Abstract
The definition of a non-mass lesion on breast ultrasound (US) is designed for everyday practice to provide unambiguous clinical management and to assist physicians and sonographers as they interpret breast US images. The field of breast imaging research requires consistent and standardized terminology for non-mass lesions identified on breast US, especially when differentiating benign from malignant lesions. Physicians and sonographers should be aware of the benefits and limitations of the terminology and use them precisely. I am hopeful that the next edition of the Breast Imaging Reporting and Data System (BI-RADS) lexicon will include standardized terminology for describing non-mass lesions detected on breast US.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Terms describing non-mass lesions on breast ultrasound (US) are widely used and accepted in screening and clinical settings. Ideally, this definition should facilitate the provision of unambiguous clinical management. However, there is an unmet need for consistent and standardized terminology to describe non-mass lesions detected on breast US to assist physicians and sonographers in interpreting US findings. Unlike mammography, US and magnetic resonance imaging (MRI) are tomographic modalities that display three-dimensional (3D) objects unaffected by the presence of adjacent dense fibroglandular tissue. In addition, contrast-enhanced breast MRI is powerful and highly sensitive for detecting breast cancer; however, its specificity is limited. This ambiguity often necessitates biopsy and histological analysis of suspicious MRI-detected lesions for optimal management [1].
Suspicious lesions with non-mass enhancement detected on MRI are frequently undetected on second-look US, with only around half appearing as non-mass lesions [2]. Integrating the US and MRI lexicon is logical and may help streamline clinical management. For example, ductal carcinoma in situ (DCIS) and invasive lobular carcinoma (ILC) usually manifest as non-mass lesions on breast US and MRI [3, 4]. Despite this, the American College of Radiology Breast Imaging Reporting and Data System (ACR BI-RADS) breast US lexicon does not contain this information. This omission may seed confusion about how to describe and manage non-mass lesions detected on breast US. I recommend using this article’s definition of a non-mass lesion on breast US to promote use of a standardized terminology.
Definition of non-mass lesions on breast US
On breast US, a non-mass lesion is a hypoechoic area that has an indistinct shape on two different projections, but that does not fit the criteria of a mass; that is, it lacks convex outer borders and conspicuity [3]. This definition was used in at least 47 articles. The proposed definition of breast non-mass lesions is based on normal breast anatomy and will inform physicians’ and sonographers’ interpretations of US findings.
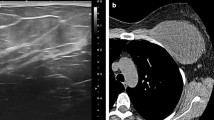
Breast US can identify ducts and lobules, with surrounding hard stroma depicted in gray. US also depicts fibrous tissue, including edematous and fat-containing stroma, as a white area in the breast (Fig. 1) [5]. Fibroglandular tissue is depicted as a mixture of gray and white (Fig. 1). The gray dendritic structures present normal ducts and lobules on breast US (Fig. 1). On breast US, non-mass lesions will feature abnormal duct and lobule patterns (Fig. 2).
Suspicious duct and lobule patterns on breast US in a 71-year-old woman. This US image shows a non-mass lesion (arrows) at the edge of the breast with disrupted duct tapering. Normally, duct and lobule patterns tapering off at the edge of the breast are noted (see Fig. 1). The results of the pathological examination confirmed LCIS. N indicates the nipple
Importantly, DCIS and ILC frequently manifest as non-mass lesions on breast US [3, 4] and feature focal discontinuities and disruptions in the gray dendritic structures that otherwise characterize normal ducts and lobules. Unfortunately, the classification scheme for non-mass image-forming findings on breast US proposed by the Japan Association of Breast and Thyroid Sonology since 2004 [6] is unnecessarily complicated. This classification scheme differs from the simple and practical definition of non-mass lesions on breast US offered here.
Associated findings of non-mass lesions on breast US
Calcifications
Non-mass lesions often include echo patterns that extend over hypoechoic or heterogeneous echogenicity. Internal echoic characteristics with small hyperechoic foci (i.e., calcifications) differ from surrounding normal gray dendritic structures, which depict normal ducts and lobules. Therefore, non-mass lesions with associated calcifications on breast US (Fig. 3) tend to be malignant [7,8,9,10] and should be classified as suspicious and biopsied.
Non-mass lesions with associated calcifications on breast US in a 61-year-old woman. a The US image shows a non-mass lesion (arrowheads) with internal hypoechoic echogenicity and small hyperechoic foci (arrows). b The mammogram shows segmental fine pleomorphic and line-branching calcifications. A subsequent pathological examination confirmed high-grade DCIS
Architectural distortion
Architectural distortion on breast US means that fibroglandular tissue is distorted by non-mass lesions. This may include thin straight lines, radiating spiculations from non-masses, and focal retraction at the fibroglandular tissue's anterior or posterior edge. While non-mass lesions with associated architectural distortion on breast US tend to be malignant [9, 10], they can be benign lesions (Fig. 4). These findings should be classified as suspicious and biopsied.
Non-mass lesions with associated architectural distortion on breast US in a 70-year-old woman. a The US image shows a non-mass lesion with thin straight lines (arrows) and radiating spiculations (arrowheads). b The breast tomosynthesis image shows spiculations (arrow). A subsequent pathological examination confirmed sclerosing adenosis
High elasticity
Shear wave elastography quantifies a tissue's elasticity in kilopascals or meters per second, and the color-coded images are generated in real time by local estimation of shear wave propagation speed. This method improves the diagnostic specificity of breast US for non-mass lesions [9]. Real-time elastography can assist in diagnosis, given its increased specificity for distinguishing benign from malignant non-mass lesions (Fig. 5).
US strain and B-mode images in split-screen mode in a 70-year-old woman. The right B-mode image shows a non-mass lesion with radiating spiculations (arrows). The left real-time elastography image depicts the lesion in blue, indicating high strain and an elasticity score of 4. A subsequent pathological examination confirmed ILC
Hypervascularity
Color or power Doppler US is used to depict blood vessels in non-mass lesions. Malignant non-mass lesions feature significantly higher vascularity (more than two vessels) than benign non-mass lesions (Fig. 6). Color Doppler US improves the specificity of breast US for characterizing non-mass lesions [9]. Elasticity and vascularity results can help characterize non-mass lesions on breast US better than morphological features on B-mode US.
Correlation between MRI and US depictions of non-mass lesions
Contrast-enhanced breast MRI is a powerful and highly sensitive breast imaging tool; however, its specificity is limited [1]. Suspicious MRI-detected lesions require a biopsy and histological examination to determine the optimal management. Suspicious MRI-detected lesions with non-mass enhancement are frequently undetected on second-look US. Approximately half of the malignant lesions with non-mass enhancement on MRI appeared as non-mass lesions on second-look US [2]. MRI-detected lesions can appear so subtle on breast US that they tend to be classified as non-mass lesions [1, 11, 12]. The ACR BI-RADS breast US lexicon inadequately describes such lesions, and their management and follow-up still need to be standardized.
DCIS is often diagnosed as non-mass enhancement on MRI [13]. Non-mass findings on breast US should be considered analogous to non-mass findings according to the ACR BI-RADS breast MRI lexicon [3]. Wherever possible and appropriate, we should use standardized terminology to describe non-mass lesions on both breast MRI and US.
Non-mass enhancement distribution
There are several different non-mass enhancement distribution patterns on breast MRI. Segmental or linear enhancement patterns are typical of DCIS on MRI; the corresponding images on breast US are usually in agreement with MRI findings (Figs. 7 and 8) [13].
High-grade DCIS in the left upper outer breast in a 61-year-old woman. a The axial postcontrast T1-weighted image shows clumped linear enhancement with clustered ring enhancements (arrows). b The corresponding US image shows a clumped linear non-mass lesion (arrowheads) with associated calcifications (arrows)
Conclusion
Understanding the clinical relevance of non-mass findings on breast US is imperative for managing a patient's diagnosis. Categorizing hypoechoic areas with indistinct boundaries as “mass” or “non-mass” lesions can be problematic. A mass lesion with indistinct margins may appear as a non-mass lesion to other readers. Physicians and sonographers should be aware of the benefits and limitations of finding classification schemes. Because we have no standardized definition of “non-mass” lesions included in the ACR BI-RADS, confusion about how to describe and manage these lesions is expected. I am hopeful that the new BI-RADS breast US lexicon will include standardized terminology for describing non-mass lesions on breast US.
Data availability
The author has no relationships relevant to the contents of this paper to disclose.
References
Uematsu T, Takahashi K, Nishimura S, et al. Real-time virtual sonography examination and biopsy for suspicious breast lesions identified on MRI alone. Eur Radiol. 2016;26:1064–72.
Coskun Bilge A, Demir PI, Aydin H, et al. Dynamic contrast-enhanced breast magnetic resonance imaging findings that affect the magnetic resonance-directed ultrasound correlation of non-mass enhancement lesions: a single-center retrospective study. Br J Radiol. 2022;95:20210832.
Uematsu T. Non-mass-like lesions on breast ultrasonography: a systematic review. Breast Cancer. 2012;19:295–301. https://doi.org/10.1007/s12282-012-0364-z
Uematsu T. Ultrasonographic findings of missed breast cancer: pitfalls and pearls. Breast Cancer. 2014;21:10–9.
Izumori A, Horii R, Akiyama F, et al. Proposal of a novel method for observing the breast by high-resolution ultrasound imaging: understanding the normal breast structure and its application in an observational method for detecting deviations. Breast Cancer. 2013;20:83–91.
Japan Association of Breast and Thyroid Sonology. Guideline for breast ultrasound: management and diagnosis. 4th ed. Tokyo: Nankodo; 2020.
Kim SJ, Park YM, Jung HK. Nonmasslike lesions on breast sonography: comparison between benign and malignant lesions. J Ultrasound Med. 2014;33:421–30.
Choi JS, Han BK, Ko EY, et al. Additional diagnostic value of shear-wave elastography and color Doppler US for evaluation of breast non-mass lesions detected at B-mode US. Eur Radiol. 2016;26:3542–9.
Park JW, Ko KH, et al. Non-mass breast lesions on ultrasound: final outcomes and predictors of malignancy. Acta Radiol. 2017;58:1054–60.
Yamaguchi R, Watanabe H, Mihara Y, Yamaguchi M, Tanaka M. Histopathology of non-mass-like breast lesions on ultrasound. J Med Ultrason. 2023. https://doi.org/10.1007/s10396-023-01286-y.
Uematsu T. Real-time virtual sonography (RVS)-guided vacuum-assisted breast biopsy for lesions initially detected with breast MRI. Jpn J Radiol. 2013;31:826–31.
Nakashima K, Uematsu T, Harada TL, et al. MRI-detected breast lesions: clinical implications and evaluation based on MRI/ultrasonography fusion technology. Jpn J Radiol. 2019;37:685–93.
Greenwood HI, Heller SL, Kim S, et al. Ductal carcinoma in situ of the breasts: review of MR imagingfeatures. Radiographics. 2013;33:1569–88.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflicts of interest to declare.
Ethical statements
All procedures followed the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Uematsu, T. Non-mass lesions on breast ultrasound: why does not the ACR BI-RADS breast ultrasound lexicon add the terminology?. J Med Ultrasonics 50, 341–346 (2023). https://doi.org/10.1007/s10396-023-01291-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-023-01291-1