Summary
Background
Large incisional hernias (LIH) are challenging conditions, often necessitating complex surgical procedures such as transversus abdominis muscle release (TAR). We evaluated the feasibility and effectiveness of tension-free abdominal wall repair of LIH with an innovative modified Rives–Stoppa procedure employing a composite free lateral polypropylene (FLaPp) prosthesis.
Methods
Symptomatic patients affected by LIH and treated with FLaPp composite prosthesis between April 2010 and December 2016 were retrospectively analyzed. The FLaPp prosthesis is made up of two layers: an internal layer based on a polypropylene film that can be used in contact with the intestinal loops to address the posterior peritoneal defect, and an external layer based on a macroporous lightweight mesh, with which a classic repair according to Rives–Stoppa is carried out.
Results
Forty-three patients were enrolled in the study. All hernias were W3. Early complications were seroma (16.3%), hematoma (11.6%), wound infection (7.0%), and bowel injury (2.3%). Late complications were sinus tract (4.7%), occasional pain (2.3%), and stiff abdomen (9.3%). The median operative time was 126 min and median hospitalization was 8 days. At the median follow-up of 40 months (range 37.5–117), the recurrence rate was 9.3% (4/43).
Conclusion
Use of FLaPp mesh with a tension-free surgical approach is an effective strategy for managing LIH in selected cases with the presence of a posterior defect, with low rates of complications and recurrences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Incisional hernias are a heterogeneous issue and different repair methods have been proposed for specific defects or locations. In large incisional hernia (LIH), the abdominal wall is qualitatively and quantitatively compromised, due mainly to the loss of muscular and fascial tissue and peritoneum; therefore, correct and complete containment of the intestine in the abdominal cavity no longer exists and surgical reduction might be challenging [1]. In these cases, the surgical technique aims to restore the anatomy of the abdominal wall by integrating the loss of substance and by limiting tension on the suture line of the mesh herniorrhaphy [2]. Patients with the abovementioned alteration are frequently obese, often experience severe abdominal symptoms, and may present cardiopulmonary or other systemic diseases. Moreover, the incisional hernia is often recurrent. Incorrect reconstruction of the abdominal wall can lead to an increase in intraabdominal pressure and cause severe postoperative respiratory complications [3, 4]. There is no consensus regarding the optimal treatment option, despite new and evolving surgical techniques applied by surgeons with different skills. Treatment of LIH is in fact a major and unsolved issue, with associated potentially life-threatening complications [5]. Options for mesh implantation in LIH include bridging the defect without approximation of the defect edges in extreme conditions, performance of an intraperitoneal onlay mesh (IPOM) repair, or mesh augmentation with approximation of the tissue and fascial edges. It is noteworthy that sublay positioning of the mesh, perhaps in combination with a component separation technique (CST), may be advantageous compared with other surgical techniques for LIH repair [2]. Nevertheless, the techniques including CST are associated with considerable morbidity and significantly longer operative time, another acknowledged risk factor for patients with severe or multiple comorbidities and/or previous unsuccessful operations [1].
Several new prosthetic materials and meshes have been proposed to overcome these concerns. Composite meshes are nowadays widely used worldwide in different ventral hernias for their moderate complication rates and low infection and recurrence rates [5]. Starting from our previous experience, we retrospectively analyzed the feasibility and efficacy of an innovative tension-free reconstruction approach with a composite mesh, namely the free lateral polypropylene (FLaPp; Dipro Medical Devices, DIPROMED s.r.l., San Mauro Torinese TO, Italy) prosthesis, in a larger high-risk LIH patient cohort in terms of early postoperative complications and late outcomes [6].
Materials and methods
We performed an update of a retrospective multicentric cohort analysis from a prospectively maintained database of high-risk patients affected by incisional hernia in two Italian teaching hospitals. High-risk patients were considered patients with one or more comorbidities reaching an American Society of Anesthesiologists (ASA) preoperative assessment score of III or IV [7]. From April 2010 to December 2016, consecutive symptomatic patients with midline LIH were eligible for inclusion in the study, which was conducted according to the Declaration of Helsinki. The work is reported in line with the STrengthening the Reporting of Observational studies in Epidemiology (STROBE) criteria [8]. All patients gave their consent to participate this observational study after being appropriately informed. In the current study, LIH was defined as a fascial defect (hernial orifice) measuring 10 cm or more in any direction according to the definition of the European Hernia Society (EHS) [9].
Exclusion criteria were patients affected by lateral incisional hernias (L1-L2-L3-L4) according to EHS [8], collagen diseases (i.e., scleroderma, Ehler–Danlos and Marfan syndromes), patients who have recently undergone chemotherapy and/or radiation therapy for cancer, immunosuppressive therapy, and patients with acquired immunodeficiency. Patients with associated problems of infected mesh in situ from previous repair and/or intraabdominal infection, and patients with associated parastomal hernia were also excluded from the study [10].
Preoperative evaluation
The preoperatively recorded variables were sex, age, body mass index (BMI), coexisting diseases, preoperative ASA assessment score, type of incisional hernia, operative time, and length of hospital stay. All patients underwent preoperative computed tomography (CT) of the abdomen with functional maneuvers that increase intraabdominal pressure (Valsalva, lateral decubitus) and, particularly, multidetector CT, which allows three-dimensional reconstruction to assess the presence of loss of domain.
Characteristics of the prosthesis
The FLaPp is composed of two layers: an upper light polypropylene mesh (PM) and a thin polypropylene transparent film (PPF). The two layers possess different properties in order to best perform their functions. The mesh for the parietal side is macroporous, with 91% porosity and 48 gr/m2, and is made of polypropylene monofilament of 120 μm diameter. The woven polypropylene is designed for tissue ingrowth for adequate prosthesis integration, due to its 2D scaffold feature which improves cell proliferation [11, 12]. The film for the visceral side is composed of nonporous, smooth, and transparent polypropylene with a thickness of 50 μm (type IV) [13] to minimize adhesion formation at the intestinal side due to its plane surface. In fact, the fibroblasts on the film do not proliferate, and, as a consequence, undergo apoptosis, a physiological death [13, 14]. The transparency of the polypropylene film enables easy identification of blood vessels, nerves, and underlying tissues during prosthesis placement and allows the risk of bowel injuries to be limited. The PM and the PPF are sewn together in the central oval area of the composite prosthesis: the two free peripheral flaps of the mesh have a dimension adaptable to the defect size. Moreover, as regards to the tensile strength of the polypropylene film, mechanical tests have demonstrated that its resistance exceeds that of the thread, and the passage of the needle does not cause any tearing of the film itself. De Maria et al. analyzed the influence of the topology of polypropylene for abdominal wall repair, evaluating its ability to prevent and minimize adhesion formation and promote tissue ingrowth. Finally, they found that the mechanical behavior of mesh presents an anisotropy index similar to that of natural tissue as well as a high safety index [14].
Surgical technique
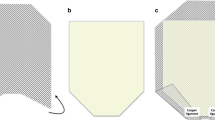
All patients received prophylactic preoperative chemotherapy with cefazolin (2 g) i.v. and all procedures were performed under general anesthesia. Once the peritoneum has been incised and the contents of the sack have been freed from adhesions, the medial edge of the rectus muscle fascia is incised on the posterior side to ensure continuity of the anterior fascial plane, which is essential to allow easy supraprosthetic closure [6]. The lateral extent of this dissection is the linea semilunaris, confirmed by visualizing the junction between the posterior and anterior rectus sheaths. Inferiorly, the space of Retzius is entered to expose the pubis symphysis and both Cooper’s ligaments. This dissection is blunt, in what is typically a bloodless plane. Since this area is below the arcuate line, the posterior layer includes peritoneum and transversalis fascia only. Proximally, the posterior fascia is transversally incised, bringing the dissection back to a preperitoneal plane, allowing it to be extended beyond the retroxiphoid region and the costal margins. Once this surgical time was over, in all the treated cases, only a posterior separation of the components would have allowed the closure of the posterior fascial due to the tissue deficit. The FLaPp prosthesis allows this necessity to be overcome, permitting realization of a simpler and faster intervention (Fig. 1, [6]). The PPF flap was sutured to the margins of the residual peritoneum and posterior rectus sheath to replace the tissue defect [15, 16]; short running sutures were used, adapting this flap to the shape and size of the defect, and the excess film was eliminated. This lower layer of the prosthesis had to be large enough to cover the defect and overlap the tissues for at least 1 cm. At this point, the prosthesis already appears well fixed and the PM flap is positioned on the large retromuscular dissection area according to the principles of Rives–Stoppa (Fig. 2). Superiorly, the PM flap can be placed beyond the costal margin and in the retroxiphoid space. It is secured with one stich to the xiphoid process; this suture is placed 4–5 cm off the edge of the PM flap to allow for large overlap, especially for subxiphoid defects. Finally, the macroporous mesh is anchored at the lateral extremities with two transparietal transfixed sutures or with full-thickness transabdominal stitches using the Reverdin needle and can be further fixed with fibrin sealant (TISSEEL; Baxter, Deerfield, IL, USA) [15, 17]. In all patients we used a FLaPp prosthesis with dimensions of 20 × 30 cm, 26 × 34 cm, or 30 × 40 cm, according to the defect size. Closure of the anterior plane with reinsertion of the rectus is performed easily, because access to the posterior fascia of the rectum is achieved while strictly maintaining continuity of the anterior fascial plane. Running sutures with an absorbable monofilament (polydioxanone) are used [6]. Two closed-suction drains were usually positioned directly over the mesh. The drains were removed when the output was < 25–30 mL of drainage over a 24-hour period. All patients used an elastic waistband for at least 2 months.
Schematic picture of FLaPp® (Dipro Medical Devices, DIPROMED s.r.l.) prosthesis application. The lower layer (transparent polypropylene film, PPF) is sutured to defect margins of the posterior plane (black line). The superior layer (macroporous polypropylene mesh, PM) is positioned in the retromuscular space according to the Rives–Stoppa procedure. Centrally, the two layers of the prosthesis are sewn together (oval)
Outcome measure
Primary outcome was the evaluation of feasibility and safety of the FLaPp prosthesis in patients affected by LIH by the assessment of early events such as postoperative surgical site occurrence (SSO), morbidity, mortality, and pain. Secondary outcome was the assessment of patients’ satisfaction with the procedure and the onset of recurrence and of other late complications (i.e., chronic mesh inflammation, mesh bowel erosion, mesh migration, sinus tract formation, onset of occasional pain or stiff abdomen).
Follow-up data were obtained from analysis of the surgeons’ office records, the patients’ hospital and outpatient electronic medical records, and a survey conducted via telephone. Follow-up appointments were scheduled at 2 weeks, 1 month, 6 months, and at the end of the follow-up (3 years). In the first 30 days, the incidence of SSO such as seroma, hematoma, surgical site infection (SSI), intensity of pain, and perioperative morbidity and mortality were evaluated [18]. Moreover, the incidence of chronic mesh inflammation, mesh bowel erosion, mesh migration, sinus tract, stiff abdomen, chronic pain, and recurrence rate were also recorded. The degree of pain was determined using a 10 cm visual analog scale (VAS) [19] (0 = no pain and 10 = worst possible pain) on postoperative day 15 and day 30, and by assessing the number of oral analgesic drugs (ketorolac 30 mg) taken by each patient. In fact, all patients received a prescription for analgesic drugs to be taken as required and they duly recorded analgesic use on a paper to be given back to the surgeon. Hernia recurrence was diagnosed by physical examination, which was performed serially in the outpatient setting.
To assess patients’ satisfaction, a quality of life (QoL) questionnaire (the so-called 36-Items Short-Form Health Survey, SF-36) was administered by an external observer (i.e., a surgical fellow) 7–10 days before surgery, and at the third year of follow-up. [20, 21]. The SF-36 defines eight domains of health status: physical functioning (PF), physical role limitations (PR), bodily pain (BP), general health perceptions (GH), energy/vitality (EV), social functioning (SF), emotional role limitations (ER), and mental health (MH). The number of questions contributing to each domain varies from 2–10. Response values for each question range from 1–6. All domain scales are standardized from 0–100, with higher scores corresponding to better health status.
The database of this study was completed in January 2020 in order to achieve a minimum follow-up time of 3 years for each patient, including the last one operated in 2016.
Statistical analysis
Continuous variables were expressed by mean and standard deviation (SD), categorical variables were reported in terms of median and interquartile range (IQR; 25th–75th percentile). The two-tailed Mann–Whitney U test or the chi-square test was applied to analyze the relationship between each preoperative characteristic and recurrence, according to variable type. The one-tailed paired Student t-test was applied to evaluate the increase in SF-36 score recorded preoperatively and at long-term follow-up for each of the eight domains separately. A p-value < 0.05 was considered statistically significant. Statistical analysis was carried out using MATLAB® 2021a (MathWorks; Natick, MA, USA) software. Analysis of SF-36 scores was performed according to guidelines developed by Ware and Sherbourne and Apolone and Mosconi [20, 21].
Results
The study population included 43 symptomatic LIH patients (26 women and 17 men) with a median age of 60 years (IQR: 57–66 years) and a mean BMI of 29.8 ± 2.5 kg/m2. Demographic data, clinical details, and the characteristics of incisional hernias are summarized in Table 1. All patients had at least one comorbidity and 14 (32.6%) had more than one coexistent disease. Based on the type of comorbidity, 38 patients (88.4%) had been classified as ASA III and 5 (11.6%) as ASA IV. Out of 43 patients, 32 (74.4%) were affected by midline xipho-pubic incisional hernia (M1–M5) and 11 (25.6%) by midline xipho-subumbilical incisional hernia (M1–M4). All incisional hernias had been classified as class W3 [6]. The median craniocaudal and transverse dimensions of the rectus sheet fascial defect were 22 cm (IQR: 20–23 cm) and 14 cm (IQR: 11–18 cm), respectively. Thirty-two patients (74.4%) had undergone the previous repair with mesh reinforcement; in 18 patients (41.9%) the incisional hernias were irreducible. The median operative time was 126 min (IQR: 93–160 min) and the median hospital stay was 8 days (IQR: 7–9 days). Median follow-up was 40 months (range 37.5–117) and follow-up has been completed in all 43 patients (100%).
Primary outcome
No major complications related to the operation or to anesthesia were observed, and no mortality occurred. SSO included 7 seromas (16.3%), 5 hematomas (11.6%), and 3 wound infections (7.0%; Table 2). Three of the 7 patients with seroma were treated by 3 or 4 needle aspirations, while the other 4 patients healed spontaneously; of the 5 patients with hematoma, only one was treated with a small skin incision under local anesthesia and with surgical drainage. All patients with wound infection were treated with antibiotic drugs after microbiological and antibiogram examinations. Two of them also required surgical drainage of the subcutaneous space and negative pressure wound therapy (VAC therapy). Early postoperative pain was controlled for the first 72 h with infusion therapy using an elastomeric pump prepared as follows: 50 mg of morphine (5 ampoules) + 50 mg of metoclopramide (5 ampoules) + 210 mg of ketorolac (7 ampoules) + 300 mg of clonidine (2 ampoules), at the infusion rate of 2 ml/hour. At postoperative day 15, 39 patients (90.7%) had a VAS score lower than 4 (range 1–3.5) and only 6 patients took one analgesic pill (ketorolac 30 mg) per day for another six days. At 30 days, the patient’s VAS score was less than 3 (range 0–2) and no patient required analgesic drugs parenterally or orally. Medical complications occurred in 6/43 patients (14.0%): particularly prolonged postoperative ileus occurred in 3 subjects (7.0%), pneumonia in one (2.3%), and urinary tract infection in 2 patients (4.7%). Moreover, all three patients with chronic renal failure (7.0%) suffered from exacerbation of renal disease and were treated with intravenous infusions of hydroelectrolyte solutions.
Secondary outcome
No patients presented chronic mesh inflammation, mesh bowel erosions, or mesh migration. No patients suffered from chronic pain: only one (2.3%) described pain that occasionally limited activities such as gardening or light gym work. However, we documented two cases (4.7%) of sinus tract and 4 patients (9.3%) complained about a stiff abdomen. Recurrence rate was 9.3% (4/43; Table 2). Of the abovementioned cases, one elderly patient required reoperation for total mesh excision 48 h after surgery for biliary peritonitis due to ischemic bowel perforation; this patient relapsed after 3 months. Two patients underwent partial mesh excision for development of a chronic sinus tract and one of these two developed a recurrence 8 months after primary surgery. Finally, the other two patients relapsed at 15 and 18 months after surgery, respectively. None of the preoperative patient characteristics were significant for the development of hernia recurrence (p > 0.2); however, two patients were obese and two patients were diabetic, and all four cases were irreducible IHs.
QoL results are summarized in Table 3. In terms of the preoperative functional status, significant deficiencies (mean score < 50) were reported in four SF-36 domains: physical function (34.9 ± 1.7), physical role limitations (35.1 ± 2.1), bodily pain (27.8 ± 1.5), and emotional role limitations (39.4 ± 1.6). At 3‑year follow-up, we documented a significant improvement in all domains (p < 0.0001) except for energy/vitality (p = 0.45).
Discussion
Rives–Stoppa surgery has been the reference for surgical repair of the abdominal wall for decades, and, in some respects, continues to be so. The intuition that only reinsertion of the rectus into the midline allows for a solid and lasting repair remains the basic principle to which all parietal surgery refers and positioning of the prosthesis at the retromuscular site is universally accepted as the best site for the best prosthetic integration [1,2,3]. However, the weak point of the Rives–Stoppa emerges in LIH or in incisional hernias, in which a wall defect is created (for example, after removal of previous prostheses), which is given by the difficulty of closing both the posterior and anterior planes to ensure a valid reinsertion of the rectus in the midline. To try to overcome this difficulty, various stratagems have been devised: fascial releases and myofascial advancement flaps, use of peritoneal patches in Vycril, use of progressive preoperative pneumoperitoneum that saw Stoppa’s Amiens school one of the most convinced proponents. The IPOM solution is also possible; however, it does not realize the abdominal wall reconstruction [22].
The “component separation” techniques, in both their anterior and posterior meanings, have filled this gap by offering the surgeon a technical and cultural background that allows him to face complex abdomens more safely. In this context, the use of botulinum toxin and the return of interest in preoperative progressive pneumoperitoneum have represented further, important progress in recent years. The origin of the limitation of the technique is the fact that Rives and Stoppa had turned their attention only to the central compartment consisting of the rectus muscles and their fascial sheath, not extending the interest to the lateral compartment and to the advantages that could derive from a muscle aponeurotic mobilization of the lateral muscles of the abdominal wall [14].
The anterior component separation (ACS) described by Ramirez et al. in 1990 is considered the actual referral procedure in the treatment of LIH worldwide [23]. However, its great efficacy does not come for free and is paid for by the complexity of the procedure with a high rate of postoperative wound complications and a non-neglectable recurrence rate even with mesh reinforcement (1–5-year recurrence rate is about 10%) [23]. Additionally, the main criticism of the posterior component separation (PCS) technique described by Carbonell et al. [24] is the access to the space between the transverse abdominis muscles and the internal oblique muscles. This space contains the branches of the lateral cutaneous nerve, which, if damaged, would lead to lateral muscle paralysis and laterodorsal bulging. Moreover, PCS with transversus abdominis release (TAR) expects a dissection that preserves all the spraying and innervation of the abdominal wall [25]. However, the extent of the dissection, the difficulty in finding a valuable peritoneal plane in pluri-operated patients, or the frequent presence of a very thin peritoneal plane could lead to repair with two prostheses (resorbable and not resorbable). Novitsky et al. [25], in a study conducted in a total of 42 patients, reported a wound complication rate of 24% (10/42) and 7% of patients had major wound infection requiring surgical debridement; 32 (76.2%) had recurrent hernias. Winder et al. [26] reported wound complications in 24.3% of patients and Krpata et al. [27] in 48.21% of patients treated with ACS. Hood et al. [28] observed 32% wound infections; of these complications, 18% required wound VAC placement and 5% needed formal debridement in the operating room. Moreover, Kumar et al. [29] reported 17.85% seroma and Torregrosa-Gallud et al. [30] 35.1% seroma and 8.8% skin necrosis.
In the current series, we proposed an innovative surgical approach that employs the FLaPp composite mesh for hernia repair even in cases of abdominal wall defects with difficult closure of the posterior plane, when conventional prosthetic meshes might be unsuitable, as with absorbable or PTFE prosthesis. We focused our attention on high-risk patients who might be unsuitable for long or laparoscopic procedures. Completely absorbable meshes are affected by a high risk of recurrence and thereby considered a bridge procedure. Conversely, using a composite mesh, as in our series, with an anterior polypropylene layer, a high structural and tensile resistance is guaranteed, allowing the limits due to the large tissue defect that should request the more complex component separation to be overcome. Previously published in vitro studies on a composite mesh with the same characteristics and materials (Clear Mesh Composite, CMC; Dipromed srl, Italy) have shown that on the one side, the macroporous morphology of the polypropylene lightweight mesh, which is in contact with muscle tissue, allows the colonization and integration of fibroblasts [11]; while on the other side, the smooth morphology of the polypropylene film shows selective colonization, avoiding colonization of fibroblasts [11] and allowing colonization of mesothelial cells [31], which allows for regeneration of the peritoneum. Moreover, a previously published in vivo animal study on the same composite mesh showed lower adhesions rate with the internal organs, using the CMC devices, respect to a simple polypropylene lightweight mesh [32]. A similar anti-adhesion property of polypropylene shaped as a flat film was also reported several years ago in an in vivo animal study by Amid et al. [12]. The proposed technique is far from being considered a bridge procedure, since the lower face of the mesh is fixed to the peritoneum residual borders and to the posterior rectus sheath; the upper macroporous face of the mesh is circumferentially stitched to the retromuscular layer as in the conventional Roves technique. This latter polypropylene face is the one that contributes majorly to the restoration of abdominal wall function.
We reported a recurrence rate of 9.3% (4/43) at a median follow-up of 40 months, a result similar to that reported by Novitsky et al. [25], Winder et al. [26], Krpata et al. [27], and Posielski et al. [33] (4.7, 2.7, 3.6, and 6.3%, respectively). All the recurrences were in patients with midline xipho-pubic incisional hernia (M1–M5) and irreducible hernias. Moreover, two of these patients were diabetic and two were obese. However, none of the preoperative patient characteristics were significant for development of hernia recurrence (p > 0.2). Therefore, to limit the postoperative risk of recurrence, it appears of utmost importance to ensure metabolic control of the patient and have a detailed preoperative and intraoperative definition of the dimensions of IH to avoid underestimation of the dimensions of the mesh and the defect overlap.
The incidences of wound infections, seromas, and hematomas were low: 7.0% (3/43), 16.3% (7/43), and 11.6% (5/43), respectively. More complex operations, such as ACS and PCS with or without TAR, involve a longer operative time. The operating times reported by Carbonell et al. [24], Novistky et al. [25], Winder et al. [26], and Krpata et al. [27] are 258 min (median range 190–480), 235 min (mean range 138–400), 359 min (median range 255–683), and 228 min (mean range 10–549) for the PCS technique and 285 min (mean range 180–600) for the ACS technique, respectively. Moreover, Hood K et al. [28], Kumar R et al. [29], and Torregrosa-Gallud A et al. [30], using a modified component separation technique without “transverse muscle release,” report operating times of 182.6 min (mean range 120–480), 180 min (mean), and 194 min (mean ± 95 SD), respectively. In our experience, the median operative time was lower (126 min; IQR: 93–160). In our opinion, a long operative time is an additional risk factor in these frail patients. Therefore, use of the FLaPp prosthesis and a tension free abdominal wall reconstruction allows results similar to those of other authors in terms of recurrence rate, but with an important reduction in operative time and fewer early and late postoperative complications, making the procedure suitable for frail subjects—a frequent characteristic among LIH patients. An interesting and unexpected observation was the recorded pain intensity: 15 days after surgery, 39 patients (90.7%) had a VAS score lower than 4 and at day 30 the score was less than 3. These results can be explained by the fact that the composite FLaPp mesh allows for tension-free abdominal wall repair. Our data on quality of life reported low preoperative values of all eight domains of the SF-36 questionnaire, as symptomatic large incisional hernia is a highly debilitating pathology. The improvement obtained in PF, PR, and BP domains, all related to the perception of pain, show that the surgical approach was effective in repairing LIH. The absence of postoperative improvement in EV domains can be explained by the presence of severe comorbidity in our patients, which may be responsible for the reduction in physical activity and psychological manifestations. The advantage of this prosthesis is that it enables “tailored surgery” thanks to the two free flaps which can be shaped according to the hernia defect.
The novelty of the proposed technique can be summarized in a short sentence: such a surgical approach allows a relatively easy Rives–Stoppa to be performed in cases in which the wall defect would normally require alternative solutions (e.g., posterior component separation), which, however, cannot be implemented for various reasons. We think of emergency interventions in which the surgeon on duty does not have sufficient knowledge and skills to perform this type of intervention, of fragile patients who should be subjected to simple and rapid interventions with reduced operating time, and of all those clinical conditions in which transverse dissection becomes complex (multiple surgical operations, previous prostheses). The lower flap in polypropylene film replaces the missing peritoneum as much as necessary and allows positioning of the upper flap in the classic retromuscular seat. The incision of the medial fascial edge of the rectum posteriorly leaves the anterior fascial plane intact, allowing its systematic closure and the reinsertion of the rectum. Moreover, abdominal compartment syndrome, which is sometimes hard to avoid in complex incisional hernia cases but was not investigated in the current study, might be a direct advantage of this technique.
Recently, several authors have assessed the efficacy and feasibility of laparoscopic anterior and posterior component separation in large midline defects. The literature still lacks definitive results. However, Balla A et al., in a systematic review, reported worse results for anterior CST with midline closure by laparotomy in terms of postoperative surgical complications and recurrence in comparison to pure minimally invasive anterior and posterior CST [34]. These encouraging results were also confirmed Dauser B et al. in 15 patients [35].
This retrospective analysis is subject to some limitations, such as the limited number of cases, the enrollment of only patients with midline defects to obtain more homogeneous results, and the retrospective design of the study.
Conclusion
Considering the current series, the FLaPp composite prosthesis for large incisional hernia repair in cases of abdominal wall defects with difficult closure of the posterior plane is safe and reproducible. This procedure appears extremely promising, especially for frail patients not suitable for longer and more complex surgery such as component separation, guarantying low recurrence rates and an important improvement of patients’ QoL. Further larger comparative studies are necessary to understand the trajectory of this patient population and to quantify the risk of long-term recurrences.
References
Azar FK, Crawford TC, Poruk KE, Farrow N, Cornell P, Nadra O, et al. Ventral hernia repair in patients with abdominal loss of domain: an observational study of one institution’s experience. Hernia. 2017;21:245–52.
Deerenberg EB, Timmermans L, Hogerzeil DP, Slieker JC, Eilers PH, Jeekel J, et al. A systematic review of the surgical treatment of large incisional hernia. Hernia. 2015;19:89–101.
Munegato G, Brandolese R. Respiratory physiopathology in surgical repair for large incisional hernias of the abdominal wall. J Am Coll Surg. 2001;192:298–304.
Munegato G, Grigoletto G, Brandolese R. Respiratory mechanics in abdominal compartment syndrome and large incisional hernias of the abdominal wall. Hernia. 2000;4:282–5.
Eriksson A, Rosenberg J, Bisgaard T. Surgical treatment for giant incisional hernia: a qualitative systematic review. Hernia. 2014;18:31–8.
Munegato G, Fei L, Schiano di Visconte M, Da Ros D, Moras L, Bellio G. A new technique for tension-free reconstruction in large incisional hernia. Updates Surg. 2017;69(4):485–91. https://doi.org/10.1007/s13304-017-0493-1.
Sidi A, Lobato EB, Cohen JA. The American Society of Anesthesiologists’ Physical Status: category V revisited. J Clin Anesth. 2000;12:328–34.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. https://doi.org/10.1016/j.jclinepi.2007.11.008.
Muysoms FE, Miserez M, Berrevoet F, Campanelli G, Champault GG, Chelala E, et al. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13:407–41.
Slater NJ, Montgomery A, Berrevoet F, Carbonell AM, Chang A, Franklin M, et al. Criteria for definition of a complex abdominal wall hernia. Hernia. 2014;18:7–17.
Canuto RA, Saracino S, Oraldi M, Festa V, Festa F, Muzio G, et al. Colonization by human fibroblasts of polypropylene prosthesis in a composite form for hernia repair. Hernia. 2013;17:241–8.
Amid PK, Shulman AG, Lichtenstein IL, Sostrin S, Young J, Hakakha M. Experimental evaluation of a new composite mesh with the selective property of incorporation to the abdominal wall without adhering to the intestines. J Biomed Mater Res. 1994;28:373–5.
De Maria C, Burchielli S, Salvadori C, Santoro V, Montemurro F, Orsi G, et al. The influence of mesh topology in the abdominal wall repair process. J Biomed Mater Res B Appl Biomater. 2016;104:1220–8.
Campanelli G, Bastazza M, Ruca A, Senni Buratti M, Casirani R, Nicolosi FM, et al. Surgical treatment of incisional hernias with marked loss of substance. Hernia. 2000;4:202–5.
Canziani M, Frattini F, Cavalli M, Agrusti S, Somalvico F, Campanelli G, et al. Sutureless mesh fibrin glue Incisional hernia repair. Hernia. 2009;13:625–9.
Anthony T, Bergen PC, Kim LT, Henderson M, Fahey T, Rege RV, et al. Factors affecting recurrence following incisional herniorrhaphy. World J Surg. 2000;24:95–100.
Shahan CP, Stoikes NF, Webb DL, Voeller GR. Sutureless onlay hernia repair: a review of 97 patients. Surg Endosc. 2016;30(8):3256–61. https://doi.org/10.1007/s00464-015-4647-2.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–31.
Ware JE, Sherbourne CC. The MOS 36-item short-form health survey (SF-36) I. Conceptual framework and item selection. Med Care. 1992;30:473–83.
Apolone G, Mosconi P. The Italian SF-36 health survey: translation, validation and norming. J Clin Epidemiol. 1998;51:1025–36.
Petersen S, Henke G, Zimmermann L, Aumann G, Hellmich G, Ludwig K. Ventral rectus fascia closure on top of mesh hernia repair in the sublay technique. Plast Reconstr Surg. 2004;114:1754–60.
Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86:519–26.
Carbonell AM, Cobb WS, Chen SM. Posterior components separation during retromuscular hernia repair. Hernia. 2008;12:359–62.
Novitsky YW, Elliott HL, Orenstein SB, Rosen MJ. Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. Am J Surg. 2012;204:709–16.
Winder JS, Behar BJ, Juza RM, Potochny J, Pauli EM. Transversus abdominis release for abdominal wall reconstruction: early experience with a novel technique. J Am Coll Surg. 2016;223:271–8.
Krpata DM, Blatnik JS, Novitsky YW, Rosen MJ. Posterior and open anterior components separations: a comparative analysis. Am J Surg. 2012;203:318–22.
Hood K, Millikan K, Pittman T, Zelhart M, Secemsky B, Rajan M. Abdominal wall reconstruction: a case series of ventral hernia repair using the component separation technique with biologic mesh. Am J Surg. 2013;205:322–7.
Kumar R, Shrestha AK, Basu S. Giant midline abdominal incisional herniae repair through combined retro-rectus mesh placement and components separation: experience from a single centre. Hernia. 2014;18:631–6.
Torregrosa-Gallud A, Sancho Muriel J, Bueno-Lledo J, García Pastor P, Iserte-Hernandez J, Bonafé-Diana S. Modified components separation technique: experience treating large, complex ventral hernias at a University Hospital. Hernia. 2017;21:601–8.
Muzio G, Perero S, Miola M, et al. Biocompatibility versus peritoneal mesothelial cells of polypropylene prostheses for hernia repair, coated with a thin silica/silver layer. J Biomed Mater Res B Appl Biomater. 2017;105(6):1586–93.
De Maria C, Burchielli S, Salvadori C, et al. The influence of mesh topology in the abdominal wall repair process. J Biomed Mater Res B Appl Biomater. 2016;104(6):1220–8.
Posielski NM, Yee ST, Majumder A, Orenstein SB, Prabhu AS, Novitsky YW. Repair of massive ventral hernias with “quilted” mesh. Hernia. 2015;19:465–72.
Balla A, Alarcón I, Morales-Conde S. Minimally invasive component separation technique for large ventral hernia: which is the best choice? A systematic literature review. Surg Endosc. 2020;34(1):14–30.
Dauser B, Ghaffari S, Ng C, Schmid T, Köhler G, Herbst F. Endoscopic anterior component separation: a novel technical approach. Hernia. 2017;21(6):951–5.
Funding
Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to the present research and reviewed the entire manuscript. All authors have read and approved the final manuscript. L. Fei: participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data; also participated substantially in the drafting and editing of the manuscript. G. Munegato: participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data; also participated substantially in the drafting and editing of the manuscript. A. Allaria: participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data. A. Catauro: participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data. S. Rosati: participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data. F. Giordano: participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data; G. Balestra: participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data. L. Docimo: participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data. C. Gambardella: participated substantially in conception, design, and execution of the study and in the analysis and interpretation of data; also participated substantially in the drafting and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
L. Fei, G. Munegato, A. Allaria, A. Catauro, S. Rosati, F. Giordano, G. Balestra, L. Docimo, and C. Gambardella declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Clinical trial number: Clinical Trial.gov no. NCT04801394.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the Unit of Gastrointestinal Surgery, University of Campania “Luigi Vanvitelli,” Naples, Italy, on reasonable request.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fei, L., Munegato, G., Allaria, A. et al. A modified Rives–Stoppa technique with composite mesh (FLaPp) in large incisional hernia: a multicentric retrospective cohort study. Eur Surg 55, 149–157 (2023). https://doi.org/10.1007/s10353-023-00805-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-023-00805-y