. Blank, F. Castellani, P. T. Vernier, and J. Zimmerberg. Dye transport through bilayers agrees with lipid electropore molecular dynamics. Biophys. J. 119:1724–1734, 2020.
Tinkle, M. D., and S. E. Barlow. Image charge forces inside conducting boundaries. J. Appl. Phys. 90:1612–1624, 2001.
Tinkle, M. D., and S. E. Barlow. Workshop on Non-neutral Plasmas, 2001.
Turq, P., F. Lantelme, and H. L. Firedman. Brownian dynamics: its application to ionic solutions. J. Chem. Phys. 66:3039–3044, 1977.
Van Gunsteren, W. F., and H. J. Berendsen. Algorithms for Brownian dynamics. Mol. Phys. 45:637–647, 1982.
Verlet, L. Computer, “Experiments” on classical fluids. I. Thermodynamical properties of Lennard-Jones molecules. Phys. Rev. 159:98–103, 1967.
Weaver, J. C. Electroporation of cells and tissues. IEEE Trans. Plasma Sci. 28:24–33, 2000.
Weaver, J. C., K. C. Smith, A. T. Esser, R. S. Son, and T. R. Gowrishankar. A brief overview of electroporation pulse strength-duration space: a region where additional intracellular effects are expected. Bioelectrochemistry. 87:236–243, 2012.
**ang, X., P. B. Grosshans, and A. G. Marshall. Image charge induced ion cyclotron orbital frequency shift for orthorhombic and cylindrical FT-ICR ion traps. Int. J. Mass Spectrom. Ion Process. 125:33–43, 1993.
Young, H., and R. Freedman. Sears and Semansky’s University Physics. New York: Addison Wesley, 1996.
Acknowledgments
This research work was funded by Consejo Nacional de Ciencia y Tecnología (CONACYT-Paraguay), Grant Number 14-INV-189. The authors would like to thank the Gustave Roussy Institute for allowing the use of equipment, cells and reactants which were used in conducting the experimental validation of the model.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Additional information
Associate Editor Rafael Vidal Davalos oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
Derivations of Induced Image Charges at the Pores
Green’s function of the interior of a cylinder of radius ρ0 and length 2z0 [63, 64] is given by:
where \({\epsilon }_{m}=2-{\delta }_{m,0}\). \({\delta }_{\mathrm{0,0}}=1\) , and \({\delta }_{m,0}=0\) otherwise. jm,n is the nth zero of the Bessel function Jm(x); \(\alpha ={z}_{0}/{\rho }_{0}\) is the aspect ratio of the cylinder. The ρ and z cylindrical coordinates are scaled by ρ0 and their origin is the center of the cylinder. The coordinate z< is the lesser of z and z′, and z> is the greater.
The z component on a charge q can be found by evaluating Green’s function G [Eq. (42)] at \(\rho ^{\prime}= \rho ,\varnothing ^{\prime}=\varnothing ,z^{\prime}=z\) and taking the gradient following Smythe [59]:
Since this approach yields a divergent series when calculating the radial force Fρ, a different technique by **ang et al. (**a93) is followed using an alternate form for the Green’s function:
where
\({I}_{m}\) y \({K}_{m}\) are hyperbolic Bessel functions. Scaling is by z0. The charge distribution σ on the cylinder surface S can be found from Green’s function [Eq. (44)]:
where the gradient is taken with respect to \(\rho ,\varnothing ,z\). Using the identity
the surface charge on the cylinder is:
The surface charge creates a pressure directed outwards of the conducting membrane pore:
Thus, the force on the ion is given by:
The radial force on the ion from the above equation is:
Considering the identity:
the radial force becomes [70]:
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
González-Cuevas, J.A., Argüello, R., Florentin, M. et al. Experimental and Theoretical Brownian Dynamics Analysis of Ion Transport During Cellular Electroporation of E. coli Bacteria. Ann Biomed Eng 52, 103–123 (2024). https://doi.org/10.1007/s10439-023-03353-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-023-03353-4