Abstract
The red and far-red light photoreceptor phytochrome B (phyB) transmits light signals following cytosol-to-nuclear translocation to regulate transcriptional networks therein. This necessitates changes in protein–protein interactions of phyB in the cytosol, about which little is presently known. Via introduction of a nucleus-excluding G767R mutation into the dominant, constitutively active phyBY276H (YHB) allele, we explore the functional consequences of expressing a cytosol-localized YHBG767R variant in transgenic Arabidopsis seedlings. We show that YHBG767R elicits selective constitutive photomorphogenic phenotypes in dark-grown phyABCDE null mutants, wild type and other phy-deficient genotypes. These responses include light-independent apical hook opening, cotyledon unfolding, seed germination and agravitropic hypocotyl growth with minimal suppression of hypocotyl elongation. Such phenotypes correlate with reduced PIF3 levels, which implicates cytosolic targeting of PIF3 turnover or PIF3 translational inhibition by YHBG767R. However, as expected for a cytoplasm-tethered phyB, YHBG767R elicits reduced light-mediated signaling activity compared with similarly expressed wild-type phyB in phyABCDE mutant backgrounds. YHBG767R also interferes with wild-type phyB light signaling, presumably by formation of cytosol-retained and/or otherwise inactivated heterodimers. Our results suggest that cytosolic interactions with PIFs play an important role in phyB signaling even under physiological conditions.
Key message
Cytoplasmic phytochrome B activity is revealed by expression of a cytosol-tethered, constitutively active YHBG767R mutant allele.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant phytochrome (phy) family are reversibly photochromic dimeric biliproteins that sense the red and far-red light spectrum of the ambient environment to regulate optimal growth and development (Rockwell et al. 2006; Franklin and Quail 2010). Light absorption by the covalently attached bilin chromophore triggers conformational changes that underlie reversible photoisomerization of phys between their red-absorbing, physiologically inactive Pr and far-red-absorbing, active Pfr states. Within the phy family in Arabidopsis thaliana, phyA and phyB play major roles in seedling photomorphogenesis—phyA mediates very-low-fluence responses and the high-irradiance response to far-red light, whereas phyB mediates low-fluence (red/far-red reversible) responses. Our understanding of phy signaling has been greatly aided by the identification of gain-of-function (GOF) alleles. The most well studied of such alleles encodes the missense Y276H variant of Arabidopsis phyB (YHB) (Fischer and Lagarias 2004; Su and Lagarias 2007). YHB is poorly photoactive and strongly fluorescent, and YHB-expressing transgenic plants exhibit dominant constitutive photomorphogenesis (cop) phenotypes (Su and Lagarias 2007; Hu et al. 2009). Since YHB function is light independent, YHB-expressing plants have widely been used to interrogate phyB signaling without activation of other light-dependent processes (Galvao et al. 2012; Jung et al. 2016; Huang et al. 2019; Alves et al. 2020; Hu et al. 2020; Chen et al. 2022).
Canonical early phyB signaling mechanisms have been revealed through three decades of studies. Newly synthesized phyB holoprotein resides in the cytosol as the inactive Pr form. The photoconversion from Pr to Pfr unmasks an intrinsic, yet undetermined, nuclear localization signal within its C-terminal half that permits its interaction with nuclear transport facilitators and subsequent cytosol-to-nucleus translocation (Chen et al. 2005; Pfeiffer et al. 2012). Nuclear Pfr-phyB aggregates as discrete photobodies most notable at high fluences of red light (Yamaguchi et al. 1999; Chen et al. 2022). Such photobodies become the orchestrating hub through which phyB interacts with an array of transcription factors such as PHYTOCHROME-INTERACTING FACTORs (PIFs) and other signaling components to profoundly rewire the transcriptional regulation of photomorphogenesis (Ni et al. 1998; Leivar and Quail 2011; Cheng et al. 2021; Kim et al. 2023). In the nucleus, Pfr-phyB induces rapid phosphorylation and consequent ubiquitin-26S proteasome pathway-mediated degradation of PIFs to abolish their growth-promoting and skotomorphogenesis-sustaining functions (Al-Sady et al. 2006; Leivar et al. 2008; Shin et al. 2009; Ni et al. 2017; Pham et al. 2018). Nuclear import thus appears to be a prerequisite for phyB to execute these regulatory roles (Huq et al. 2003; Fankhauser and Chen 2008; Klose et al. 2015). Consistent with its constitutive signaling activity, YHB always forms a few large nuclear photobodies even in the absence of light (Su and Lagarias 2007; Chen et al. 2010).
During dark-to-light transitions, nascent cytosolic Pfr-phy species must disengage from cytosolic retention complexes and productively engage with nuclear translocation factors—processes that are poorly understood. Both phenomena likely impact the observed cytosolic signaling responses reviewed by Hughes (2013). These include phy-mediated (1) transient increase in cytosolic Ca2+ levels (Shacklock et al. 1992), (2) interaction with cytosolic protein PENTA1 to suppress the translation of protochlorophyllide reductase (PORA) mRNA (Paik et al. 2012), and (3) interaction with phototropins at the plasma membrane to modulate directional response to light and gravity (Rosler et al. 2010; Jaedicke et al. 2012). More recently, the acute red-light dependent spike of cytosolic Ca2+ levels was shown to activate Ca2+-dependent kinases that in turn phosphorylate Pfr-phyB and promote phyB nuclear import during early de-etiolation transition (Zhao et al. 2023). Whereas these phenomena are most evident during seedling de-etiolation, their contributions to de novo phyB-mediated seedling photomorphogenesis are difficult to distinguish from processes affected by photosynthesis.
To address the importance of cytoplasmic signaling functions of phyB, we introduced a loss-of-function (LOF) G767R mutation into the constitutively active YHB allele. Among the strongest known PHYB LOF missense alleles, the phyBG767R variant is defective in nuclear import and exhibits imperceptible light-signaling activity (Wagner and Quail 1995; Matsushita et al. 2003; Pfeiffer et al. 2012). Addition of a nuclear localization signal (NLS) to a phyBG767R-GFP chimera not only confers phyBG767R nuclear localization in darkness, but also restores its photoregulatory activity (Matsushita et al. 2003). Based on this evidence, we reason that the YHBG767R protein will adopt a ‘constitutively active’ state (like YHB) without being translocated to the nucleus. The present studies address the physiological consequence of YHBG767R expression in the phyABCDE null mutant of Arabidopsis to test the effects of sustained cytosolic activation of phyB in darkness while also avoiding interference from photosynthesis and/or potential protein–protein interactions with other phys. We also examine the influence of YHBG767R expression on light-grown seedling development in wild-type and various phy mutant backgrounds in which photosynthesis is restored. These studies provide novel insights into phyB-dependent processes in the cytoplasm which influence signaling pathways occurring in the nucleus.
Materials and methods
Constructs and transgenic plants
The G767R mutation was introduced into YHB and PHYB genomic sequences in the pJM78 plasmid (Su and Lagarias 2007) by site-directed mutagenesis using primers 5ʹ-GTCGGCGTTTGTTTTGTTCGACAAGACGTTACTAGTC-3ʹ and 5ʹ-GACTAGTAACGTCTTGTCGAACAAAACAAACGCCGAC-3ʹ. Upon sequencing validation, the YHBG767R and PHYBG767R inserts were excised with SacII and PstI, and subcloned into the pJM63-PHYBg binary vector (Su and Lagarias 2007) (Fig. S1A). pJM63-YHBg-G767R was then transformed into the phyABCDE null mutant, the phyB-5 mutant and the Ler WT using the floral dip method (Clough and Bent 1998; Hu et al. 2013; Jones et al. 2015). In addition, pJM63-PHYBg-G767R was transformed into the phyB-5 mutant, and pJM63-PHYBg was transformed into the phyABCDE mutant. Standard genetic procedures were employed to secure multiple single-insertion, homozygous transgenic lines. YHBg/phyABCDE and YHBg/phyB-5 were derived from YHBg/phyA-201phyB-5 line #5 by outcrossing (Su and Lagarias 2007; Hu et al. 2009; Jones et al. 2015). 35S::YHB/phyB-5 #10 was described previously (Su and Lagarias 2007). The pBI-pPHYB:PHYBC357S/phyB-1 (No-0 ecotype) was a generous gift from Prof. Robert Sharrock (Montana State University) (Clack et al. 2009). All wild type and other transgenic lines were in the Ler ecotype background.
Growth conditions and phenotypic analyses
Seeds were surface sterilized with 75% ethanol for 12 min, then suspended with 0.1% phytagar and sown on 1 × MS medium (pH 5.7, 0.8% phytagar). Following 4-day stratification, plates were exposed to ≥ 3 h white light (~ 80 µmol m−2 s−1) to induce synchronized germination. Seedlings were grown in continuous red light (Rc, 50 µmol m−2 s−1), or in ‘true darkness’ where plates were additionally exposed to 5 min of FR pulse (20 µmol m−2 s−1) before wrap** with aluminum foil (Leivar et al. 2008). Seedlings were scanned or photographed and measured digitally in the NIH ImageJ software (https://imagej.nih.gov/ij/). For seed germination assays, approximately 100 seeds of each genotype for each replicate experiment were surface sterilized and sown on 0.75% phytagar plates (pH 5.7) within an hour and then allowed to germinate within a five-day period in darkness, under continuous cool fluorescent white light (~ 50 µmol m−2 s−1), exposed to 5 min far-red light pulses (FRp, 12 µmol m−2 s−1) before darkness, or exposed to additional 5 min red light pulses (Rp, 20 µmol m−2 s−1) following FRp before placing in darkness (Fig. 1D). Seeds were deemed germinated if the radicle emerged. For seedling growth direction quantification, plates were placed vertically; the growth angles from 100 seedlings of each genotype measured in ImageJ were plotted as circular histograms.
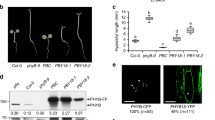
The G767R mutation strongly, yet incompletely suppresses the constitutive activity of YHB in the phyABCDE mutant background. A Immunoblot analyses of YHBG767R/phyABCDE lines. B Phenotypic comparison of 4-day-old seedlings grown in true darkness or under continuous red light (50 µmol m−2 s−1); # denotes YHBG767R seedlings with etiolated morphology. C Quantification of hypocotyl lengths, cotyledon separation angles and cotyledon areas of two overexpressed YHBG767R/phyABCDE lines (#10, #16) and two less expressed lines (#9, #14) in comparison to other control genotypes; *denotes statistical significance compared to phyABCDE grown under the same condition (p < 0.0001; Student’s t-test), ns, not statistically significant; n = ~ 30. D Immunoblot analyses of dark-grown seedlings reveal significant, but not complete loss of PIF3 protein by YHBG767R in the overexpressing line #16 and the less expressing line #14. E YHBG767R promotes light-independent seed germination; six transgenic lines were used for YHBG767R and three replicates for other genotypes, each replicate used ~ 100 seeds. F Circular histograms depict seedling growth directions in darkness or under continuous red light (Rc50); YHBG767R data were from two independent lines, n = ~ 100
Immunoblot assay and confocal fluorescence microscopy
Protein extraction and immunoblot assays were performed as previously described (Su and Lagarias 2007; Jones et al. 2015; Hu and Lagarias 2017). Blot intensities were quantified using LI-COR Image StudioLite software (for phyB and tubulin) or ImageJ (for PIF3). Confocal fluorescence microscopy was performed as described previously (Hu and Lagarias 2017).
Subcellular fractionation and immunoblot assay
Overexpression PHYB/phyABCDE line #3 and YHBG767R/phyABCDE line #2 were used for fractionation. Approximately 10–12 leaves of short day-grown (8 h L/16 h D), 50-d-old plants were collected at mid-day to isolate mesophyll protoplasts using a slightly modified Tape-Arabidopsis-Sandwich protocol (Wu et al. 2009). Leaves peeled off of the abaxial epidermis were incubated in 8 ml of digestion enzyme solution with gentle shaking for an hour under ambient room light. Released protoplasts were collected by centrifugation (100 g × 3 min) and rinsed three times with W5 solution; after the final spin, the protoplasts were gently resuspended in 1 ml pre-cooled NIBA buffer (CelLytic™ PN Isolation/Extraction Kit, Sigma-Aldrich; Cat. # CELLYTPN1). Good harvest of intact protoplasts was confirmed by observation under light microscope. Protoplasts were lysed by adding 0.3% (v/v, final) Triton X-100 followed by incubation on ice for 10 min with intermittent gentle shaking. Lysates were spun at 12,000 g × 10 min to separate cytoplasmic (supernatant) and nuclear (pellet) fractions. Supernatant proteins were purified using the hot SDS extraction buffer followed by methanol-chloroform purification (Su and Lagarias 2007). Nuclear pellets were resuspended in 1 ml NIBA buffer and re-centrifuged. The re-centrifuged nuclear pellets were then lysed with 200 µl hot SDS extraction buffer followed by methanol-chloroform protein purification (Su and Lagarias 2007). Total protein was extracted from aliquots of protoplasts as described previously (Su and Lagarias 2007). Protein was quantified by BCA assay, and 50 µg of each sample were loaded for SDS-PAGE. Mouse anti-RNA Polymerase II (RPII) monoclonal antibody (Abcam, cat. #ab5408) was used in 1/1000 dilution for blotting. Immunoblotting procedure and antibodies against phyB and Actin were as described previously (Jones et al. 2015; Hu and Lagarias 2017).
Results
YHB G767R retains selective light-independent signaling activity in the phyABCDE null mutant background
To explore the extent of suppression by the G767R mutation on the constitutively active YHB allele, we introduced both Y276H and G767R missense mutations into the genomic PHYB expression construct pJM63-PHYBg (Fig. S1A) (Su and Lagarias 2007) and transformed the resultant double mutant construct into the phyABCDE null mutant (Hu et al. 2013). Immunoblot assay of seven YHBG767R/phyABCDE lines revealed a range of expression from 2.8- to 6.9-fold that of the endogenous phyB level (Fig. 1A). We also transformed the null mutant with the pJM63-PHYBg construct as a wild-type (WT) phyB-only control, recovering six highly overexpressing pJM63-PHYBg/phyABCDE lines and one line with ~ threefold endogenous expression level (Fig. S1B, C). Despite the use of the native PHYB promoter, our observed overexpression results agree with previous studies showing that the strong promoter/enhancer driving expression of the selectable marker frequently overrides the specificity of the transgene promoter residing within the same construct (Yoo et al. 2005; Hu and Lagarias 2017).
In contrast to YHB-expressing lines that exhibit background-independent strong cop phenotypes (Su and Lagarias 2007; Hu and Lagarias 2017), all YHBG767R/phyABCDE lines grown for 4 days in true darkness possessed elongated hypocotyls, confirming that the G767R mutation suppressed the GOF signaling activity of YHB on hypocotyl growth (Fig. 1B, C). However, dark-grown YHBG767R/phyABCDE seedlings lacked apical hooks and possessed open cotyledons, in contrast to dark-grown Ler WT, phyABCDE null, phyB-5 and PHYB-complemented phyABCDE seedlings, all of which retained apical hooks and closed cotyledons (Fig. 1B, C). These results indicate that YHBG767R selectively retains GOF signaling activity.
Compared to the etiolated phenotype of phyABCDE null mutants under 50 µmol m−2 s−1 continuous red light (Rc50), YHBG767R/phyABCDE seedlings had fully opened and green cotyledons despite retaining elongated hypocotyls (Fig. 1B, C). Such seedlings had larger cotyledons than Rc-grown phyB-5 single mutants, and the two overexpressed lines #10 and #16 were even comparable to Rc-grown WT—a result indicating significant rescue of R-dependent photomorphogenesis to the null mutant (Fig. 1C). A transgene dosage effect was observed among Rc-grown seedlings, with higher YHBG767R expressing lines having larger cotyledons, e.g. #2, #4, #10 and #16 vs #8, #9 and #14 (Fig. 1B, C). These results show that although the G767R mutation nearly eliminates YHB inhibition of hypocotyl growth under red light, significant red light-dependent photomorphogenesis is retained in the YHBG767R transgenic seedlings.
Previous studies have established that the protein level of the PIF3 transcription factor in dark-grown seedlings is a sensitive molecular indicator for YHB function (Hu and Lagarias 2017). We therefore examined the PIF3 levels present in the YHBG767R/phyABCDE seedlings by immunoblot analysis. Representative high and relatively low YHBG767R-expressing lines, i.e. #16 and #14 respectively, both exhibited greatly reduced PIF3 levels that were more similar to those of Rc-grown WT than dark-grown WT (Fig. 1D). These results suggest that YHBG767R, like YHB and photoactivated WT phyB, elicits the loss of PIF3 protein.
Other YHB-conferred traits include light-independent seed germination and randomized growth direction both in darkness and under red light. We therefore examined the influence of YHBG767R on these two developmental responses in the phyABCDE background. On phytagar medium lacking mineral nutrients, WT Ler showed R/FR-reversible seed germination and phyB-5 only germinated efficiently under Wc, while phyABCDE failed to germinate under all light conditions (Fig. 1E) unless GA was added (Hu et al. 2013). By contrast, seeds of both YHB and YHBG767R lines germinated well in darkness and under all light conditions (Fig. 1E). Hypocotyls of WT Arabidopsis seedlings display negative gravitropism (upward growth) in darkness, whereas their growth is randomized, or agravitropic, under red and far-red light—a response known to be mediated by multiple phytochromes (Robson and Smith 1996; Kim et al. 2011). As expected, phyABCDE mutants exhibited constitutive negative gravitropism and YHB seedlings grew with randomized orientation both in the dark and under Rc (Fig. 1F). By comparison, the growth direction of dark-grown YHBG767R seedlings were more random than those of Ler and phyABCDE, but still were largely negatively gravitropic. Under Rc, YHBG767R seedlings exhibited greatly randomized growth, although its orientation range was less than those of Ler and YHB (Fig. 1F). These results show that YHBG767R also retains signaling activity to promote light-independent seed germination and to inhibit negative gravitropism.
YHB G767R is retained in the cytoplasm
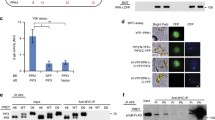
Previous studies have established that the G767R mutation inhibits light-dependent nuclear localization of phyB while not altering its chromophorylation, photochemistry, dark reversion or dimerization (Wagner and Quail 1995; Matsushita et al. 2003; Shin et al. 2011). This inability to migrate into the nucleus accounts for the loss of function of phyBG767R, since addition of an NLS restores the nuclear localization and photoregulatory activity of a GFP-fusion of this LOF allele (Matsushita et al. 2003). We reasoned that introduction of the G767R mutation into YHB might similarly affect its subcellular localization while not altering its chromophorylation or light-independent activation. Since Arabidopsis YHB is a red fluorescent protein and constitutively forms a few large nuclear photobodies independent of light conditions (Su and Lagarias 2007; Hu and Lagarias 2017), we took advantage of confocal microscopy to probe the localization of YHBG767R. Unlike the constitutive nuclear localization pattern of YHB (Fig. 2A), no red fluorescent signal was detected from nuclei of YHBG767R-expressing seedlings grown in the dark or under Rc. These results indicate that YHBG767R is not imported into the nucleus. To corroborate this interpretation, we performed fractionation immunoblot assays for light-grown plants. Using photoactivated phyB as the positive control for the nuclear fractions, the assays showed that YHBG767R was only detectable in the cytosolic fraction and absent from the nuclear fraction (Fig. 2B). Therefore, the G767R mutation inhibits nuclear migration of YHB, analogous to its effect on WT phyB. Taken together, these results strongly suggest that the cytoplasmic signaling activity of YHBG767R accounts for its influence on seedling photomorphogenesis shown in Fig. 1.
YHBG767R is localized in cytoplasm. A Confocal fluorescence microscopy reveals the absence of nuclear photobodies in YHBG767R/phyABCDE grown in the dark or under continuous red light, whereas YHB/phyABCDE displays steady nuclear photobodies; Chl., chlorophyll autofluorescence. B Fractionation immunoblotting reveals cytosolic distribution of YHBG767R from light-grown transgenic plants. T, total soluble protein; C, cytoplasmic fraction; N, nuclear fraction. RPII, RNA Polymerase II
Both YHB G767R and PHYB G767R can complement phyB mutants under red light in a dosage-dependent manner
To examine the signaling activities of YHBG767R and phyBG767R in the presence of other phys, we next introduced their corresponding genomic constructs into the phyB-5 single mutant. Immunoblot analysis revealed that some of these lines had expression levels similar to or even lower than that of endogenous phyB (Fig. 3A). In darkness, all of the PHYBG767R lines remained fully etiolated, while only the most strongly overexpressing YHBG767R lines (#2 and #5) exhibited partial GOF hypocotyl growth suppression and cotyledon opening (Fig. 3B, bottom). By comparison, YHBG767R lines with endogenous expression levels (#11) or lower (#4 and #8) displayed marginal to undetectable cotyledon opening, with no significant influence on hypocotyl growth (Fig. 3B, bottom). Whereas all dark-grown YHBG767R lines had greatly reduced PIF3 levels, PIF3 abundance remained high in the PHYBG767R lines (Fig. 3D). These results corroborate the observed GOF activity of YHBG767R in the phyABCDE null background and also show that phyBG767R is signaling inactive in darkness as expected.
YHBG767R and PHYBG767R complement the phyB-5 mutant in a dosage-dependent manner. A Immunoblot analysis of nine genetically single-insertion homozygous transgenic lines. B Four-day-old seedlings grown in continuous red light (50 µmol m−2 s−1) or in darkness. * denotes transgenic lines with discernable shorter hypocotyls than phyB-5 mutants; #denote lines with discernable cop phenotype in the dark. C Quantification (mean ± S.D.) of hypocotyl lengths (top) and cotyledon sizes (bottom) of Rc50-grown seedlings; * denotes statistical significance in comparison to phyB-5 (p < 0.01; Student’s t-test), n = 20. D Immunoblot analysis of PIF3 protein levels in dark-grown seedlings. E Immunoblot analysis of red light-induced PIF3 loss in PHYBG767R/phyB-5 lines #2 and #8 with comparable expression levels to endogenous phyB. F Phenotypic comparison of true dark-grown YHBG767R transgenics in the phyB-5 and phyABCDE mutant backgrounds
Under Rc, both YHBG767R and PHYBG767R alleles were able to significantly complement the phyB-5 mutant; however, only overexpressing lines were able to phenocopy WT as evaluated by hypocotyl lengths, cotyledon sizes, or both (Fig. 3B upper and 3C). The effect of R light on PIF3 levels in two PHYBG767R lines with endogenous expression levels (#2 and #8; see Fig. 3A) was compared with WT Ler. After 20 min of R-light exposure on dark-grown seedlings, both WT and PHYBG767R lines experienced significant PIF3 loss, consistent with its light-dependent turnover (Fig. 3E). The R-dependent loss of PIF3 was nearly complete in Ler, whereas both PHYBG767R lines still retained some residual PIF3 levels. This reduction of PIF3 levels also was sustained following prolonged R-light exposure where the role of phyA is suppressed by its turnover (Fig. 3E, R24h and Rc). Indeed, these data corroborate those of a previous study which established that overexpression of PHYBG767R was able to complement the phyA-211;phyB-9 double mutant (Col accession) under Rc, ruling out a role for phyA in the sustained loss of PIF3 (Park et al. 2018). Overall, our results support the hypothesis that cytosol-tethered phyBG767R, like YHBG767R in darkness (Fig. 3C), can trigger PIF3 turnover and/or inhibit PIF3 translation under Rc. Despite the great loss of PIF3 in all Rc-grown PHYBG767R and YHBG767R lines, overexpression is required to rescue seedling photomorphogenesis of the phyB-5 mutant.
Finally, we also performed a side-by-side comparison of true dark-grown YHBG767R/phyB-5 and YHBG767R/phyABCDE lines. Since no discernable difference was observed in YHBG767R-dependent weak cop phenotypes, the presence of other phys does not appreciably modulate YHBG767R function in darkness (Fig. 3F).
Dosage-dependent effect of YHB G767R in the presence of functional phyB alleles
YHBG767R was also introduced into Ler WT to test its activity in the presence of WT phyB, and lines with varied transgene expression levels were obtained (Fig. 4A). In lines #1, #6, #8 and #10, in which YHBG767R accumulated to levels higher than endogenous phyB, PIF3 levels in dark-grown seedlings were drastically reduced, whereas a significant amount of PIF3 protein was detected in the lowest expressing line #2 (Fig. 4A). The four moderately and highly expressing YHBG767R lines also displayed weak cop phenotypes in the dark, whilst line #2 did not (Fig. 4B). When grown under Rc, only line #8 with the highest YHBG767R expression (more than ten-fold of endogenous phyB) exhibited statistically significant inhibition of hypocotyl growth, whereas line #2 exhibited the opposite trend with more elongated hypocotyls (Fig. 4B, C). Because of the dimerization nature of phyB, we propose that formation of cytosol-retained YHBG767R:phyB heterodimers might be responsible for these dosage-dependent observations.
Dosage-dependent effects of YHBG767R in the presence of functional phyB alleles. A Immunoblot analysis of dark-grown seedlings. B Four-day-old seedlings grown in continuous red light (50 µmol m−2 s−1) or in the dark; # denotes lines with discernable cop phenotypes (partially opened cotyledons). C Hypocotyl lengths (mean ± S.D.) of Rc50-grown, 4-d-old seedlings; * denotes statistical significance in comparison to Ler (p < 0.001; Student’s t-test), n = 20. D Dark-grown F1 seedlings of genetic crosses between a weak-expressing YHBg/phyB-5 or an overexpressing 35S::YHB/phyB-5 line with Ler WT, phyB-5 and two cytoplasm-retained PHYB mutation lines
To further test this hypothesis, we crossed both low- and high-expressing YHB lines, i.e. YHBg and 35S::YHB, respectively, with Ler WT, the phyB-5 mutant, and YHBG767R- and PHYBC357S-expressing lines (Fig. 4D). YHBg outcrosses with either Ler WT or phyB-5 yielded F1 seedlings with slightly longer hypocotyls in darkness, consistent with the expected decrease in YHBg transgene dosage. By contrast, YHBg outcrosses with YHBG767R or with chromophore-less PHYBC357S-expressing lines significantly promoted F1 seedling elongation, suggesting that both cytosol-retained phyB variants dampen nuclear import of some YHB molecules by heterodimerization (Fig. 4D). As predicted, neither variant proved effective in suppressing the GOF activity of overexpressed YHB, presumably due to the presence of saturating levels of YHB:YHB homodimers in these lines (Fig. 4D).
Genetic background independence and dosage dependence of YHB G767R function
Finally, to further confirm that the photomorphogenesis-promoting role of YHBG767R is dosage-dependent and largely independent of the presence of other phys, we outcrossed the overexpressing YHBG767R/phyABCDE line #16 with Ler WT and with other phy mutants. Consistent with the decrease by half of the YHBG767R levels in various F1 seedlings harboring a phyB mutation, cotyledon opening angles of these dark-grown F1 seedlings were reduced to half the degrees seen in the parental YHBG767R/phyABCDE line (Fig. 5). Interestingly, F1 seedlings resulting from the outcross with Ler WT exhibited slightly greater cotyledon angles than those F1 seedlings retaining a phyB mutant allele, which also may reflect the effect of increased number of dimers containing YHBG767R by heterodimerization with phyB. Overall, this outcross experiment supports that the YHBG767R signaling activity is dosage-dependent and independent of the presence of other non-phyB phys.
Discussion
The G767R mutation has long been known to profoundly suppress phyB signaling function (Wagner and Quail 1995; Matsushita et al. 2003). Since this mutation is located outside the N-terminal photosensory module (PSM) of the full-length photoreceptor, it does not alter the photochemical property of phyB in vivo or in vitro (Wagner and Quail 1995; Shin et al. 2011). Instead, this mutation inhibits phyB nuclear import (Matsushita et al. 2003; Pfeiffer et al. 2012). Our confocal microscopy and subcellular fractionation work also establishes that YHBG767R is retained in the cytosol (Fig. 2). For this reason, the cop phenotypes of dark-grown YHBG767R-expressing Arabidopsis seedlings likely reflect the signaling action of a cytosol-localized phyB species. Beyond the limits of the detection sensitivity of our measurement, however, we cannot rule out the possibility that a trace portion of YHBG767R might reside in the nucleus exerting the observed signaling function. Given the significant loss of PIF3, strong seed germination de-repression and cotyledon opening, our data support a light-independent cytosolic action of YHBG767R in these photomorphogenetic responses.
YHBG767R/phyABCDE enables studying the function of cytosolic photoactive phyB without interference from endogenous phy signaling or from light-triggered physiological activities when grown in darkness. Although earlier work using dark-grown YHBG767R/phyABCDE demonstrated that this cytosolic phyB allele could not maintain circadian robustness (Jones et al. 2015), the present studies show that dark-grown YHBG767R/phyABCDE seedlings display partial de-etiolation, notably hook opening and cotyledon expansion, and reduced gravitropic growth (Fig. 1). Moreover, YHBG767R transgenic lines exhibit light-independent seed germination and PIF3 protein loss (Fig. 1). These observations imply that nuclear translocation is not essential for these signaling aspects of photoactivated phyB.
Overexpressed tagged PIF3 was observed to diffusely distribute in the nucleus of dark-grown transgenic plants (Bauer et al. 2004; Al-Sady et al. 2006) and also in nuclei of transfected onion epidermal cells (Ni et al. 1998). Upon red light irradiation, YFP-PIF3 swiftly forms nuclear photobodies, followed by rapid phosphorylation and turnover—a process mainly mediated by phyA. Such PIF3 behavior is consistent with the light-induced rapid nuclear import and subsequent turnover of phyA (Al-Sady et al. 2006). PIF3 is known to interact with both N- and C-terminal regions of phyB, showing much stronger interaction with the N-terminal PSM of photoactivated phyB than with the C-terminal regulatory domain (Ni et al. 1999). In contrast to FHY1/FHL-mediated nuclear translocation of photoactivated phyA (Hiltbrunner et al. 2005, 2006), nuclear-targeting of photoactivated phyB is less well understood. We know that the C-terminal fragment of phyB is constitutively localized to nuclear bodies (Yamaguchi et al. 1999), that phyB nuclear targeting requires the C-terminal regulatory domain of the photoreceptor (Chen et al. 2005), and that early nuclear photobody formation of phyB requires the presence of PIF3 (Bauer et al. 2004). PIF3 was also shown to be needed to mediate full-length phyB import into the nuclei of the model green alga Acetabularia, and the G767R mutation prevented PIF3-induced nuclear import of the C-terminal phyB fragment (Pfeiffer et al. 2012). Together with the evidence that other PIF proteins influence the nuclear accumulation of phyB-YFP chimera (Pfeiffer et al. 2012) and that PIF3 was initially identified using a yeast two hybrid screen with the C-terminal phyB fragment (Ni et al. 1998), these studies support that PIF3 and other PIFs mediate phyB nuclear translocation via interaction with the C-terminus of phyB in a light-dependent manner. Consistent with this hypothesis, removal of the C-terminal region of phyB prevents its nuclear translocation in planta, despite the known strong light-dependent interaction between PIFs and the N-terminal PSM of phyB (Ni et al. 1999; Matsushita et al. 2003).
Based on this knowledge, we propose that PIFs interact with cytosol-constrained YHBG767R via its N-terminal PSM that has been constitutively activated by the Y276H mutation. We further speculate that a significant portion of PIF3 resides in the cytoplasm of dark-grown seedlings, poised to interact with newly photoactivated phyB to implement early nuclear import of phyB-PIF3 complex. That fluorescent protein tagged PIF3 was not reported to be seen in the cytoplasm likely is due to spatial dilution of such chimera protein in the vast cytosolic volume in comparison to the nucleus. We conclude that YHBG767R-induced PIF3 loss occurs in the cytosol, as cytosol-retained YHBG767R cannot physically interact with nuclear PIF3. The smaller amount of PIF3 detected in dark-grown YHBG767R lines may be those comparted in the nucleus and inaccessible to YHBG767R.
The observed reduction of PIF3 levels in dark-grown YHBG767R-expressing seedlings suggests that YHBG767R either targets PIF3 for degradation and/or inhibits translation of the PIF3 mRNA in the cytosol. The first scenario implies the residual PIF3 would be nuclear localized, whereas the latter scenario implies it would be cytosolic. The latter possibility already has been reported for the PORA transcript (Paik et al. 2012), so the potential role of cytosolic phyB inhibiting PIF3 translation remains a viable hypothesis. Photoactivated phyB, likely of the nuclear portion, has been shown to enhance retention of an intron in the PIF3 5’UTR to inhibit PIF3 protein translation (Dong et al. 2020). Based on these considerations, measurement of residual PIF3 localization in these plants will not resolve this issue. Although definitive resolution of the two mechanistic hypotheses is beyond the scope of this study, we note that PIF3 transcript levels are not significantly altered in dark-grown YHB-expressing plants compared with WT Ler (Hu et al. 2009). This result argues that the inhibition of PIF3 transcription unlikely accounts for the reduced PIF3 protein levels in dark-grown YHBG767R plants.
Given that cytosolic photoexcited phyB rapidly induces transient spike of cytosolic Ca2+ levels during seedling de-etiolation (Zhao et al. 2023), it is not surprising some phy signaling cascades occur in the cytoplasm (Hughes 2013). Indeed, in addition to PIF3 turnover, our studies show that light-independent seed germination is also elicited by cytosolic YHBG767R. Since PIF1 is a key negative regulator of seed germination, which is targeted for turnover by photoactivated phyB (Oh et al. 2004, 2006; Shen et al. 2005), PIF1 is likely to be degraded by YHBG767R in the cytoplasm as well. This interpretation is supported by the work of Park et al. (2018) showing that PIF1 levels are greatly reduced in red light-grown PHYBG767R/phyA-211;phyB-5 seedlings. Their results and our own data argue that the loss of PIF1 is caused by photoactivated, cytosol-tethered phyB. Based on these results, models of phyB action may need to consider the role of cytosolic interactions between phyB and PIFs in the regulation of gene expression.
In summary, YHBG767R is a valuable germplasm to study signaling actions of cytosolic phyB in both darkness and the presence of light. Our studies demonstrate that the GOF activities of YHBG767R in various phyB mutant backgrounds exhibit a protein dosage dependence. We also show that G767R alleles of phyB can suppress the regulatory activities of other phytochromes likely through heterodimerization. Whereas it is clear that maximal phyB signaling in develo** seedlings requires nuclear translocation, our studies argue that cytosolic signaling by phyB must not be discounted even under physiological light conditions. Moreover, the relatively small effect of YHBG767R on hypocotyl growth suppression suggests that cytosolic interactions with PIFs and possibly other factors that influence seed germination, hook-opening and cotyledon development take precedence to processes such as hypocotyl, stem and root growth regulated by other PIFs, such as PIF4 and PIF5. This makes sense since rapid elongation growth is needed for the radicle to breach the seed coat during germination. The recent reports that PIF3 plays a critical role in root penetration into soil via interacting with the cytosolic transmembrane receptor FERONIA (Xu et al. 2024), and that FERONIA can phosphorylate phyB (Liu et al. 2023), suggest that the interplay between phyB, PIF3 and FER in the cytosol are important for early seedling establishment in Arabidopsis.
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information, and also from the authors upon reasonable request.
References
Al-Sady B, Ni W, Kircher S, Schafer E, Quail PH (2006) Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell 23:439–446
Alves FRR, Lira BS, Pikart FC, Monteiro SS, Furlan CM, Purgatto E, Pascoal GB, Andrade S, Demarco D, Rossi M, Freschi L (2020) Beyond the limits of photoperception constitutively active PHYTOCHROME B2 overexpression as a means of improving fruit nutritional quality in tomato. Plant Biotechnol J 18:2027–2041
Bauer D, Viczian A, Kircher S, Nobis T, Nitschke R, Kunkel T, Panigrahi KC, Adam E, Fejes E, Schafer E, Nagy F (2004) Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16:1433–1445
Chen M, Galvao RM, Li M, Burger B, Bugea J, Bolado J, Chory J (2010) Arabidopsis HEMERA/pTAC12 initiates photomorphogenesis by phytochromes. Cell 141:1230–1240
Chen D, Lyu M, Kou X, Li J, Yang Z, Gao L, Li Y, Fan LM, Shi H, Zhong S (2022) Integration of light and temperature sensing by liquid-liquid phase separation of phytochrome B. Mol Cell 82(3015–3029):e3016
Chen M, Tao Y, Lim J, Shaw A, Chory J (2005) Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr Biol 15:637–642
Cheng MC, Kathare PK, Paik I, Huq E (2021) Phytochrome signaling networks. Annu Rev Plant Biol 72:217–244
Clack T, Shokry A, Moffet M, Liu P, Faul M, Sharrock RA (2009) Obligate heterodimerization of Arabidopsis phytochromes C and E and interaction with the PIF3 basic helix-loop-helix transcription factor. Plant Cell 21:786–799
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Dong J, Chen H, Deng XW, Irish VF, Wei N (2020) Phytochrome B induces intron retention and translational inhibition of phytochrome-interacting factor3. Plant Physiol 182:159–166
Fankhauser C, Chen M (2008) Transposing phytochrome into the nucleus. Trends Plant Sci 13:596–601
Fischer AJ, Lagarias JC (2004) Harnessing phytochrome’s glowing potential. Proc Natl Acad Sci U S A 101:17334–17339
Franklin KA, Quail PH (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61:11–24
Galvao RM, Li M, Kothadia SM, Haskel JD, Decker PV, Van Buskirk EK, Chen M (2012) Photoactivated phytochromes interact with HEMERA and promote its accumulation to establish photomorphogenesis in Arabidopsis. Genes Dev 26:1851–1863
Hiltbrunner A, Tscheuschler A, Viczian A, Kunkel T, Kircher S, Schafer E (2006) FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol 47:1023–1034
Hiltbrunner A, Viczian A, Bury E, Tscheuschler A, Kircher S, Toth R, Honsberger A, Nagy F, Fankhauser C, Schafer E (2005) Nuclear accumulation of the phytochrome A photoreceptor requires FHY1. Curr Biol 15:2125–2130
Hu W, Figueroa-Balderas R, Chi-Ham C, Lagarias JC (2020) Regulation of monocot and dicot plant development with constitutively active alleles of phytochrome B. Plant Direct 4:e00210
Hu W, Franklin KA, Sharrock RA, Jones MA, Harmer SL, Lagarias JC (2013) Unanticipated regulatory roles for Arabidopsis phytochromes revealed by null mutant analysis. Proc Natl Acad Sci U S A 110:1542–1547
Hu W, Lagarias JC (2017) A tightly regulated genetic selection system with signaling-active alleles of phytochrome B. Plant Physiol 173:366–375
Hu W, Su YS, Lagarias JC (2009) A light-independent allele of phytochrome B faithfully recapitulates photomorphogenic transcriptional networks. Mol Plant 2:166–182
Huang H, McLoughlin KE, Sorkin ML, Burgie ES, Bindbeutel RK, Vierstra RD, Nusinow DA (2019) PCH1 regulates light, temperature, and circadian signaling as a structural component of phytochrome B-photobodies in Arabidopsis. Proc Natl Acad Sci U S A 116:8603–8608
Hughes J (2013) Phytochrome cytoplasmic signaling. Annu Rev Plant Biol 64:377–402
Huq E, Al-Sady B, Quail PH (2003) Nuclear translocation of the photoreceptor phytochrome B is necessary for its biological function in seedling photomorphogenesis. Plant J 35:660–664
Jaedicke K, Lichtenthaler AL, Meyberg R, Zeidler M, Hughes J (2012) A phytochrome-phototropin light signaling complex at the plasma membrane. Proc Natl Acad Sci U S A 109:12231–12236
Jones MA, Hu W, Litthauer S, Lagarias JC, Harmer SL (2015) A constitutively active allele of phytochrome B maintains circadian robustness in the absence of light. Plant Physiol 169:814–825
Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Khattak AK, Box MS, Charoensawan V, Cortijo S, Kumar M, Grant A, Locke JC, Schafer E, Jaeger KE, Wigge PA (2016) Phytochromes function as thermosensors in Arabidopsis. Science 354:886–889
Kim C, Kwon Y, Jeong J, Kang M, Lee GS, Moon JH, Lee HJ, Park YI, Choi G (2023) Phytochrome B photobodies are comprised of phytochrome B and its primary and secondary interacting proteins. Nat Commun 14:1708
Kim K, Shin J, Lee SH, Kweon HS, Maloof JN, Choi G (2011) Phytochromes inhibit hypocotyl negative gravitropism by regulating the development of endodermal amyloplasts through phytochrome-interacting factors. Proc Natl Acad Sci U S A 108:1729–1734
Klose C, Viczian A, Kircher S, Schafer E, Nagy F (2015) Molecular mechanisms for mediating light-dependent nucleo/cytoplasmic partitioning of phytochrome photoreceptors. New Phytol 206:965–971
Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, Huq E, Quail PH (2008) Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol 18:1815–1823
Leivar P, Quail PH (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16:19–28
Liu X, Jiang W, Li Y, Nie H, Cui L, Li R, Tan L, Peng L, Li C, Luo J, Li M, Wang H, Yang J, Zhou B, Wang P, Liu H, Zhu JK, Zhao C (2023) FERONIA coordinates plant growth and salt tolerance via the phosphorylation of phyB. Nat Plants 9:645–660
Matsushita T, Mochizuki N, Nagatani A (2003) Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature 424:571–574
Ni M, Tepperman JM, Quail PH (1998) PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95:657–667
Ni M, Tepperman JM, Quail PH (1999) Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 400:781–784
Ni W, Xu SL, Gonzalez-Grandio E, Chalkley RJ, Huhmer AFR, Burlingame AL, Wang ZY, Quail PH (2017) PPKs mediate direct signal transfer from phytochrome photoreceptors to transcription factor PIF3. Nat Commun 8:15236
Oh E, Kim J, Park E, Kim JI, Kang C, Choi G (2004) PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16:3045–3058
Oh E, Yamaguchi S, Kamiya Y, Bae G, Chung WI, Choi G (2006) Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J 47:124–139
Paik I, Yang S, Choi G (2012) Phytochrome regulates translation of mRNA in the cytosol. Proc Natl Acad Sci U S A 109:1335–1340
Park E, Kim Y, Choi G (2018) Phytochrome B requires PIF degradation and sequestration to induce light responses across a wide range of light conditions. Plant Cell 30:1277–1292
Pfeiffer A, Nagel MK, Popp C, Wust F, Bindics J, Viczian A, Hiltbrunner A, Nagy F, Kunkel T, Schafer E (2012) Interaction with plant transcription factors can mediate nuclear import of phytochrome B. Proc Natl Acad Sci U S A 109:5892–5897
Pham VN, Kathare PK, Huq E (2018) Phytochromes and phytochrome interacting factors. Plant Physiol 176:1025–1038
Robson PR, Smith H (1996) Genetic and transgenic evidence that phytochromes A and B act to modulate the gravitropic orientation of Arabidopsis thaliana hypocotyls. Plant Physiol 110:211–216
Rockwell NC, Su YS, Lagarias JC (2006) Phytochrome structure and signaling mechanisms. Annu Rev Plant Biol 57:837–858
Rosler J, Jaedicke K, Zeidler M (2010) Cytoplasmic phytochrome action. Plant Cell Physiol 51:1248–1254
Shacklock PS, Read ND, Trewavas AJ (1992) Cytosolic free calcium mediates red light-induced photomorphogenesis. Nature 358:753–755
Shen H, Moon J, Huq E (2005) PIF1 is regulated by light-mediated degradation through the ubiquitin-26S proteasome pathway to optimize photomorphogenesis of seedlings in Arabidopsis. Plant J 44:1023–1035
Shin J, Kim K, Kang H, Zulfugarov IS, Bae G, Lee CH, Lee D, Choi G (2009) Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc Natl Acad Sci U S A 106:7660–7665
Shin AY, Kim HS, Lee SS, Kim MG, Han YJ, Song PS, Kim JI (2011) Photochemical characterization of phytochrome missense mutants. Biosci Biotechnol Biochem 75:2411–2414
Su YS, Lagarias JC (2007) Light independent phytochrome signaling mediated by dominant GAF-domain tyrosine mutants of Arabidopsis phytochromes in transgenic plants. Plant Cell 19:2124–2139
Xu F, Chen J, Li Y, Ouyang S, Yu M, Wang Y, Fang X, He K, Yu F (2024) The soil emergence-related transcription factor PIF3 controls root penetration by interacting with the receptor kinase FER. Dev Cell 59(434–447):e438
Yamaguchi R, Nakamura M, Mochizuki N, Kay SA, Nagatani A (1999) Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J Cell Biol 145:437–445
Wagner D, Quail PH (1995) Mutational analysis of phytochrome-B identifies a small Cooh-terminal-domain region critical for regulatory activity. P Natl Acad Sci USA 92:8596–8600
Wu FH, Shen SC, Lee LY, Lee SH, Chan MT, Lin CS (2009) Tape-Arabidopsis sandwich—a simpler Arabidopsis protoplast isolation method. Plant Methods 5:16
Yoo SY, Bomblies K, Yoo SK, Yang JW, Choi MS, Lee JS, Weigel D, Ahn JH (2005) The 35S promoter used in a selectable marker gene of a plant transformation vector affects the expression of the transgene. Planta 221:523–530
Zhao Y, Shi H, Pan Y, Lyu M, Yang Z, Kou X, Deng XW, Zhong S (2023) Sensory circuitry controls cytosolic calcium-mediated phytochrome B phototransduction. Cell 186(1230–1243):e1214
Acknowledgements
This work was supported in part by the National Institutes of Health grants RO1:GM068552 and R35GM139598 awarded to J.C.L. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH. We thank Profs. Meng Chen (UC Riverside) and Peter Quail (Plant Gene Expression Center) for the PIF3 and phyB antibodies, respectively.
Funding
National Institutes of Health, RO1 GM068552, J. Clark Lagarias, R35GM139598, J. Clark Lagarias
Author information
Authors and Affiliations
Contributions
WH and JCL conceived the project and wrote the manuscript; WH designed and performed the research, and analyzed the data; JCL provided supervision and financial supports.
Corresponding author
Ethics declarations
Competing interests
There are no conflicts of interest or competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, W., Lagarias, J.C. A cytosol-tethered YHB variant of phytochrome B retains photomorphogenic signaling activity. Plant Mol Biol 114, 72 (2024). https://doi.org/10.1007/s11103-024-01469-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11103-024-01469-2