Abstract
Conditions encountered during early development affect future survival and reproduction in many bird species. For parents, it is important what body condition the nestlings will achieve at fledging because the condition affects the offspring’s chances to survive and reproduce in the future. However, there is a trade-off between the number of nestlings and their condition. We studied parental behaviour and nestling body condition in uniparental Penduline Tits. In this small passerine, the parental care (incubation and food provisioning) is provided by the female only (49% of clutches in the study population) or the male only (15%). In addition, over a third of clutches are deserted by both parents before the start of incubation. We found that female-only cared clutches had more eggs and nestlings and produced more fledglings than male-only cared clutches. The incubation behaviour and incubation temperature in both types of clutches were similar. The provisioning rate per brood was positively, and the provisioning rate per nestling was negatively, correlated with brood size. Although males cared for smaller clutches, parent sex was not significant in both models of provisioning rates (per brood and per nestling). Moreover, the provisioning rate did not predict the brood’s average nestling condition. However, nestlings reared in broods with male care were in better condition than those reared by females. At the age of 13 days, they had a higher scaled mass index (describes the relative size of energy reserves) and higher haemoglobin levels. The results suggest that the lower productivity of male-only cared clutches, compared to those cared for only by females, may be compensated by the higher condition of nestlings. Information about the recruitment success of broods cared for by males and females will be necessary to test this prediction.
Zusammenfassung
Bei uniparentalen Beutelmeisen sind von Männchen aufgezogene Nestlinge in besserer körperlicher Verfassung als von Weibchen aufgezogene.
Bei vielen Vogelarten wirken sich die Lebensumstände in der frühen Entwicklungsphase auf das künftige Überleben und die Fortpflanzung aus. Für die Elterntiere ist die körperliche Verfassung ihrer Jungen zum Zeitpunkt des Ausfliegens wichtig, weil sie die Überlebens- und zukünftigen Fortpflanzungschancen beeinflusst. Aber es gibt einen trade-off zwischen der Anzahl an Nestlingen und ihrer Verfassung. Wir untersuchten das Verhalten der Eltern und den Körperzustand der Nestlinge bei uniparentalen Beutelmeisen. Bei diesem kleinen Singvogel liegt die elterliche Fürsorge (Bebrütung und Nahrungsbeschaffung) entweder nur bei den Weibchen (49% der Gelege in der untersuchten Population) oder nur bei den Männchen (15%). Außerdem wird mehr als ein Drittel der Gelege von beiden Elternteilen vor Beginn der Brutzeit aufgegeben. Wir stellten fest, dass nur von Weibchen betreute Gelege mehr Eier und Nestlinge hatten und mehr Nachwuchs produzierten als nur von Männchen betreute Gelege. Das Brutverhalten und die Bebrütungstemperatur waren bei beiden Gelege-Varianten ähnlich. Der Versorgungsgrad pro Brut war positiv, die Verpflegungsrate pro Nestling negativ mit der Brutgröße korreliert. Obwohl die Männchen kleinere Gelege betreuten, war das Geschlecht der Eltern in beiden Varianten für den Versorgungsgrad (pro Brut und pro Nestling) nicht signifikant wichtig. Außerdem konnte anhand des Versorgungsgrads keine Vorhersage zum durchschnittlichen Nestlingszustand der Brut gemacht werden. Allerdings waren Nestlinge aus Bruten mit männlicher Betreuung in besserer körperlicher Verfassung als von Weibchen aufgezogene. Im Alter von 13 Tagen hatten sie einen höheren skalierten Massenindex (beschreibt die relative Größe der Energiereserven) und höhere Hämoglobinwerte. Diese Ergebnisse deuten darauf hin, dass die geringere Produktivität von nur von Männchen betreuten Gelegen im Vergleich zu solchen, die nur von Weibchen betreut werden, eventuell durch die bessere physische Verfassung der Nestlinge ausgeglichen werden kann. Zur Überprüfung dieser Vorhersage bräuchte man mehr Informationen über den Bruterfolg der von Männchen und von Weibchen betreuten Bruten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the light of life-history theory, individuals must balance the effort they put into looking after the offspring and save energy for surviving the next winter to have the opportunity to produce offspring in the following breeding season. Intergenerational trade-offs are important as well, e.g. between parental effort and the probability of offspring survival to the next breeding season (Stearns 1989). Therefore, the condition of the reared brood might be crucial because a better condition gives chicks higher chances to survive before leaving the nest as well as after fledging (Coulson and Porter 1985; Tinbergen and Boerlijst 1990; Hochachka and Smith 1991). However, there is also a trade-off between the number of nestlings and their condition (Stearns 1989). Moreover, life-history differences between sexes can influence the selection of parental investment by females and males and result in sex-specific trade-offs (Royle et al. 2012; Santos and Nakagawa 2012).
It has been shown that body condition may affect the life history of an individual, such as reproductive output (Chastel et al. 1995) and/or survival (Lindström 1999). In passerines, an adult’s body condition was found to affect its reproductive success by influencing the brood size and/or laying date (Drent and Daan 1980; Price et al. 1988; Rowe et al. 1994; Christians 2000). Similarly, body condition during early development has consequences for subsequent fecundity and survival (Haywood and Perrins 1992; Merilä and Svensson 1997). Many factors were found to influence the condition of nestlings directly or indirectly by both genetic predisposition and environmental factors (Smith and Wettermark 1995; Merilä et al. 1999; Jensen et al. 2003), such as parent age (Daunt et al. 2001; Janiszewski et al. 2017), food providing (Brommer et al. 2003), amount of nourishment (Bańbura et al. 2007), quality of the territory (Przybylo et al. 2001), habitat structure (Sánchez et al. 2007), offspring sex (Rosivall et al. 2010), relationship with the social father (Freeman-Gallant et al. 2006) or the temperature of incubation (Hepp et al. 2015). Moreover, offspring reared in a highly competitive environment attain lower body condition than offspring from reduced aggression and competition environments (Pryke and Griffith 2009).
We studied the Eurasian Penduline Tit Remiz pendulinus, a small passerine bird (body mass ~ 9 g) with a complex breeding system, and uniparental care (it can be either female-only or a male-only) (Persson and Öhrström 1989). Most clutches are under female care (49% in the study population), the nests where the male takes care are rarer (15%), and a considerable number of clutches are deserted by both parents before incubation starts (36%). Desertion occurs during egg-laying, usually when the second, third or fourth egg is laid (Valera et al. 1997). In a situation of male desertion, a female may either continue egg-laying (mean clutch size of female-only cared clutches = 6.47) or desert an incomplete clutch (mean clutch size of clutches deserted by female = 3.85). If a female deserts before a male, he either starts to incubate or abandons the eggs (van Dijk et al. 2007). Therefore, caring males can only increase their reproductive success at a nest where they stayed, by providing more care, whereas females can also increase the number of young produced. Indeed, earlier studies conducted on this species have shown that female-only cared broods had more eggs and nestlings than the male-only cared broods, and in consequence, they were more productive (Persson and Öhrström 1989; Czyż 2008). However, the offspring-provisioning rate in the study population was the same in both types of broods and after counting it per chick, tended to be higher in male-only cared broods (Walczyna 2006; Czyż 2008). This result suggests that chicks fed by males may be in a better condition than chicks from nests with female care, because they probably get more food. Moreover, the influence on the chicks’ condition can also be caused by competition between the siblings, which would be higher in broods with a bigger number of nestlings, i.e. in broods with female-only care. As a result, the fledgling survival after leaving the nest may be different in female-only and male-only cared broods.
This study aimed to test if there is a difference in the amount of parental care by female and male Eurasian Penduline Tits that provide care, which would cause dissimilarity in nestling body condition reared by only a female or male. We argue that life-history trade-offs might be different for the sexes regardless of their similar sex roles (ambisexual uniparental care). Therefore, we predict that:
-
1)
Clutch size of female-only cared brood will be greater than in male-only cared broods,
-
2)
Female-only cared broods will produce more fledglings compared to male-only broods,
-
3)
Since experiments made by Szentirmai et al. (2005) showed that the clutch size influences the cooling rate in the study species, i.e. smaller clutches lose heat more rapidly than bigger ones, we expect that males would have to spend more time incubating and/or make shorter recesses to maintain similar incubation temperature, or the incubation temperature will be higher in female-only cared clutches,
-
4)
Provisioning rate per nestling will be higher in male-only cared broods than in female-only broods,
-
5)
Nestling condition in male-only cared broods will be higher than in female-only cared broods.
Methods
Field data collection
We studied a European Penduline Tit population from middle April until middle August in 2011–2016, in the nature reserve Milicz Fishponds (SW Poland). The study site (1200 ha) covered 28 ponds (each 18–64 ha) and surrounding land in the Stawno system (51°33’ N, 17°21’ E). The edges of the ponds were overgrown with reedbeds. The dykes were vegetated with deciduous trees, oaks Quercus sp., willows Salix sp., birch Betula pendula, and poplars Populus sp., amongst others. Penduline Tits build their nests predominately in the latter three species.
We searched for new nests every day, and most (97%) of nests were found at the building or laying stage. Birds were captured during nest building with the use of mist nets and a song playback, and individually marked with three colour rings and a metal ring. Some individuals were ringed during incubation (after day 8 of incubation). European Penduline Tit nests are very often difficult to reach (may hang very high above the ground or open water; up to 11 m over the ground on average 3.9 m) and we were not able to look inside all the nests at our study site. We also limited the nest inspection we were able to reach to one (13-day post-hatch) or two visits (during incubation and 13-day post-hatch). The nests were visited every day during building and laying to determine the day of incubation start. We considered the clutch as incubated when the parent started to spend more time inside the nest than previously, mostly without visual indication of building behaviour (although the bird may continue some building of the entrance tube), and made only short recesses outside (typically less than 5 min). In 2011–2012, on the seventh day of incubation, we put temperature loggers (Thermochron iButton Device DS1921G) inside the nests we could reach. The loggers recorded the temperature every 2 min for 56 h starting at noon. In the morning (7:00–11:00) on the day 8 of incubation, we video-recorded (~ 1.5 h, Me = 91.5 min, Q1–Q3 = 90–96, n = 92) the incubation behaviour of the parent (years 2011–2016). Beginning on the twelfth day of incubation, we visited the nests each day to determine the date of hatching. During these visits, we observed if the parent brought food to the nest, which meant that the nestlings had started to hatch. In case we had any doubt (e.g. the parent entered the nest too quick to observe any food in the beak) or if the nest could be easily reached, we looked inside the nest using an inspection camera. In the years 2011–12 and 2014–2015, on day 12 post-hatch, we video-recorded (~ 1.5 h, Me = 92 min, Q1–Q3 = 81–96, n = 50 between 7:00–11:00) offspring provisioning and on the next day (day 13 post-hatch) the nestling maximum tarsus length (to the nearest 0.1 mm using digital callipers) according to Gosler (2004) and mass (to the nearest 0.2 g using Pesola spring balance scale) were measured. We also took two drops of blood from the brachial vein. The first drop was used to measure the haemoglobin concentration (g/L) using the HemoCue HB 201 + analyser (HemoCue AB, Angelholm, Sweden). The second blood drop was preserved in 96% ethanol and used for sex determination of the nestling (see below). The nests were then visited every 2–3 days, and beginning from day 19 of the nestlings’ life, visits were made every day to determine the nest’s success and the fledging date. The number of fledglings (productivity) was accessed either by the number of 13-day-old nestlings or counts of young returning to nests for roosting on the day they fledged or 1 day later. These two measures were highly correlated in our population (rs = 0.94, n = 36, P < 0.001). The number of 13-day-old nestlings present in the nest was decreased by the number of, if present, dead nestlings found inside the nest. The bodies of nestlings that died after the age of 13 days are not removed from the nest by the parents; they, thus, remain inside (authors’ unpubl. data).
In 2015 and 2016, we video-recorded the families entering their nests for roosting starting with the day of fledging and after 5 and 10 days. From the recordings, we counted the number of fledglings that survived at each nest. We included only families that used their nests for roosting at least for 10 days (14 nests with female-only care and 7 nests with male-only care).
Nestling sex determination
We determined the sex of nestlings by polymerase chain reaction (PCR) amplification of the CHD-W and CHD-Z genes using sex1’ and sex2 primers (Wang et al. 2010). DNA was extracted from blood with the use of GeneMatrix Tissue DNA Purification Kit (Eurx) following the manufacturer’s protocol designed for tissues. We performed PCR amplification in a final volume of 10 μL using 1 × PCR buffer (10 × Pol Buffer A, Eurx), 2.0 mM MgCl 2, 0.2 mM of each dNTP, 0.25 U polymerase (Yellow OptiTaq DNA Polymerase, 1U/μL, Eurx), 0.4 μM of each primer, and about 50 ng of DNA. PCR condition were as follows: initial denaturation at 94 °C for 3 min, then 35 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 45 s and extension at 72 °C for 50 s, followed by a final extension at 72 °C for 10 min. PCR products were separated on 3% agarose gels stained with ethidium bromide (2011–2014) or SimplySafe (Eurx), and visualised under UV light. We sexed the nestlings according to the presence of two bands for females and one band for males. We performed molecular sexing of 31 adult Eurasian Penduline Tits of known sex. All adult birds were sexed correctly.
Data processing and statistical analysis
Sample sizes vary amongst analyses because we used the maximum number of data we have for each analysis (Table 1). To compare the female and male parental effort, we analysed (1) nest attentiveness, i.e. total time spent incubating per hour (min/h), (2) mean incubation bout length (a single stay inside the nest, min), (3) mean incubation recess (the period when incubating parent was off of the nest, min), (4) temperature inside the nest during incubation and (5) provisioning rates (per brood and nestling). From the video recordings made during incubation, we noted the time each bird arrived at the nest and left the nest to the nearest second. We define incubation as the time parent spent inside the nest. To calculate nestling provisioning rates (as nest visits per hour and nest visits per hour per nestling), we used total number of visits to the nest by a parent bird during recordings expressed per hour. We analysed the incubation and provisioning behaviour using general linear models. The explanatory variables in the initial models were brood size, parent sex, year, date and hour of observation. Models of incubation behaviour included ambient temperature (measured during video recording) as well. We used the data (recorded at two hour intervals) from the meteorological station (Davis Vantage Pro2) placed at the Ornithological Station of the University of Wrocław in Ruda Milicka at the border of our study area. Some of the dependent variables were transformed to normalise the residuals. Provisioning rates were log-transformed, whilst incubation bout length (mean values per nest) and recess length (mean values per nest) were transformed using Box–Cox transformation. The model selection was based on Akaike’s information criterion (AIC) with the function stepAIC from the MASS library (Venables and Ripley 2002). We calculated the mean temperature inside each nest during incubation from temperature loggers’ data. We made a linear model with temperature as the dependent variable (Box-Cox transformed) and parent sex, ambient temperature during incubation, clutch size and year as explanatory variables. The final model selection was based on Akaike’s information criterion (AIC) using function stepAIC.
Hatching success was calculated as the proportion of eggs that produced 13-day-old nestlings since we did not have data on the number of hatchlings. Differences between female-only and male-only cared broods in clutch size, the number of nestlings and the number of fledglings (productivity) were tested with the Wilcoxon rank sum test, since the data did not meet the requirement for using parametric tests. Correlations were calculated either using Pearson (r) (two measures of nestling body condition; see below) or Spearman (rs) correlation coefficient (relation between number of nestlings and fledglings). We performed an exact binomial test to determine whether the sex ratio (proportion of males) in the analysed sample of nestlings deviated from parity. The Chi-square test with Yeats’ correction was used to compare the sex ratio between female-only cared and male-only cared clutches. To identify survival differences between fledglings from female-only and male-only cared broods, we used the survdiff function implemented in the R package survival. This function uses log-rank statistic and can be used with data containing low number of events and groups with no events. For the fledglings that died between 1 and 5 days post-fledge, the time of event was set at 2.5 days. Similarly, the time of event was set at 7.5 for fledglings that died between 5 and 10 days post-fledge.
Nestling condition was analysed using general linear mixed models (GLMM) with the R package lme4 (Bates et al. 2015). We used two measures of nestling body condition, i.e. scaled mass index (SMI) described in Peig and Green (2009) and haemoglobin concentration (g/L), which were the explanatory variables in the models. The fixed effects were date of hatching (1 = 1 May), brood size, type of parental care (female- or male-only) and nestling sex. Year and nest ID were introduced as random variables. The scaled mass index was calculated as follows:
where Mi is the body mass of the individual, Li is the linear body measurement (tarsus length), bSMA is the scaling exponent estimated by the standardised major axis (SMA) regression of M on L and L0 is the arithmetic mean of tarsus length (Peig and Green 2009). Haemoglobin concentration in blood is a good measure of the physiological condition of nestlings and has been found to be related to trophic conditions (Bańbura et al. 2007; Kaliński et al. 2009). Using haemoglobin concentration as a reliable method for checking body condition is strongly supported by experimental studies that showed a positive relationship between diet quality and haemoglobin level both in adults and chicks (Pryke and Rollins 2012; Minias 2015). In the models, haemoglobin concentration was power-transformed to normalise the residuals. The model selection was based on Akaike’s information criterion corrected for small sample sizes (AICc) using function dredge from MuMIn package (Bartoń 2023). We considered top models to be the best models. Simple linear regression was used to test if the provisioning rate per nestling significantly predicted the body condition of nestlings. In this analysis, we used brood averages of haemoglobin concentration and SMI. The residual plots of the models suggested that both the homoscedasticity and normality assumptions were satisfied. The explanatory variables of GLMMs were tested for multicollinearity by examining the variance inflation factor (VIF) (Fieberg 2022). The VIFs ranged from 1 to 1.59 indicating lack of multicollinearity amongst the predictor variables. P values were calculated using the lmerTest package (Kuznetsova et al. 2017). Statistical analyses were performed using R (version 3.4.4) software (R Development Core Team 2018), and provided probabilities are two-tailed.
Results
Clutch and brood size
Clutch size in female-only cared clutches (median = 6, Q1–Q3 = 6–7, n = 49) was higher than in male-only cared clutches (median = 3, Q1–Q3 = 3–4, n = 21 (Wilcoxon rank sum test: W = 945.5, P < 0.001). Hatching success in female-only cared clutches (0.71, n = 229 eggs in 33 nests) and in male-only cared clutches (0.73, n = 56 eggs in 15 nests) was similar (Chi-squared test with Yates’ correction: χ2 = 0.00006, df = 1, P = 0.99). Consequently, there was a significant difference in the number of nestlings in these two types of brood. Thus, females had to provide food for more nestlings (median = 5, Q1–Q3 = 4–6, n = 87) than males (median = 3, Q1–Q3 = 2–3, n = 25) (Wilcoxon Rank Sum Test: W = 1738, P < 0.001). Female-only cared broods produced more fledglings (median = 4, Q1–Q3 = 0–6, n = 111) than male-only cared broods (median = 3, Q1–Q3 = 0–3, n = 35) (Wilcoxon rank sum test: W = 2620, P = 0.002). The same was observed when only successful broods were analysed. The productivity of successful broods was higher in female-only (median = 5, Q1–Q3 = 4–6, n = 81) than in male-only cared broods (median = 3, Q1–Q3 = 3–4, n = 22) (Wilcoxon rank sum test: W = 1372, P < 0.001).
Sex-specific incubation and feeding behaviour
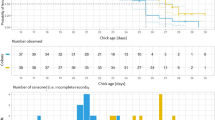
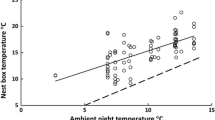
The temperature in the nest during incubation did not differ between female-only cared and male-only cared clutches (GLM: t = 1.489, P = 0.15, Fig. 1), and it did not depend on the ambient temperature (GLM: t = 1.609, P = 0.12), but it was positively correlated with clutch size (GLM: t = 3.328, P = 0.002). The incubation behaviour of females and males was similar. Nest attentiveness (total time spent incubating per hour), mean incubation bout length (a single stay inside the nest) and mean incubation recess (the period when incubating parent was off the nest) did not differ significantly between females and males (Table 2). The incubation behaviour was independent of time of day and year but incubating parents reacted to ambient temperature by reducing the time spent incubating and increasing the recess length with higher ambient temperature (Table 2).
Variation in food provisioning rates (per brood and per nestling) was best explained by brood size and differed between years but did not depend on the sex of the parent providing care (Table 3). The provisioning rate per brood was positively, and the provisioning rate per nestling was negatively, correlated with brood size.
Nestling condition and post-fledgling survival
The two used measures of body condition (i.e. scaled mass index and haemoglobin level) were weakly positively correlated (r = 0.18, P = 0.002, n = 288). Nestling condition was unrelated to the date of hatching and brood size, but it depended on the sex of the parent providing the brood. Nestlings reared in broods with male care were in better condition than those reared by females. At the age of 13 days, they had a higher scaled mass index (Fig. 2, Table 4) and higher haemoglobin levels (Fig. 3, Table 5) compared to nestlings in female-only cared broods. The haemoglobin concentration and SMI were also nestling sex dependent. Male nestlings had higher haemoglobin levels (mean ± se 131.7 g/L ± 1.9, n = 144) and higher SMI (mean ± se 9.62 g ± 0.05, n = 147) than female nestlings (haemoglobin level: mean ± se 128.8 ± 1.54, n = 143; SMI: mean ± se 9.49 g ± 0.05, n = 144) (Tables 3 and 4). The sex ratio (proportion of males) in analysed sample was 0.51 (binomial test: P = 0.91, n = 291). There was no difference in sex ratio between male-only (0.54) and female-only (0.50) cared clutches (Chi-squared test with Yates’ correction: χ2 = 0.09, df = 1, P = 0.77). The provisioning rate per nestling did not predict haemoglobin concentration (r2 = 0.04, F1, 44 = 2.065, P = 0.16) and SMI of nestlings (r2 = 0.04, F1, 44 = 2.065, P = 0.20).
From 90 fledglings that were observed after leaving the nest, 84 (93%) survived until 10 days post-fledge. The remaining six fledglings died: three before 5 and three before 10 days post-fledge. All birds that did not survive until 10 days after fledglings were raised in female-only cared broods (χ2 = 2.4, df = 1, P = 0.1).
Discussion
This paper presents evidence that the lower productivity of male-only cared broods in uniparental Eurasian Penduline Tits may be compensated by a higher condition of nestlings compared to female-only cared broods. The result supports our prediction that nestling condition in male-only cared broods will be higher than in female-only cared broods. We also showed that variation in provisioning rates between broods was best explained by differences in brood size but not by the sex of the parent providing care. Moreover, the body condition of nestlings was not related to the provisioning rate. This suggests that other factors might be responsible for the difference in body condition of nestlings between male-only and female-only cared broods. First, there could be an initial difference in egg size between male-only and female-only-care clutches. Egg size is often used as a measure of the amount of energy and nutrients that female parents invest in each egg. It was found that egg size was correlated with offspring mass and size up to 2 weeks after hatching (Christians 2002). However, there are no differences in egg size in clutches of different parental care types in our population (author’s unpubl. data). Other maternal effects could potentially affect the nestling size or begging behaviour like testosterone, corticosteroids or non-hormonal compounds (e.g. carotenoids) deposited in eggs (Paquet and Smiseth 2015). Investigating the differences in maternal effects between male-only and female-only cared clutches may be considered for further research. Second, food loads per trip brought to nestlings by males might be larger. Yet, there are no data on the prey size or food loads dissimilarity between the sexes in Penduline Tit. Some studies, however, reported such differences between the sexes in passerine birds. Rytkönen et al. (1996) found that load sizes of food brought by male Willow Tits Parus montanus were larger than those of females. The same pattern was observed in Blue Tits Cyanistes caeruleus, in which caterpillars delivered by males were larger (Bańbura et al. 2001). In contrast, male Superb Fairy-wren Malarus cyaneus brought smaller prey items to the nest than females. This was probably a result of the beak size difference between the sexes. Male Superb Fairy-wrens have narrower beaks which are better tools for capturing small prey items (Colombelli-Négrel and Kleindorfer 2010). We were, however, unable to measure the prey type and size from our recordings. Third, our measure of provisioning rates was made once during only 1.5 h of recording, which may be insufficient to accurately estimate the real provisioning behaviour. The difference in body condition between male-only and female-only-care broods could be explained by synchronous vs. asynchronous hatching. In female-only-care broods, one nestling is often younger since females may start incubation after laying the penultimate egg, whilst males start incubation after female desertion, i.e. after clutch completion. However, removing from the data the smallest nestlings from female-only broods did not change the main results of the analysis, i.e. nestlings in male-only cared broods were in better condition. Another explanation for the dissimilarity of chicks’ body condition reared by females and males may be higher sibling competition in female-only cared broods. Previous studies in birds showed that nestlings reared in more competitive environments (e.g. enlarged broods) attain lower body conditions (Neuenschwander et al. 2003; Pryke and Griffith 2009).
According to our predictions, clutch size and productivity in female-only cared broods were higher than in male-only cared broods. Female European Penduline Tits continue egg laying after their mate desertion, and hence can maximise their reproductive success by producing several medium-quality offspring. Caring males, however, may enhance the reproductive success of a brood they provide parental care to by producing higher quality offspring. Most studies conducted on this species have already shown the initial difference in clutch size between female-only and male-only cared clutches (Persson and Öhrström 1989; Czyż 2005; Pogány et al. 2012). This initial difference resulted in different productivity of female-only and male-only clutches in some populations being studied (Persson and Öhrström 1989; Czyż 2008), whilst in others, the productivity of nests did not depend on the type of parental care (Pogány et al. 2012). That means higher brood reduction of female-only compared to male-only cared broods in the latter population, resulting in no difference between the number of fledglings reared in female-only and male-only cared broods. In our study population, brood reduction in female-only cared clutches was lower than in the Hungarian population (medians were 6/5/4 in our population vs. 6/4/3 eggs/nestlings/fledglings, respectively, for the Hungarian population, Pogány et al. 2012) whilst these parameters for male-only cared clutches were similar. These differences in populations could result from many factors, e.g. lower food availability in the Hungarian population compared to the Swedish and Polish populations. Yet, we do not have any data regarding this issue, but it is a rather unlikely situation taking into account the similar characteristics of the study area in Milicz (Poland) and Fehértó (Hungary) fishpond systems (extensive reedbeds and dykes vegetated by deciduous trees). Alternatively, life-history traits might be sex specific and population specific, which may affect how adults partition their breeding effort across broods resulting in both sex and population differences.
In birds, the temperature of incubation was found to influence the body size, growth, metabolism and thermoregulatory ability of embryos and nestlings as well as their survival and future breeding success (Hepp et al. 2015). Differences in nestling body condition reared by males and females could potentially have arisen from differences in incubation temperature. However, we did not find any dissimilarity regarding this parameter in male-only and female-only cared clutches. Contrary to our expectations, the incubation behaviour and mean incubation temperature of both sexes were similar, although the temperature inside the nest was positively correlated with clutch size. Experiments made by Szentirmai et al. (2005) showed that the clutch size influences the cooling rate in the study species, i.e. smaller clutches lose heat more rapidly than bigger ones. Thus, we expected that males (that care for smaller clutches than females) would have to spend more time incubating and/or make shorter recesses to maintain a similar incubation temperature as females, but none of this was observed.
Our results contribute to a better understanding of the evolution of the complex breeding system in the Penduline Tit, which is the result of conflict between sexes. Szentirmai et al. (2007) showed that by deserting a clutch, males increase their own and reduce their mate’s reproductive success. The analogous analysis for females was, however, less powerful than for males and, as mentioned by the authors, should be treated with caution. In our population, females that deserted all of their clutches had lower reproductive success compared to females that took care once in the breeding season, whilst the highest annual number of fledglings was produced by females that cared for two clutches during the breeding season (Czyż 2008). Considering the difference in body condition of fledglings from male-only cared clutches and female-only cared ones helps explain how the desertion behaviour of females may be as beneficial in terms of reproductive success as caring behaviour. Higher body condition of fledglings from male-only cared clutches (i.e. deserted by females) could result in a better offspring annual survival, which would compensate, at least to some extent, for the lower number of fledglings in this type of broods. Information about the recruitment success of broods cared for by males and females will be necessary to test this prediction. This would, however, be very challenging because of the very low local recruitment in this species (authors’ unpubl. data).
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Bańbura J, Perret P, Blondel J, Sauvages A, Galan M-J, Lambrechts M (2001) Sex differences in parental care in a Corsican Blue Tit Parus caeruleus population. Ardea 89:517–526
Bańbura J, Bańbura M, Kaliński A, Skwarska J, Słomczyński R, Wawrzyniak J, Zieliński P (2007) Habitat and year-to-year variation in haemoglobin concentration in nestling blue tits Cyanistes caeruleus. Comp Biochem Physiol A Mol Integr Physiol 148:572–577
Bartoń K (2023) MuMIn: Multi-Model Inference. R package version 1.47.5. https://CRAN.R-project.org/package=MuMIn
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Brommer JE, Karell P, Pihlaja T, Painter JN, Primmer CR, Pietiäinen H (2003) Ural owl sex allocation and parental investment under poor food conditions. Oecologia 137:140–147
Chastel O, Weimerskirch H, Jouventin P (1995) Body condition and seabird reproductive performance: a study of three petrel species. Ecology 76:2240–2246
Christians JK (2000) Producing extra eggs does not deplete macronutrient reserves in European starlings Sturnus vulgaris. J Avian Biol 31:312–318
Christians JK (2002) Avian egg size: variation within species and inflexibility within individuals. Biol Rev 77:1–26
Colombelli-Négrel D, Kleindorfer S (2010) Video nest monitoring reveals male coloration-dependant nest predation and sex differences in prey size delivery in a bird under high sexual selection. J Ornithol 151:507–512
Coulson J, Porter J (1985) Reproductive success of the kittiwake Rissa tridactyla: the roles of clutch size, chick growth rates and parental quality. Ibis 127:450–466
Czyż B (2005) Abundance, distribution and breeding ecology of the Penduline Tit Remiz pendulinus in the Stawno complex, the “Milicz Fishponds” reserve. Not Orn 46:203–212
Czyż B (2008) Reproductive strategies of male and female Penduline Tit Remiz pendulinus at the "Stawy Milickie" nature reserve. PhD thesis, University of Wrocław (Polish with English summary)
Daunt F, Monaghan P, Wanless S, Harris M, Griffiths R (2001) Sons and daughters: age-specific differences in parental rearing capacities. Funct Ecol 15:211–216
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding. Ardea 55:55–28
Fieberg J (2022). Statistics for Ecologists: A Frequentist and Bayesian Treatment of Modern Regression Models. An open-source online textbook. https://fw8051statistics4ecologists.netlify.app/. Accessed 5 May 2023
Freeman-Gallant CR, Wheelwright NT, Meiklejohn KE, Sollecito SV (2006) Genetic similarity, extrapair paternity, and offspring quality in Savannah sparrows (Passerculus sandwichensis). Behav Ecol 17:952–958
Gosler AG (2004) Birds in the hand. In: Sutherland WJ, Newton I, Green RE (eds) Bird Ecology and Conservation. A Handbook of Techniques. Oxford University Press, Oxford
Haywood S, Perrins CM (1992) Is clutch size in birds affected by environmental conditions during growth? Proc Royal Soc B 249:195–197
Hepp G, DuRant S, Hopkins W (2015) Influence of incubation temperature on offspring phenotype and fitness in birds. In: Deeming DC, Raynolds SJ (eds) Nests, eggs and incubation: New ideas about avian reproduction. Oxford University Press, Oxford
Hochachka W, Smith JN (1991) Determinants and consequences of nestling condition in song sparrows. J Anim Ecol 60:995–1008
Janiszewski T, Minias P, Lesner B, Kaczmarek K (2017) Age effects on reproductive success, nest-site location, and offspring condition in the Great Cormorant Phalacrocorax carbo sinensis. J Ornithol 158:193–202
Jensen H, Sæther B-E, Ringsby TH, Tufto J, Griffith S, Ellegren H (2003) Sexual variation in heritability and genetic correlations of morphological traits in house sparrow (Passer domesticus). J Evol Biol 16:1296–1307
Kaliński A, Wawrzyniak J, Bańbura M, Skwarska J, Zieliński P, Bańbura J (2009) Haemoglobin concentration and body condition of nestling Great Tits Parus major: a comparison of first and second broods in two contrasting seasons. Ibis 151:667–676
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: Tests in linear mixed effects models. J Stat Softw 82:1–26
Lindström J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348
Merilä J, Svensson E (1997) Are fat reserves in migratory birds affected by condition in early life? J Avian Biol 28:279–286
Merilä J, Przybylo R, Sheldon BC (1999) Genetic variation and natural selection on blue tit body condition in different environments. Genet Res 73:165–176
Minias P (2015) The use of haemoglobin concentrations to assess physiological condition in birds: a review. Conserv Physiol 3:cov007
Neuenschwander S, Brinkhof MWG, Kölliker M, Richner H (2003) Brood size, sibling competition, and the cost of begging in great tits (Parus major). Behav Ecol 14:457–462
Paquet M, Smiseth PT (2015) Maternal effects as a mechanism for manipulating male care and resolving sexual conflict over care. Behav Ecol 27:685–694
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891
Persson O, Öhrström P (1989) A new avian mating system: ambisexual polygyny in the Penduline Tit Remiz pendulinus. Ornis Scand 20:105–111
Pogány Á, Dijk REV, Horváth P, Székely T (2012) Parental behavior and reproductive output in male-only cared and female-only cared clutches in the Eurasian Penduline Tit (Remiz pendulinus). Auk 129:773–781
Price T, Kirkpatrick M, Arnold SJ (1988) Directional selection and the evolution of breeding date in birds. Science 240:798–799
Pryke SR, Griffith SC (2009) Socially mediated trade-offs between aggression and parental effort in competing color morphs. Am Nat 174:455–464
Pryke SR, Rollins LA (2012) Mothers adjust offspring sex to match the quality of the rearing environment. Proc Royal Soc B 279:4051–4057
Przybylo R, Wiggins DA, Merilä J (2001) Breeding success in Blue Tits: good territories or good parents? J Avian Biol 32:214–218
R Core Team (2018) R: A Language and Environment for Statistical Computing. https://www.R-project.org/
Rosivall B, Szöllősi E, Hasselquist D, Török J (2010) Males are sensitive — sex-dependent effect of rearing conditions on nestling growth. Behav Ecol Sociobiol 64:1555–1562
Rowe L, Ludwig D, Schluter D (1994) Time, condition, and the seasonal decline of avian clutch size. Am Nat 143:698–722
Royle NJ, Smiseth PT, Kölliker M (2012) The evolution of parental care. Oxford University Press, Oxford
Rytkönen S, Koivula K, Orell M (1996) Patterns of per-brood and per-offspring provisioning efforts in the willow Tit Parus montanus. J Avian Biol 27:21–30
Sánchez S, Javier Cuervo J, Moreno E (2007) Does habitat structure affect body condition of nestlings? A case study with woodland Great Tits Parus major. Acta Ornithol 42:200–204
Santos E, Nakagawa S (2012) The costs of parental care: A meta-analysis of the trade-off between parental effort and survival in birds. J Evol Biol 25:1911–1917
Smith HG, Wettermark KJ (1995) Heritability of nestling growth in cross-fostered European Starlings Sturnus vulgaris. Genetics 141:657–665
Stearns SC (1989) Trade-offs in life-history evolution. Func Ecol 3:259–268
Szentirmai I, Székely T, Liker A (2005) The influence of nest size on heat loss of penduline tit eggs. Acta Zool Acad Sci Hung 51:59–66
Szentirmai I, Komdeur J, Szekely T (2007) Sexual conflict over care: antagonistic effect of clutch desertion on reproductive success of penduline tits. J Evol Biol 20:1739–1744
Tinbergen J, Boerlijst M (1990) Nestling weight and survival in individual great tits (Parus major). J Anim Ecol 59:1113–1127
van Dijk R, Szentirmai I, Komdeur J, Székely T (2007) Sexual conflict over parental care in Penduline Tits Remiz pendulinus: the process of clutch desertion. Ibis 149:530–534
Venables WN, Ripley BD (2002) Modern Applied Statistics with S, 4th edn. Springer, New York
Walczyna M (2006) Food provisioning in penduline tit Remiz pendulinus broods. Master thesis, University of Wrocław. (Polish with English summary)
Wang N, Li J, Liu Y, Zhang Z (2010) Improvement on molecular sex identification primers for Passeriform bird species. Chin Birds 1:65–69
Acknowledgements
We thank Robert Pawliszko, Katarzyna Mazur, Alicja Kwinecka and Dominik Kuśnierzowski for help during the field work. The analyses and procedures of this research conformed to the current laws of Poland. Blood sampling was approved by II Local Ethics Committee in Wrocław, Poland, decision no. 180/2010. The field work in the nature reserve “Stawy Milickie” was conducted under the permit from the Regional Directorate for Nature Protection in Wrocław (decision no. WPN.6205.52.2011.MR). The work was supported by the University of Wrocław.
Author information
Authors and Affiliations
Contributions
BC: conceptualization, methodology, investigation (equal), formal analysis, writing—original draft (lead), writing—review and editing (lead). AW: investigation (equal); writing—review and editing (supporting). KW: investigation (equal), writing—original draft (supporting), writing—review and editing (supporting).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Czyż, B., Wasińska, A. & Lukoszek, K. Nestlings reared by males are in better body condition than those reared by females in uniparental European Penduline Tits. J Ornithol 165, 461–471 (2024). https://doi.org/10.1007/s10336-023-02131-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02131-2