Abstract
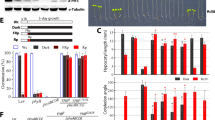
Redox regulation of chloroplast proteins is necessary to adjust photosynthetic performance with changes in light. The thioredoxin (Trx) system plays a central role in this process. Chloroplast-localized classical Trx is a small redox-active protein that regulates many target proteins by reducing their disulfide bonds in a light-dependent manner. Arabidopsis thaliana mutants lacking f-type Trx (trx f1f2) or m-type Trx (trx m124-2) have been reported to show delayed reduction of Calvin cycle enzymes. As a result, the trx m124-2 mutant exhibits growth defects. Here, we characterized a quintuple mutant lacking both Trx f and Trx m to investigate the functional complementarity of Trx f and Trx m. The trx f1f2 m124-2 quintuple mutant was newly obtained by crossing, and is analyzed here for the first time. The growth defects of the trx m124-2 mutant were not enhanced by the lack of Trx f. In contrast, deficiencies of both Trxs additively suppressed the reduction of Calvin cycle enzymes, resulting in a further delay in the initiation of photosynthesis. Trx f appeared to be necessary for the rapid activation of the Calvin cycle during the early induction of photosynthesis. To perform effective photosynthesis, plants seem to use both Trxs in a coordinated manner to activate carbon fixation reactions. In contrast, the PROTON GRADIENT REGULATION 5 (PGR5)-dependent cyclic electron transport around photosystem I was regulated by Trx m, but not by Trx f. Lack of Trx f did not affect the activity and regulation of the PGR5-dependent pathway. Trx f may have a higher specificity for target proteins, whereas Trx m has a variety of target proteins to regulate overall photosynthesis and other metabolic reactions in the chloroplasts.






Similar content being viewed by others
References
Benitez-Alfonso Y, Cilia M, San Roman A, Thomas C, Maule A, Hearn S, Jackson D (2009) Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc Natl Acad Sci USA 106:3615–3620
Brezeale VD, Buchanan BB, Wolosiuk RA (1978) Chloroplast sedoheptulose 1,7-bisphosphatase: evidence for regulation by the ferredoxin/thioredoxin system. Z Naturforsch 33c:521–528
Buchanan BB (2016) The path to thioredoxin and redox regulation in chloroplasts. Annu Rev Plant Biol 67:1–24
Cejudo FJ, Gonzalez MC, Perez-Ruiz JM (2021) Redox regulation of chloroplast metabolism. Plant Physiol 186:9–21
Courteille A, Vesa S, Sanz-Barrio R, Cazale AC, Becuwe-Linka N, Farran I, Havaux M, Rey P, Rumeau D (2013) Thioredoxin m4 controls photosynthetic alternative electron pathways in Arabidopsis. Plant Physiol 161:508–520
DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schunemann D, Finazzi G, Joliot P, Barbato R, Leister D (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132:273–285
Endo T, Shikanai T, Sato F, Asada K (1998) NAD(P)H dehydrogenase-dependent, antimycin A-sensitive electron donation to plastoquinone in tobacco chloroplasts. Plant Cell Physiol 39:1226–1231
Furutani R, Ohnishi M, Mori Y, Wada S, Miyake C (2021) The difficulty of estimating the electron transport rate at photosystem I. J Plant Res. https://doi.org/10.1007/s10265-021-01357-6
Geigenberger P, Thormahlen I, Daloso DM, Fernie AR (2017) The unprecedented versatility of the plant thioredoxin system. Trends Plant Sci 22:249–262
Genty B, Briantais JM, Baker NR (1989) The Relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Hashimoto M, Endo T, Peltier G, Tasaka M, Shikanai T (2003) A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J 36:541–549
Hertle AP, Blunder T, Wunder T, Pesaresi P, Pribil M, Armbruster U, Leister D (2013) PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol Cell 49:511–523
Jacquot J-P, Vidal J, Gadal P, Schürmann P (1978) Evidence for the existence of several enzyme-specific thioredoxins in plants. FEBS Lett 96:243–246
Kanazawa A, Kramer DM (2002) In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc Natl Acad Sci USA 99:12789–12794
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis—the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li XP, Bjorkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403:391–395
Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110:361–371
Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429:579–582
Murai R, Okegawa Y, Sato N, Motohashi K (2021) Evaluation of CBSX proteins as regulators of the chloroplast thioredoxin system. Front Plant Sci 12:530376
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nalin CM, Mccarty RE (1984) Role of a disulfide bond in the gamma-subunit in activation of the atpase of chloroplast coupling factor-I. J Biol Chem 259:7275–7280
Naranjo B, Diaz-Espejo A, Lindahl M, Cejudo FJ (2016) Type-f thioredoxins have a role in the short-term activation of carbon metabolism and their loss affects growth under short-day conditions in Arabidopsis thaliana. J Exp Bot 67:1951–1964
Naranjo B, Penzler JF, Ruhle T, Leister D (2021) NTRC effects on non-photochemical quenching depends on PGR5. Antioxidants (Basel). https://doi.org/10.3390/antiox10060900
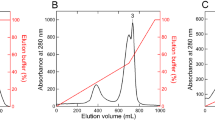
Okegawa Y, Motohashi K (2015) Chloroplastic thioredoxin m functions as a major regulator of Calvin cycle enzymes during photosynthesis in vivo. Plant J 84:900–913
Okegawa Y, Motohashi K (2016) Expression of spinach ferredoxin-thioredoxin reductase using tandem T7 promoters and application of the purified protein for in vitro light-dependent thioredoxin-reduction system. Protein Expr Purif 121:46–51
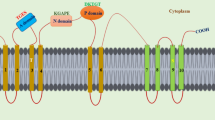
Okegawa Y, Motohashi K (2020) M-type thioredoxins regulate the PGR5/PGRL1-dependent pathway by forming a disulfide-linked complex with PGRL1. Plant Cell 32:3866–3883
Okegawa Y, Long TA, Iwano M, Takayama S, Kobayashi Y, Covert SF, Shikanai T (2007) A balanced PGR5 level is required for chloroplast development and optimum operation of cyclic electron transport around photosystem I. Plant Cell Physiol 48:1462–1471
Okegawa Y, Kagawa Y, Kobayashi Y, Shikanai T (2008) Characterization of factors affecting the activity of photosystem I cyclic electron transport in chloroplasts. Plant Cell Physiol 49:825–834
Okegawa Y, Basso L, Shikanai T, Motohashi K (2020) Cyclic electron transport around psi contributes to photosynthetic induction with thioredoxin f. Plant Physiol 184:1291–1302
Okegawa Y, Tsuda N, Sakamoto W, Motohashi K (2022) Maintaining the chloroplast redox balance through the PGR5-dependent pathway and the Trx system is required for light-dependent activation of photosynthetic reactions. Plant Cell Physiol 63:92–103
Perez-Ruiz JM, Spinola MC, Kirchsteiger K, Moreno J, Sahrawy M, Cejudo FJ (2006) Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. Plant Cell 18:2356–2368
Perez-Ruiz JM, Naranjo B, Ojeda V, Guinea M, Cejudo FJ (2017) NTRC-dependent redox balance of 2-Cys peroxiredoxins is needed for optimal function of the photosynthetic apparatus. Proc Natl Acad Sci USA 114:12069–12074
Ruhle T, Dann M, Reiter B, Schunemann D, Naranjo B, Penzler JF, Kleine T, Leister D (2021) PGRL2 triggers degradation of PGR5 in the absence of PGRL1. Nat Commun 12:3941
Schurmann P, Buchanan BB (2008) The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid Redox Signal 10:1235–1274
Serrato AJ, Perez-Ruiz JM, Spinola MC, Cejudo FJ (2004) A novel NADPH thioredoxin reductase, localized in the chloroplast, which deficiency causes hypersensitivity to abiotic stress in Arabidopsis thaliana. J Biol Chem 279:43821–43827
Serrato AJ, Fernandez-Trijueque J, Barajas-Lopez JD, Chueca A, Sahrawy M (2013) Plastid thioredoxins: a “one-for-all” redox-signaling system in plants. Front Plant Sci 4:463
Tagawa K, Tsujimoto HY, Arnon DI (1963) Role of chloroplast ferredoxin in the energy conversion process of photosynthesis. Proc Natl Acad Sci USA 49:567–572
Taira Y, Okegawa Y, Sugimoto K, Abe M, Miyoshi H, Shikanai T (2013) Antimycin A-like molecules inhibit cyclic electron transport around photosystem I in ruptured chloroplasts. FEBS Open Bio 3:406–410
Wolosiuk RA, Buchanan BB (1977) Thioredoxin and glutathione regulate photosynthesis in chloroplasts. Nature 266:565–567
Wolosiuk RA, Crawford NA, Yee BC, Buchanan BB (1979) Isolation of three thioredoxins from spinach leaves. J Biol Chem 254:1627–1632
Yamamoto H, Shikanai T (2019) PGR5-dependent cyclic electron flow protects photosystem i under fluctuating light at donor and acceptor sides. Plant Physiol 179:588–600
Yokochi Y, Fukushi Y, Wakabayashi KI, Yoshida K, Hisabori T (2021) Oxidative regulation of chloroplast enzymes by thioredoxin and thioredoxin-like proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2114952118
Yoshida K, Hisabori T (2016) Two distinct redox cascades cooperatively regulate chloroplast functions and sustain plant viability. Proc Natl Acad Sci USA 113:E3967-3976
Yoshida K, Hisabori T (2017) Distinct electron transfer from ferredoxin-thioredoxin reductase to multiple thioredoxin isoforms in chloroplasts. Biochem J 474:1347–1360
Yoshida K, Hara S, Hisabori T (2015) Thioredoxin selectivity for thiol-based redox regulation of target proteins in chloroplasts. J Biol Chem 290:14278–14288
Yoshida K, Hara A, Sugiura K, Fukaya Y, Hisabori T (2018) Thioredoxin-like2/2-Cys peroxiredoxin redox cascade supports oxidative thiol modulation in chloroplasts. Proc Natl Acad Sci USA 115:E8296–E8304
Acknowledgements
We thank Dr. Krishna K Niyogi (University of California) for providing the npq4 mutant seeds.
Funding
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (grant numbers JP19H04733, 20H02961, and JP21K06219 to Y.O.). We also thank the Oohara Foundation for financial support of our research group.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YO. The first draft of the manuscript was written by YO and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Okegawa, Y., Sakamoto, W. & Motohashi, K. Functional division of f-type and m-type thioredoxins to regulate the Calvin cycle and cyclic electron transport around photosystem I. J Plant Res 135, 543–553 (2022). https://doi.org/10.1007/s10265-022-01388-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-022-01388-7