Abstract
Tannic acid–acetic acid is proposed as novel and green chemicals for cobalt and lithium recycling from spent lithium-ion batteries through a leaching process. The synergism of both acids was documented through batch and continuous studies. Tannic acid promotes cobalt dissolution by reducing insoluble Co3+ into soluble Co2+, while acetic acid is critical to improve the dissolution and stabilize the metals in the pregnant leach solution. Based on batch studies, the optimum conditions for metal recovery at room temperature are acetic acid 1 M, tannic acid 20 g/L, pulp density 20 g/L, and stirring speed 250 rpm (94% cobalt and 99% lithium recovery). The kinetic study shows that increasing temperature to 80 °C improves cobalt and lithium recovery from 65 to 90% (cobalt) and from 80 to 99% (lithium) within 4 h at sub-optimum condition (tannic acid 10 g/L). Kinetic modeling suggests the leaching process was endothermic, and high activation energy indicates a surface chemical process. For other metals, the pattern of manganese and nickel recovery trend follows the cobalt recovery trend. Copper recovery was negatively affected by tannic acid. Iron recovery was limited due to the weak acidic condition of pregnant leach solution, which is beneficial to improve leaching selectivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
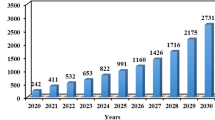
Lithium-ion battery (LIB) is a type of rechargeable battery widely used as energy storage in electronic devices [1]. Since energy storage technology is improving, the demand for certain elements, especially cobalt (Co) and lithium (Li), has been increasing. Unfortunately, natural resources for both elements are not evenly distributed in nature. In the case of Li, most natural resources are concentrated in few countries, e.g., Argentina, Chile, and China, leading to its criticality. Due to their scarcity and geopolitical position, recycling technology for Co and Li is warranted. It is estimated that in 2020, spent LIB produced would reach 400 million tons [2]. Apart from securing the supply, recycling is also practiced to reduce the burden to the environment due to toxic, yet valuable content in LIB.
There are three extraction schemes applied in metal recycling from spent LIB: pyrometallurgy, hydrometallurgy, and biometallurgy. In pyrometallurgy, the spent LIB is mixed with reductant and fluxes and is melted at high temperatures. During melting, the transition metals such as Co will be reduced to a metal alloy, while Li will be transferred into the slag phase as silicate [3]. Li recovery from the slag requires further treatment, e.g., roasting, before it is finally hydrometallurgically processed [4]. High energy consumption, toxic gas emission, and Li loss in the processing limit the applicability of pyrometallurgy in spent LIB recycling. Biometallurgy uses microorganisms to produce in situ lixiviants, which dissolve elements in spent LIB. Although the approach is considered cost-effective and environmentally friendly [5, 6], the adaptation to industrial scale is still limited [7]. Hydrometallurgy is widely employed due to lower energy input. By using chemicals (lixiviants), the target metals are dissolved out from the spent LIB and further separated and purified from pregnant leach solution (PLS) using solvent extraction [8], precipitation [9], and solid-phase extraction [10].
Literature survey reveals that lixiviants used to recover Co and Li include inorganic and organic lixiviants. Inorganic lixiviants such as sulfuric acid [7, 11], nitric acid [12], hydrochloric acid [13], and phosphoric acid [14] are commonly combined with a reductant, e.g., hydrogen peroxide to dissolve Co and Li from the LIB cathode. Due to the aggressive nature of the acids, several research groups have shifted their attention to safer organic lixiviants, e.g., malic acid [15], lactic acid [16], citric acid [17], and oxalic acid [18, 19]. Organic compounds are used to stabilize Co as complexes in PLS, and some also serve as reductants [20]. Aside from hydrogen peroxide [21, 22], very few reductants have been proposed for Co and Li leaching. The reductant is critical in the leaching process to promote solubility of Co by reducing insoluble Co3+ in the spent LIB cathode (LiCoO2) into soluble Co2+ (1). Inorganic reductants suggested so far include bisulfite [23], sodium sulfite [24], and divalent iron [25], while organic reductants recommended were glucose [26], ascorbic acid [27], and ethanol [28].
Although organic reductants pose lower hazard, the leaching efficiency still needs improvement. This is related to the reducing capacity of each reductant, i.e., the amount of electron released by each gram of reductant during a redox reaction, e.g., glucose 133.2 mmol/g and ethanol 43.31 mmol/g [29]. To improve leaching efficiency and consider economic reasons, we propose tannic acid (TA) as a novel and green reductant for Co and Li extraction from spent LIB. TA as a polyphenolic compound with a high estimated reducing capacity (203.5 mmol/g) [29] is inexpensively extracted from plant tissue.
To the best of our knowledge, there is no report available investigating the effect of TA as a reagent for spent LIB leaching. Therefore, in this study, the ability of TA as reductant will be tested in dissolving Co and Li from spent LIB. The use of TA will be combined with acetic acid as a pH regulator to create an acidic condition favorable for maintaining Co in the PLS. In addition, the selection of acetic acid is meant to comply with the green and sustainable principle in the recycling process (Fig. 1). For that reason, the main objective of this research is to investigate the efficacy of tannic acid-acetic acid as a lixiviant for Co and Li leaching from spent LIB. The correlation between initial leaching conditions, i.e., tannic acid concentration, acetic acid concentration, pulp density, stirring speed, and kinetic study (leaching time and temperature) to dissolution rate of Co, Li, and other metals from spent LIB will be examined and discussed, including the possible leaching mechanism.
Materials and methods
Material and instrumentation
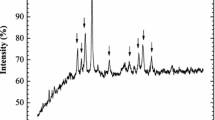
Spent LIB (18,650 type) was obtained from a local scrapyard in Jakarta, Indonesia. The batteries were discharged and manually dismantled to separate their components. The cathode component was ball milled and sieved. A size fraction less than 270 mesh (53 μm) was used further in the leaching studies. XRD characterization (Panalytical, Expert3 Powder) revealed the major phase in the raw materials to be graphite (C) and LiCoO2. After leaching, XRD analysis showed the residue consisted of graphite (Fig. 2). Acid digestion of cathode powder (HNO3–HCl 1:3) followed by elemental determination by ICP-OES (Analytik Jena, Plasma Quant 9000 Elite, Germany) resulted in metal concentration listed in Table 1.
Tannic acid was obtained from Bean Town Chemicals, USA. Acetic acid glacial, sodium hydroxide, and nitric acid were purchased from Merck, Darmstadt, Germany, all in analytical grade. Solution pH/Eh was measured using a pH/ORP meter (Oakton 45, Vernon Hills, IL, USA). Deionized (MilliQ) water was used throughout the experiments.
Leaching experiment
The batch method was applied to optimize the leaching condition and investigate the correlation between the initial leaching condition, i.e., tannic acid concentration, acetic acid concentration, pulp density, and stirring speed to Co and Li recovery. Typically, 1 g of cathode powder was mixed with lixiviant (tannic acid-acetic acid mixture) in a sealed conical flask. The mixture was homogenized using an orbital shaker for 12 h at room temperature. After equilibrium, the PLS was separated from solid residue by filtration (Whatman 42 filter paper) and analyzed using ICP-OES for metal concentration determination. Metal recovery was calculated using a mass balance (2).
where CE is the metal concentration in filtrate/lixiviant (mg/L), Co themetal content in cathode powder (mg/g), m the mass of cathode powder used in leaching (g), and V the leaching agent volume (L).
To evaluate the effect of leaching time and temperature on Co and Li recovery, 5 g of cathode powder was equilibrated with 250 ml lixiviant (20 g/L pulp density) in a 1000 ml boiling flask. The mixture was stirred, and the temperature was controlled using a water bath (temperature variation within 1 °C). PLS was sampled at a particular time interval, and the metal content was determined using ICP-OES.
Results and discussion
Effect of tannic acid concentration
The effect of tannic acid was studied between 0 (control test) and 24 g/L (Fig. 3a). The figure demonstrates that the addition of tannic acid significantly increased the recovery of Co and Li. Increasing Co dissolution due to oxidation of tannic acid (3) [30] by Co3+ in LIB (4) resulted in soluble Co2+ (5).
At the control test (tannic acid 0 g/L), Co and Li recoveries were 39% and 55%, respectively, which were significant. Since Co3+ was virtually insoluble in 1 M acetic acid media, the existence of Co in PLS was most likely contributed by Co2+ as the product of acetic acid oxidation according to reaction (6). Further, when initial tannic acid concentration increased to 20 g/L, Co and Li recoveries improved to 94% and 100% (Fig. 3a). This confirms the efficacy of tannic acid as a reductant for Co and Li leaching from the spent LIB battery.
The measurements of pH and Eh at initial and equilibrium conditions (Fig. 3b) showed that PLS became more alkaline (pH increased) and more reducing (Eh decreased) during leaching. The figure also demonstrates that increasing tannic acid concentration caused the increasing pH of pregnant leach solution after leaching, which confirmed the redox reaction followed reaction (5). The measured equilibrium pH of PLS at TA 24 g/L was 3.82. This pH was still within acceptable pH to maintain metal, i.e., Co2+ in the aqueous phase (pH < 8), according to Pourbaix diagrams in Fig. 4 (generated using Hydra and Medusa, KTH Royal Institute of Technology, 2015). In terms of Eh value, as expected, increasing tannic acid concentration made the solution more reducing. At control condition (TA 0 g/L), the Eh decreased from 0.241 to 0.175 V, which was presumably contributed by acetic acid degradation (6). In contrast, at TA 24 g/L, Eh changed from 0.246 to 0.158 V. These range values, according to the Pourbaix diagram (Fig. 4), were favorable for Co reduction or solubilization (Eh > −0.3 V).
Figure 5 depicts the influence of leaching time and tannic acid on Co and Li recovery at room temperature. Increasing tannic acid clearly increased the maximum recovery of both metals, but insignificantly improved the leaching rate. Visual observation on Fig. 4 implied that the leaching reached saturation (maximum recovery) within 240 min, regardless of initial tannic acid concentration. On calculation using tannic acid-reducing capacity (203.5 mmol e−/g), Eq. (5), and leaching condition (acetic acid 1 M, solid–liquid ratio 20 g/L), to dissolve all Co, the minimum tannic acid concentration required was 0.45 g/L. Although the tannic acid concentration employed in the kinetic test exceeded the stoichiometric value, the Co recovery did not reach the maximum value. When tannic acid changed from 5 to 20 g/L, maximum recovery of Co rose from 40 to 80% and for Li from 45 to 80%. The results implied that the reaction between Co3+ in solid and tannic acid in the liquid phase was also controlled or limited by other mechanisms. A possible mechanism is the passivation of LiCoO2 by an insoluble layer of Co(OH)2 as a product of redox reaction (5, 6). The insoluble layer would prevent further reaction between Co3+ and tannic acid–acetic acid, halting the dissolution. Co(OH)2 on the surface of LiCoO2 was observed by FT-IR characterization (Fig. 6). The figure shows the FT-IR spectra of LiCoO2 before and after leaching. Peaks between 3200 and 3600 cm−1 were assigned to the stretching vibration of the O–H groups. The presence of metal hydroxide was indicated by peaks around 500 and 750 cm−1, while specifically, the presence of Co(OH)2 was confirmed by peaks at 1059 and 1123 cm−1 [31,32,33,34].
Effect of acetic acid concentration
The effect of acetic acid was tested between 0 M (control test) and 2 M. The results in Fig. 7a support the hypotheses that the increasing acetic acid concentration promotes Co and Li dissolution (more acidic condition). At the control test, Co and Li recoveries were 3.5% and 33%, respectively. The recovery improved to 82% for Co and 93% for Li at acetic acid 2 M. Acetic acid concentration 1 M was chosen as optimum concentration, since the majority of Co and Li had been leached at this concentration (Co > 60% and Li > 70%). Although the leaching efficiency could be improved at higher acetic acid concentration, acetic acid 1 M was deliberately chosen for further studies (effect of pulp density, temperature and leaching time) to stress out the effect of other leaching parameters in boosting Co and Li recovery.
Figure 7b shows the pH and Eh change during leaching according to initial acetic acid concentration. The recovery of Co at the control test was minimum due to almost neutral pH (6.75) at equilibrium, which contradicted the data in the Pourbaix diagram (Fig. 4). The chart implied that neutral pH is still favorable for Co dissolution. This can be explained by the passivation mechanism by Co(OH)2 as the product of redox reaction (5). Limited acid in PLS was insufficient to remove the insoluble layer, which prevented further solubilization of Co3+. Therefore, increasing acetic acid would be beneficial to prevent passivation. In the case of Eh value, the PLS became more reductive, which was favorable for Co dissolution (Fig. 7).
Figure 8 displays the recovery of Co and Li as a function of leaching time and acetic acid concentration. Increasing acetic acid concentration enhanced Co and Li recovery. Similar to kinetic results in Fig. 5 (effect of tannic acid), raising acetic acid concentration did not improve the initial recovery rate, albeit increased maximum recovery. Leaching saturation rose from 30 to 60% for Co and from 50 to 65% for Li when acetic acid increased from 0.2 to 1 M. Based on the figure, the leaching efficiency reached saturation within 180 min for Co and Li irrespective of the acetic acid concentration. Considering pKa of acetic acid 4.76, the acetic acid concentration used in kinetic studies (0.2, 0.5, and 1 M) was insufficient to produce the proton required to neutralize OH− produced during redox reaction between LiCoO2 and tannic acid (5). The remaining OH− would form an insoluble layer with Co2+. This supports that dissolution was controlled by surface passivation.
Effect of pulp density and stirring speed
The effect of solid–liquid ratio during leaching was tested from 2 to 100 g/L (Fig. 9a). Increasing pulp density negatively affected Co and Li recovery. Co and Li recovery at pulp density 2 g/L was 96.5% and 99.9%, respectively, which decreased to 42% and 32% at pulp density 100 g/L. Decreasing metal recovery due to increasing pulp density is caused by reducing area per unit volume solution. This, in turn, reduces reaction sites and affected the reaction rate. On the other hand, lower pulp density (higher liquid–solid ratio) encourages the diffusion of lixiviant molecules into the solid–liquid interface, promoting the leaching reaction rate [35]. The influence of stirring speed was studied from 50 to 250 rpm. Figure 9b shows that increasing stirring speed from 50 to 250 rpm only enhances the metal recovery by about 20%. Increasing recovery due to higher stirring speed was attributed to the external diffusion process [36].
Effect of leaching time and temperature (kinetic modeling)
The influence of leaching time and temperature to Co and Li recovery was observed within 6 h at 6 different temperatures (27, 40, 50, 60, 70, and 80 °C). As shown in Fig. 10, increasing temperature boosted leaching recovery, which confirmed that the leaching process was endothermic. Co leaching recovery improved from 60 to 90% and for Li from 60 to 100% when the leaching temperature rose from 27 °C to 80 °C. Figure 10 indicates the longer time required to attain maximum recovery as temperature increased. At room temperature, leaching saturation was achieved within 120 min, while at 80 °C, Co and Li maximum recovery was reached within 240 min.
To describe and analyze kinetic data and to discern the process controlling the dissolution of Co and Li from spent LIB cathode. Several kinetic models widely known, such as the shrinking core model (SCM) and shrinking particle model (SPM), will be applied. SCM is based on a reaction between the heterogeneous solid–liquid phase (outer surface) [37]. In SPM, the dissolution process is assumed to be controlled by diffusion through the inner layer [38]. Both SCM and SPM do not consider the change of reactant/product concentration in bulk solution. Another kinetic model that considers the reactant change in bulk solution is the interface transfer and diffusion model (ITD) proposed by Dickinson and Heal [39]. The fitting results in Table 2 show that SCM and SPM are not sufficient to describe the experimental kinetic data as demonstrated by lower coefficient correlation (R2). ITD was better to describe the experimental data since the model considered the change of tannic acid and acetic acid as reactants in bulk solution during Co and Li dissolution. The k value obtained from the fitting using ITD was used to calculate activation energy (Ea, kJ/mol) using the Arrhenius Eq. (7). A, T, and R are frequency factor, temperature, and gas constant, respectively.
Plot between 1/T and ln k in Fig. 11 resulted in two slopes for Co and a single slope for Li. For Co, the first slope at 27–60 °C and the second slope at 60–80 °C yielded activation energy of 9.8 kJ/mol and 92.7 kJ/mol. Low activation energy at the lower temperature (<40 kJ/mol) indicated domination of physical attraction between bulk volume and solid phase, while at the higher temperature, high activation energy (>40 kJ/mol) implied the domination of surface chemical process [40]. In the case of Li, calculated activation energy 51.7 kJ/mol suggested that Li dissolution was a surface chemical process [36, 37].
Dissolution of other metals (Cu, Ni, Mn, and Fe)
The effect of acetic acid, tannic acid, pulp density, and stirring speed on other metals dissolution is shown in Fig. 12. Increasing acetic acid concentration improved Cu, Ni, and Mn recovery (Fig. 12a). In the case of Fe, the recovery was not affected by acetic acid concentration, which was probably due to the limited solubility of iron in equilibrium pH (>3.5) (Fig. 7b). The recovery of Cu, Ni, and Mn at acetic acid 0 M was minimum due to almost neutral pH at equilibrium (pH 6.75) (Fig. 3b), which encouraged surface passivation. Mn and Ni dissolution sharply increased, even at a low concentration of acetic acid. In the case of Cu, significant dissolution occurred at minimum acetic acid 0.5 M (Fig. 12a), which corresponded to pH and Eh value 4 and 0.153 V. This condition is in line with data in the Porbaix diagram (Fig. 13), which imply minimum Eh for Cu dissolution is 0.15 V.
Leaching efficiency of Cu, Mn, Ni, and Fe according to a acetic acid concentration (constant variable tannic acid concentration 10 g/L, pulp density 20 g/L, stirring rate 250 rpm, room temperature, 12 h, b tannic acid concentration (constant variable acetic acid concentration 1 M, pulp density 20 g/L, stirring rate 250 rpm, room temperature, 12 h, c pulp density (constant variable acetic acid 1 M, tannic acid 10 g/L, stirring speed 250 rpm, room temperature) and d stirring speed (constant variable acetic acid 1 M, tannic acid 10 g/L, pulp density 20 g/L, room temperature)
The inertness of Cu to the dissolution was more pronounced in the tannic acid effect (Fig. 12b), since tannic acid decreased the Eh value (more reductive), as shown in Fig. 3b. The leaching efficiency of Ni and Mn was positively affected by tannic acid concentration, since both metals favorably existed in PLS within equilibrium Eh and pH (Pourbaix diagram, Fig. 13). The effect of pulp density and stirring speed on Cu, Ni, Mn, and Fe leaching efficiency are depicted in Fig. 12c, d, respectively. Fe recovery was severely affected by pulp density. In the case of stirring speed, metal recovery only increased about 20% if the speed rose from 50 to 250 rpm. In general, compared to previously reported lixiviants (reductant, pH regulator), the tannic acid–acetic acid combination possessed advantages in terms of consumption rate and lower hazard with competitive leaching efficiency (Table 3).
Conclusions
Tannic acid–acetic acid was successfully applied as a novel and green reagent for Co and Li recycling from spent LIB cathode. Tannic acid was proved to be an effective reducing agent by encouraging Co solubilization through reduction to divalent species. Acetic acid was confirmed as a pH regulator, while in the absence of tannic acid, acetic acid could act as a reductant. The acetic acid concentration was critical since it improved metal solubility, especially Co, by buffering the pH within acidic range, maintaining the metals in the aqueous phase, and preventing surface passivation. Batch studies revealed the optimum condition was acetic acid 1 M, tannic acid 20 g/L, pulp density 20 g/L, and stirring speed 250 rpm (Co 93% and Li 99%). Kinetic studies result at sub-optimum condition (tannic acid 10 g/L) showed that increasing temperature from 27 °C to 80 °C improved recovery from 62 to 92% for Co and 66% to 99% for Li, within 4 h. Kinetic modeling obeyed the interface and diffusion model, suggesting the leaching process was controlled by reactant and product concentration in the liquid phase. Activation energy analysis resulted in a different pattern for Co and Li leaching. Based on lnk-1/T plot, only one slope was observed for Li (Ea 51.7 kJ/mol, indicating surface chemical reaction and process rate-controlling step), while for Co, two slopes were observed. Activation energy 9.8 kJ/mol (T 27–60 °C) and 92.7 kJ/mol (T 60–80 °C) for Co indicated physical attraction dominates the lower temperature process and surface chemical process in higher temperature, respectively. Mn and Ni recovery trend follows the Co recovery trend, while Cu was negatively affected by tannic acid. Fe recovery was limited due to the weak acidic condition of PLS.
References
Gao W, Liu C, Cao H, Zheng X, Lin X, Wang H, Zhang Y, Sun Z (2018) Comprehensive evaluation on effective leaching of critical metals from spent lithium-ion batteries. Waste Manag 75:477–485. https://doi.org/10.1016/j.wasman.2018.02.023
Gu F, Guo J, Yao X, Summers PA, Widijatmoko SD, Hall P (2017) An investigation of the current status of recycling spent lithium-ion batteries from consumer electronics in China. J Clean Prod 161:765–780. https://doi.org/10.1016/j.jclepro.2017.05.181
Chen Y, Liu N, Hu F, Ye L, ** Y, Yang S (2018) Thermal treatment and ammoniacal leaching for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag 75:469–476. https://doi.org/10.1016/j.wasman.2018.02.024
Dang H, Li N, Chang Z, Wang B, Zhan Y, Wu X, Liu W, Ali S, Li H, Guo J, Li W, Zhou H, Sun C (2020) Lithium leaching via calcium chloride roasting from simulated pyrometallurgical slag of spent lithium ion battery. Sep Purif Technol 233:116025. https://doi.org/10.1016/j.seppur.2019.116025
Naseri T, Bahaloo-Horeh N, Mousavi SM (2019) Bacterial leaching as a green approach for typical metals recovery from end-of-life coin cells batteries. J Clean Prod 220:483–492. https://doi.org/10.1016/j.jclepro.2019.02.177
Bahaloo-Horeh N, Mousavi SM, Baniasadi M (2018) Use of adapted metal tolerant Aspergillus niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries. J Clean Prod 197:1546–1557. https://doi.org/10.1016/j.jclepro.2018.06.299
Li H, **ng S, Liu Y, Li F, Guo H, Kuang G (2017) Recovery of lithium, iron, and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system. ACS Sustain Chem Eng 5(9):8017–8024. https://doi.org/10.1021/acssuschemeng.7b01594
Wang WY, Yen CH, Lin JL, Xu RB (2019) Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. J Mater Cycles Waste Manag 21(2):300–307. https://doi.org/10.1007/s10163-018-0790-x
Badawy SM, Nayl AA, El Khashab RA, El-Khateeb MA (2013) Cobalt separation from waste mobile phone batteries using selective precipitation and chelating resin. J Mater Cycles Waste Manag 16:739–746. https://doi.org/10.1007/s10163-013-0213-y
Wang H, Huang K, Zhang Y, Chen X, ** W, Zheng S, Yi Z, Li P (2017) Recovery of lithium, nickel, and cobalt from spent lithium-ion battery powders by selective ammonia leaching and an adsorption separation system. ACS Sustain Chem Eng 5(12):11489–11495. https://doi.org/10.1021/acssuschemeng.7b02700
Peng C, Hamuyuni J, Wilson BP, Lundström M (2018) Selective reductive leaching of cobalt and lithium from industrially crushed waste Li-ion batteries in sulfuric acid system. Waste Manag 76:582–590. https://doi.org/10.1016/j.wasman.2018.02.052
Li L, Chen R, Sun F, Wu F, Liu J (2011) Preparation of LiCoO2 films from spent lithium-ion batteries by a combined recycling process. Hydrometallurgy 108(3–4):220–225. https://doi.org/10.1016/j.hydromet.2011.04.013
Guo Y, Li F, Zhu H, Li G, Huang J, He W (2016) Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl). Waste Manag 51:227–233. https://doi.org/10.1016/j.wasman.2015.11.036
Pinna EG, Ruiz MC, Ojeda MW, Rodriguez MH (2017) Cathodes of spent Li-ion batteries: dissolution with phosphoric acid and recovery of lithium and cobalt from leach liquors. Hydrometallurgy 167:66–71. https://doi.org/10.1016/j.hydromet.2016.10.024
Sun C, Xu L, Chen X, Qiu T, Zhou T (2018) Sustainable recovery of valuable metals from spent lithium-ion batteries using DL-malic acid: leaching and kinetics aspect. Waste Manag Res 36(2):113–120. https://doi.org/10.1177/0734242X17744273
Roshanfar M, Golmohammadzadeh R, Rashchi F (2019) An environmentally friendly method for recovery of lithium and cobalt from spent lithium-ion batteries using gluconic and lactic acids. J Environ Chem Eng 7(1):102794. https://doi.org/10.1016/j.jece.2018.11.039
Musariri B, Akdogan G, Dorfling C, Bradshaw S (2019) Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner Eng 137:108–117. https://doi.org/10.1016/j.mineng.2019.03.027
Refly S, Floweri O, Mayangsari TR, Sumboja A, Santosa SP, Ogi T, Iskandar F (2020) Regeneration of lini1/3co1/3mn1/3o2cathode active materials from end-of-life lithium-ion batteries through ascorbic acid leaching and oxalic acid coprecipitation processes. ACS Sustain Chem Eng 8(43):16104–16114. https://doi.org/10.1021/acssuschemeng.0c01006
Li L, Lu J, Zhai L, Zhang X, Curtiss L, ** Y, WuF CR, Amine K (2018) A facile recovery process for cathodes from spent lithium iron phosphate batteries by using oxalic acid. CSEE J Power Energy Syst 4(2):219–225. https://doi.org/10.17775/cseejpes.2016.01880
Astuti W, Mufakhir FR, Prasetyo E, Sumardi S, Yuda APT, Nurjaman F, Supriyatna YI, Handoko AS (2019) Reductive-atmospheric leaching of manganese from pyrolusite ore using various reducing agents. AIP Conf Proc 2097:030117. https://doi.org/10.1063/1.5098292
Lee CK, Rhee KI (2002) Preparation of LiCoO2 from spent lithium-ion batteries. J Power Sources 109(1):17–21. https://doi.org/10.1016/S0378-7753(02)00037-X
Jha MK, Kumari A, Jha AK, Kumar V, Hait J, Pandey BD (2013) Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag 33(9):1890–1897. https://doi.org/10.1016/j.wasman.2013.05.008
Meshram P, Pandey BD, Mankhand TR (2015) Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem Eng J 281:418–427. https://doi.org/10.1016/j.cej.2015.06.071
Zheng X, Gao W, Zhang X, He M, Lin X, CaoH ZY, Sun Z (2017) Spent lithium-ion battery recycling – Reductive ammonia leaching of metals from cathode scrap by sodium sulphite. Waste Manag 60:680–688. https://doi.org/10.1016/j.wasman.2016.12.007
Ghassa S, Farzanegan A, Gharabaghi M, Abdollahi H (2020) The reductive leaching of waste lithium ion batteries in presence of iron ions: Process optimization and kinetics modelling. J Clean Prod 262:121312. https://doi.org/10.1016/j.jclepro.2020.121312
Pagnanelli F, Moscardini E, Granata G, Cerbelli S, Agosta L, Fieramosca A, Toro L (2014) Acid reducing leaching of cathodic powder from spent lithium ion batteries: glucose oxidative pathways and particle area evolution. J Ind Eng Chem 20(5):3201–3207. https://doi.org/10.1016/j.jiec.2013.11.066
Gao G, Luo X, Lou X, Guo Y, Su R, Guan J, Li Y, Yuan H, Dai J, Jiao Z (2019) Efficient sulfuric acid-Vitamin C leaching system: Towards enhanced extraction of cobalt from spent lithium-ion batteries. J Mater Cycles Waste Manag 21(4):942–949. https://doi.org/10.1007/s10163-019-00850-4
Zhao J, Zhang B, **e H, Qu J, Qu X, **ng P, Yin H (2020) Hydrometallurgical recovery of spent cobalt-based lithium-ion battery cathodes using ethanol as the reducing agent. Environ Res 181:108803. https://doi.org/10.1016/j.envres.2019.108803
Prasetyo E, Purwaningsih E, Astuti W (2019) Selective-reductive leaching of manganese from low-grade manganese ore using tannic acid as reductant. Mining Metall Explor 36:1003–1012. https://doi.org/10.1007/s42461-019-00115-6
Trojanowicz M, Bojanowska-Czajka A, Bartosiewicz I, Kulisa K (2018) Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)—a review of recent advances. Chem Eng J 336:170–199. https://doi.org/10.1016/j.cej.2017.10.153
Lokhande PE, Panda HS (2015) Synthesis and characterization of Ni.Co(OH)2 material for supercapacitor application. Int J Adv 2(9):10–13. https://doi.org/10.17148/IARJSET.2015.2903
Aghazadeh M, Barmi A-AM, Yousefi T (2012) Synthesis, characterization, and supercapacitive properties of β-Co(OH)2 leaf-like nanostructures. J Iran Chem Soc 9:225–229. https://doi.org/10.1007/s13738-011-0037-4
Wang S, Wang RH, Chang J, Hu N, Xu C (2018) Self-supporting CO3O4/graphene hybrid films as binder-free anode materials for lithium ion batteries. Sci Rep 8:3182. https://doi.org/10.1038/s41598-018-21436-4
Wang J, Bao Y, Cui C, Zhang Z, Li S, Pan J, Zhang Y, Tu G, Wang J, Li Z (2019) Fabrication of dispersive α-CO(OH)2 nanosheets on graphene nanoribbons for boosting their oxygen evolution performance. J Mater Sci 54:7692–7701. https://doi.org/10.1007/s10853-019-03421-y
Fu Y, He Y, Qu L, Feng Y, Li J, Liu J, Zhang G, **e W (2019) Enhancement in leaching process of lithium and cobalt from spent lithium-ion batteries using benzenesulfonic acid system. Waste Manag 88:191–199. https://doi.org/10.1016/j.wasman.2019.03.044
He LP, Sun SY, Song XF, Yu JG (2017) Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries. Waste Manag 64:171–181. https://doi.org/10.1016/j.wasman.2017.02.011
Levenspiel O (1999) Chemical reaction engineering. Ind Eng Chem Res. https://doi.org/10.1021/ie990488g
Xue J, Zhong H, Wang S, Li C, Li J, Wu F (2016) Kinetics of reduction leaching of manganese dioxide ore with Phytolacca americana in sulfuric acid solution kinetics of reduction leaching of manganese dioxide ore. J Saudi Chem Soc 20(4):437–442. https://doi.org/10.1016/j.jscs.2014.09.011
Dickinson CF, Heal GR (1999) Solid-liquid diffusion controlled rate equations. Thermochim Acta 340–341:89–103. https://doi.org/10.1016/s0040-6031(99)00256-7
Cantu Y, Remes A, Reyna A, Martinez D, Villarreal J, Ramos H, Trevino S, Tamez C, Martinez A, Eubanks T, Parsons JG (2014) Thermodynamics, kinetics, and activation energy studies of the sorption of chromium(III) and chromium(VI) to a Mn3O4 nanomaterial. Chem Eng J 254:374–383. https://doi.org/10.1016/j.cej.2014.05.110
Li L, Lu J, Ren Y, Zhang XX, Chen RJ, Wu F, Amine K (2012) Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J Power Sources 218:21–27. https://doi.org/10.1016/j.jpowsour.2012.06.068
Chen MJ, Wang R, Qi YP, Han YH, Wang R, Fu JL, Meng FS, Yi XX, Huang JF, Shu JC (2021) Cobalt and lithium leaching from waste lithium ion batteries by glycine. J Power Sources 482:228942. https://doi.org/10.1016/j.jpowsour.2020.228942
Sun L, Qiu K (2012) Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag 32(8):1575–1582. https://doi.org/10.1016/j.wasman.2012.03.027
Meng Q, Zhang YJ, Dong P (2017) Use of glucose as reductant to recover Co from spent lithium ions batteries. Waste Manag 64:214–218. https://doi.org/10.1016/j.wasman.2017.03.017
Zeba GTC, Paulino JF, Afonso JC (2021) Recovery of metals from electroactive components of spent Li-ion batteries after leaching with formic acid. Braz J Chem Eng. https://doi.org/10.1007/s43153-021-00095-5
Wu CB, Li BS, Yuan CF, Ni S, Li LF (2019) Recycling valuable metals from spent lithium-ion batteries by ammonium sulfite-reduction ammonia leaching. Waste Manag 93:153–161. https://doi.org/10.1016/j.wasman.2019.04.039
Funding
Funding was provided by Lembaga Ilmu Pengetahuan Indonesia (Indonesian Institute of Sciences), National Priority Research Project, Fiscal Year 2021. Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prasetyo, E., Muryanta, W.A., Anggraini, A.G. et al. Tannic acid as a novel and green leaching reagent for cobalt and lithium recycling from spent lithium-ion batteries. J Mater Cycles Waste Manag 24, 927–938 (2022). https://doi.org/10.1007/s10163-022-01368-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-022-01368-y