Abstract
Context
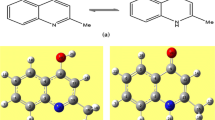
Quinolone derivatives have gathered major attention largely due to their wonderful biological activities. Quinolones are a class of molecules that are derived from quinolines and also extracted from natural sources. Most of these quinolones have significant medicinal properties ranging from antiallergenic and anticancer to antimicrobial activities. Some bacteria produce several 2-alkyl-4(1H)-quinolones. In past years, a variety of methods have been reported for the synthesis of quinolone derivatives. In this present work, structural, wave functional, and electronic properties of monomeric and dimeric forms of 2-methyl-4(1H)-quinolone are investigated. From the calculated binding energies, it was found that the formation of dimers is thermodynamically favorable. The analysis of reactivity parameters confirms that the keto form is more reactive than the enol form and keto–keto dimer is more reactive than compared to all monomeric and dimeric forms of our studied compound.
Methods
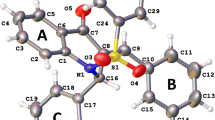
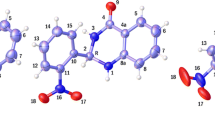
Geometry optimizations of monomers and dimers of studied molecules were carried out using the B3LYP-D3(BJ)/ma-def2-TZVPP level of theory. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies were calculated using the B3LYP/def2-TZVP level of theory. All DFT calculations were done with the ORCA 5.0.3 program. The reactivity parameters such as ionization potential, electron affinity, global hardness, global softness, electronegativity, chemical potential, and electrophilicity index were calculated. The nature of intermolecular interactions within the dimers was studied using topological analysis such as atoms in molecule (AIM) and reduced density gradient (RDG) surface analyses. To visualize the electron delocalization in the dimer electron localization function (ELF) and localized orbital locator (LOL) studies were also performed. The analyses such as AIM, RDG, ELF, and LOL were carried out by the multifunctional wavefunction analysis program Multiwfn 3.8.

















Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Maguire MP et al (1994) A new series of PDGF receptor tyrosine kinase inhibitors: 3-substituted quinoline derivatives. J Med Chem 37:2129–2137
Larsen RD et al (1996) Practical route to a new class of LTD4 receptor antagonists. J Org Chem 61:3398–3405
Chen Y-L et al (2001) Synthesis and antibacterial evaluation of certain quinolone derivatives. J Med Chem 44:2374–2377
Chu X-M et al (2019) Quinoline and quinolone dimers and their biological activities: an overview. Eur J Med Chem 161:101–117
Matada BS, Pattanashettar R, Yernale NG (2021) A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg Med Chem 32:115973
Dorababu A (2020) Report on recently (2017–20) designed quinoline-based human cancer cell growth inhibitors. Chemis Select 5:13902–13915
Heeb S et al (2011) Quinolones: from antibiotics to autoinducers. FEMS Microbiol Rev 35:247–274
Katritzky AR, Rees CW, Drayton CJ (1984) Comprehensive heterocyclic chemistry : the structure, reactions, synthesis, and uses of heterocyclic compounds. Pergamon Press, New York
Nadaraj V, Thamarai Selvi S (2007) Microwave-assisted solvent-free synthesis of 4-methyl-2-hydroxy-and 2-methyl-4-hydroxyquinolines. Indian J Chem 46B:1203–1207
Pourmousavi SA et al (2016) Synthesis, spectroscopic investigations and computational study of monomeric and dimeric structures of 2-methyl-4-quinolinol. Res Chem Intermed 42:1237–1274
Yuan S, Zhang K, **a J (2013) Microwave-assisted synthesis of 2-methyl-4-quinolinones via Combes synthesis catalyzed by acidic resin under solvent-free condition. Asian J Chem 25:5535
Nilsen A et al (2014) Discovery, synthesis, and optimization of antimalarial 4(1H)-quinolone-3-diaryl ethers. J Med Chem 57:3818–3834
Wratten SJ et al (1977) Antibiotic metabolites from a marine pseudomonad. Antimicrob Agents Chemother 11:411–414
Long RA et al (2003) 2-n-Pentyl-4-quinolinol produced by a marine Alteromonas sp. and its potential ecological and biogeochemical roles. Appl Environ Microbiol 69:568–576
Neese F et al (2020) The ORCA quantum chemistry program package. J Chem Phys 152:224108
Hanwell MD et al (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4:1–17
Becke A (1993) Density-Functional Thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305
Zheng J, Xu X, Truhlar DG (2011) Minimally augmented Karlsruhe basis sets. Theor Chem Accounts 128:295–305
Ehrlich S et al (2011) System-dependent dispersion coefficients for the DFT-D3 treatment of adsorption processes on ionic surfaces. Chem Phys Chem 12:3414–3420
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the dam** function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465
Miralrio A, Sansores LE (2016) On the search of stable, aromatic and ionic endohedral compounds of C28: a theoretical study. Comput Theoret Chem 1083:53–63
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Roy B, Mukhopadhyay B (2007) Sulfuric acid immobilized on silica: an excellent catalyst for Fischer type glycosylation. Tetrahedron Lett 48:3783–3787
Rozas I, Alkorta I, Elguero J (2000) Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J Am Chem Soc 122:11154–11161
Johnson ER et al (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Saleh G, Gatti C, Presti LL (2012) Non-covalent interaction via the reduced density gradient: independent atom model vs experimental multipolar electron densities. Computat Theoret Chem 998:148–163
Mohammed K et al (2020) Computational evaluation on the molecular conformation, vibrational spectroscopy, NBO analysis and molecular docking of betaxolol and betaxolol-chlorthalidone cocrystals. J Mol Struct 1209:127744
Savin A (2005) The electron localization function (ELF) and its relatives: interpretations and difficulties. J Mol Struct Theochem 727:127–131
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397–5403
Silvi B, Savin A (1994) Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371:683–686
Jacobsen H (2008) Localized-orbital locator (LOL) profiles of chemical bonding. Can J Chem 86:695–702
Lewis D, Ioannides C, Parke D (1994) Interaction of a series of nitriles with the alcohol-inducible isoform of P450: computer analysis of structure—activity relationships. Xenobiotica 24:401–408
Asiri AM et al (2011) Synthesis, molecular conformation, vibrational and electronic transition, isometric chemical shift, polarizability and hyperpolarizability analysis of 3-(4-Methoxy-phenyl)-2-(4-nitro-phenyl)-acrylonitrile: a combined experimental and theoretical analysis. Spectrochim Acta A Mol Biomol Spectrosc 82:444–455
Dollish FR, Fateley WG, Bentley FF (1974) Characteristic Raman frequencies of organic compounds. Wiley. https://doi.org/10.1016/0022-2860(74)87027-4
Varsányi G (2012) Vibrational spectra of benzene derivatives. Elsevier
Luque F et al (1993) SCRF calculation of the effect of water on the topology of the molecular electrostatic potential. J Phys Chem 97:9380–9384
Šponer J, Hobza P (1996) DNA base amino groups and their role in molecular interactions: ab initio and preliminary density functional theory calculations. Int J Quantum Chem 57:959–970
Colthup N, LH-Daly and SE Wiberley. (1964) Introduction to infrared and Raman spectroscopy. Academic Press, New York and London
Socrates G (2004) Infrared and Raman characteristic group frequencies: tables and charts. John Wiley & Sons
Jamróz MH, Dobrowolski JC, Brzozowski R (2006) Vibrational modes of 2, 6-, 2, 7-, and 2, 3-diisopropylnaphthalene. A DFT study. J Mol Struct 787:172–183
Singh N, Yadav R (2001) Vibrational studies of trifluoromethyl benzene derivatives 1: 2-amino, 5-chloro and 2-amino, 5-bromo benzotrifluorides. Ind J Phys 75:347–355
Altun A, Gölcük K, Kumru M (2003) Theoretical and experimental studies of the vibrational spectra of m-methylaniline. J Mol Struct Theochem 625:17–24
Fleming I (2011) Molecular orbitals and organic chemical reactions. John Wiley & Sons
Rizwana BF et al (2020) Vibrational spectroscopy, reactive site analysis and molecular docking studies on 2-[(2-amino-6-oxo-6, 9-dihydro-3H-purin-9-yl) methoxy]-3-hydroxypropyl (2S)-2-amino-3-methylbutanoate. J Mol Struct 1202:127274
Acknowledgements
The authors are thankful to Dr. Pradip Ghosh, Director, Midnapore City College to provide us with financial support and laboratory facility to carry out this work.
Funding
The authors thank Dr. Pradip Ghosh, Director, Midnapore City College to provide us with financial support.
Author information
Authors and Affiliations
Contributions
J.P. and S.S. performed the calculations. S.S. and M.B. wrote the first draft. All the authors revised the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sabud, S., Bera, M. & Pal, J. Topological analysis and reactivity study of monomeric and dimeric forms of 2-methyl-4(1H)-quinolone: a computational study. J Mol Model 29, 369 (2023). https://doi.org/10.1007/s00894-023-05779-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05779-y