Abstract
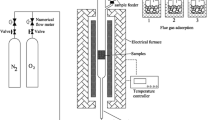
Acid tar (AT) is a distinct black viscous waste from the petrochemical industry that retains hazardous characteristics decades after creation. It is defined as an emulsion of various organic compounds, sulfuric acid, and water. Due to specific chemical composition and physical properties, the best available technique for the treatment of AT is not determined. This paper investigates the treatment of AT by CaO, resulting in its complete physicochemical transformation into a dry powder with the characteristics of inorganic material. Waste characterization of AT and obtained powder (including metals, PAH, BTEX, total hydrocarbon content, and EN12457-4 leaching test), their comparative FT-IR and SEM–EDS analyses, and XRD analysis of the powder revealed that the transformation is a complex process involving Ca(OH)2 formation, evaporation of water and BTEX, and degradation of aromatic and unsaturated hydrocarbons. The remained organic phase is encapsulated under Ca(OH)2 and CaCO3 layers forming “organic core—inorganic shell” micron-sized particles, rendering the powder suitable for further treatment. This was confirmed by solidification/stabilization treatment of AT and obtained powder using the same amount of cement and pozzolanic binder. In contrast to AT solidificates, the powder solidificates possess unconfined compressive strength above the required value and characteristics of inert waste.







Similar content being viewed by others
References
Milne DD, Clark AI, Perry R (1986) Acid tars: their production, treatment and disposal in the UK. Waste Manage Res 4:407–418
Xu H (2007) Acid tar lagoons: assessment and environmental interaction. Dissertation, University of Sheffield.
Leonard SA, Stegemann JA, Roy A (2010) Characterization of acid tars. J Hazard Mater 175:382–392. https://doi.org/10.1016/j.jhazmat.2009.10.015
Pensaert S (2011) The remediation of the acid tar lagoons, Rieme, Belgium, In: Stabilisation/Solidification Treatment and Remediation: Proceedings of the International Conference on Stabilisation/Solidification Treatment and Remediation, 12–13 April 2005. CRC Press, Cambridge, UK
Danha C (2017) Characterisation and utilisation of acid tar waste from crude benzol processing for environmental sustainability. Dissertation, Chinhoyi University of Technology
Smith C, Hao X, Talbot S, Lawson N (2008) Acid tar lagoons, In: CL:AIRE: Contaminated Land: Applications in Real Environments, Subr:im Bulletin SUB 7.
Popovych VV, Malovanyy MS, Prydatko OV, Popovych NP, Petlovanyi MV, Korol КA, Korolova OG (2021) Technogenic impact of acid tar storage ponds on the environment: a case study from Lviv, Ukraine. Ecologia Balkanica 13:35–44
Karušs J, Lamsters K, Poršņovs D, Zanderson V, Ješkins J (2021) Geophysical map** of residual pollution at the remediated Inčukalns acid tar lagoon, Latvia. Est J Earth Sci 70:140–151. https://doi.org/10.3176/earth.2021.10
Khlibyshyn YY, Pochapska IY, Grynyshyn OB, Gnativ ZY (2018) The study of the fabrication of bitumen from acid tars and oil residues. Boпpocы xимии и xимичecкoй тexнoлoгии 5:161–167
Pensaert S, De Puydt S, Janssens T (2010) Remediation of the acid tar lagoon in the port of Ghent. In: Lake CB, Hills C (eds) International solidification/stabilization technology forum. Dalhousie University
Chihobo CH, Chowdhury A, Kuipa PK, Simbi DJ (2016) Pyrolysis characteristics and kinetics of acid tar waste from crude benzol refining: A thermogravimetry–mass spectrometry analysis. Waste Manage Res 34:1258–1267. https://doi.org/10.1177/0734242X16669999
Reeb C, Pierlot C, Davy C, Lambertin D (2021) Incorporation of organic liquids into geopolymer materials-A review of processing, properties and applications. Ceram Int 47:7369–7385
Leonard SA, Stegemann JA (2010) Stabilization/solidification of acid tars. J Environ Sci Heal A 45:978–991. https://doi.org/10.1080/10934521003772394
Lagrega MD, Evans JC, Acuna CO, Zarlinski SJ, Hall DS (1990) Stabilization of acidic refinery sludges. J Hazard Mater 24:169–187. https://doi.org/10.1016/0304-3894(90)87008-6
Schifano V, Lilley F (2018) Solidification/Stabilization Remediation of Acid Organic Waste for Impoundment Units Closure. In: The International Congress on Environmental Geotechnics. Springer, Singapore
Hadadi V, Moradi A (2019) A novel method for refining of acidic sludge and mixing with vacuum bottom to improve bitumen properties. Petrol Sci Technol 37:1529–1538
Liu Z (2016) National carbon emissions from the industry process: Production of glass, soda ash, ammonia, calcium carbide and alumina. Appl Energ 166:239–244. https://doi.org/10.1016/j.apenergy.2015.11.005
Malviya R, Chaudhary R (2006) Factors affecting hazardous waste solidification/stabilization: A review. J Hazard Mater 137:267–276
Halim CE, Short SA, Scott JA, Amal R, Low G (2005) Modelling the leaching of Pb, Cd, As, and Cr from cementitious waste using PHREEQC. J Hazard Mat 125:45–61. https://doi.org/10.1016/j.jhazmat.2005.05.046
Štulović M, Radovanović D, Kamberović Ž, Korać M, Anđić Z (2019) Assessment of leaching characteristics of solidified products containing secondary alkaline lead slag. Int J Env Res Pub He 16:2005. https://doi.org/10.3390/ijerph16112005
Rulebook on categories, testing and classification of waste. The Official Gazette of the Republic of Serbia 31/2021.
Ivšić-Bajčeta D, Kamberović Ž, Korać M, Gavrilovski M (2013) A solidification/stabilization process for wastewater treatment sludge from a primary copper smelter. J Serb Chem Soc 78:725–739. https://doi.org/10.2298/JSC120716125I
Ko JH, Musson S, Townsend T (2010) Destruction of trichloroethylene during hydration of calcium oxide. J Hazard Mat 174:876–879. https://doi.org/10.1016/j.jhazmat.2009.09.043
Schifano V, MacLeod C, Hadlow N, Dudeney R (2007) Evaluation of quicklime mixing for the remediation of petroleum contaminated soils. J Hazard Mat 141:395–409. https://doi.org/10.1016/j.jhazmat.2006.05.086
Smith KA, Goins LE, Logan TJ (1998) Effect of calcium oxide dose on thermal reactions, lime speciation, and physical properties of alkaline stabilized biosolids. Water Environ Res 70:224–230
Cabrera-Penna M, Rodríguez-Páez JE (2021) Calcium oxyhydroxide (CaO/Ca(OH)2) nanoparticles: Synthesis, characterization and evaluation of their capacity to degrade glyphosate-based herbicides (GBH). Adv Powder Technol 32:237–253. https://doi.org/10.1016/j.apt.2020.12.007
Hu J, Feng Z, Huang Z, Yu J, Liu J (2020) Mechanochemical destruction of 4-bromochlorobenzene with CaO. Efficiency, kinetics, and mechanism. Environ Prot Eng 46:63–77. https://doi.org/10.37190/epe200205
Qiao W, Ge X, Zhang Y, Luo Y, Yu L, Wang H, Wang Q (2019) Degradation of endosulfan by high-energy ball milling with CaO: process and mechanism. Environ Sci Pollut R 26:18541–18553. https://doi.org/10.1007/s11356-019-05020-5
Humel S, Schritter J, Sumetzberger-Hasinger M, Ottner F, Mayer P, Loibner AP (2020) Atmospheric carbonation reduces bioaccessibility of PAHs in industrially contaminated soil. J Hazard Mat 383:121092. https://doi.org/10.1016/j.jhazmat.2019.121092
Coates J (2006) Interpretation of infrared spectra, a practical approach. In: Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation, Wiley Online Library, pp 1–23
Alhreez M, Wen D (2019) Molecular structure characterization of asphaltene in the presence of inhibitors with nanoemulsions. RSC Adv 9:19560–19570. https://doi.org/10.1039/c9ra02664a
Yerzhan I, Yerdos O, Yerbol T, Evgeniy K, Anatoly G (2017) High temperature transformation of tar-asphaltene components of bituminous sand bitumen. JOJ Material Sci 1:555552. https://doi.org/10.19080/JOJMS.2017.01.555552
Lalmuanpuii R, Muthukumaran RB (2016) Fourier transform-infrared spectroscopic characterization of the particulate phase of commercial tuibur. Science Vision 16:68–73
Khachani M, El Hamidi A, Halim M, Arsalane S (2014) Non-isothermal kinetic and thermodynamic studies of the dehydroxylation process of synthetic calcium hydroxide Ca(OH)2. J Mater Environ Sci 5:615–624
Galván-Ruiz M, Hernández J, Baños L, Noriega-Montes J, Rodríguez-García ME (2009) Characterization of calcium carbonate, calcium oxide, and calcium hydroxide as starting point to the improvement of lime for their use in construction. J Mater Civil Eng 21:694–698. https://doi.org/10.1061/(ASCE)0899-1561(2009)21:11(694)
Immobilisation Technical Note 2. Cement-based Solidification/Stabilisation Treatment of Organic Chemical Contaminants in Waste. https://www.epa.nsw.gov.au/your-environment/waste/tracking-transporting-hazardous-waste/immobilisation/technical-notes-immobilisation/immobilisation-note2. Accessed 4 November 2022
Shen Z, ** F, O’Connor D, Hou D (2019) Solidification/stabilization for soil remediation: an old technology with new vitality. Environ Sci Technol 53:11615–11617. https://doi.org/10.1021/acs.est.9b04990
Acknowledgements
This work has been financially supported by grants issued from the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Contract No. 451-03-47/2023-01/200287 and 451-03-47/2023-01/200135).
Funding
Ministarstvo Prosvete,Nauke i Tehnološkog Razvoja,451-03-47/2023-01/200287
Author information
Authors and Affiliations
Contributions
Dragana Radovanović: Conceptualization, Methodology, Investigation, Writing- Original draft preparation. Marija Štulović: Data curation, Investigation. Milisav Ranitović: Writing- Reviewing and Editing. Jovana Djokić: Visualization, Writing- Reviewing and Editing. Zoran Andjić: Validation. Željko Kamberović: Supervision.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Radovanović, D., Štulović, M., Ranitović, M. et al. Acid tar treatment—the transformation of organic waste into “organic core—inorganic shell” structure particles. J Mater Cycles Waste Manag (2024). https://doi.org/10.1007/s10163-024-02012-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10163-024-02012-7