Abstract
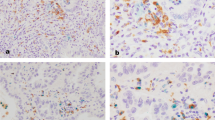
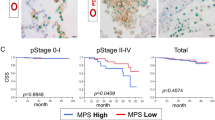
Immunotherapies that target programmed cell death protein 1 (PD-1) signals are standard therapies for advanced-stage lung cancer, and the expression of programmed death-ligand 1 (PD-L1) in cancer tissue predicts immunotherapy efficacy. Although programmed death-ligand 2 (PD-L2) is expressed in cancer cells and macrophages, similar to PD-L1, its significance in lung cancer is unclear. Double immunohistochemistry analyses using anti-PD-L2 and anti-PU.1 antibodies were carried out on tissue array sections from 231 cases of lung adenocarcinoma, and PD-L2 expression in macrophages was evaluated. High PD-L2 expression in macrophages was associated with longer progression-free survival (PFS) and cancer-specific survival (CSS) and observed more often in females, non-heavy smokers, and patients with epidermal growth factor receptor (EGFR) mutations and those at a lower disease stage. Significant correlations were found more frequently in patients with EGFR mutations. Cell culture studies revealed that cancer cell-derived soluble factors induced PD-L2 overexpression in macrophages, suggesting the involvement of the JAK-STAT signaling pathway. The present findings suggest that PD-L2 expression in macrophages predicts PFS and CSS in lung adenocarcinoma without immunotherapy.



Similar content being viewed by others
Availability of data and materials
The data presented in this study are available in this article and its supplementary materials.
Abbreviations
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death 1 ligand 1
- JAK:
-
Janus kinase
- STAT:
-
Signal transducer and activator of transcription
- IHC:
-
Immunohistochemistry
- PFS:
-
Progression-free survival
- CSS:
-
Cancer-specific survival
- EGFR:
-
Epidermal growth factor receptor
- NSCLC:
-
Non-small-cell lung cancer
- TAMs:
-
Tumor-associated macrophages
- ELISA:
-
Enzyme-linked immunosorbent assay
- CM:
-
Conditioned medium
References
Hoffman PC, Mauer AM, Vokes EE (2000) Lung cancer. Lancet 355(9202):479–485
Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS (2021) Lung cancer. Lancet 398(10299):535–554
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833
Hellmann MD, Paz-Ares L, Caro RB et al (2020) Atezolizumab for first-line treatment of PD-L1 selected patients with NSCLC. N Engl J Med 383(14):1328–1339
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264
Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367(6477):eaax0182.
Koomen BM, Badrising SK, Heuvel MM, Willems SM (2020) Comparability of PD-L1 immunohistochemistry assays for non-small-cell lung cancer: a systematic review. Histopathology 76(6):793–802
Herbst RS, Soria JC, Kowanetz M et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563–567
Shinchi Y, Komohara Y, Yonemitsu K et al (2019) Accurate expression of PD-L1/L2 in lung adenocarcinoma cells: a retrospective study by double immunohistochemistry. Cancer Sci 110(9):2711–2721
Kozuma Y, Takada K, Toyokawa G, Kohashi K, Shimokawa M, Hirai F, Tagawa T, Okamoto T, Oda Y, Maehara Y (2018) Indoleamine 2,3-dioxygenase 1 and programmed cell death-ligand 1 co-expression correlates with aggressive features in lung adenocarcinoma. Eur J Cancer 101:20–29
Horlad H, Ma C, Yano H et al (2016) An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Sci 107(11):1696–1704
Shinchi Y, Ishizuka S, Komohara Y et al (2022) The expression of PD-1 ligand 1 on macrophages and its clinical impacts and mechanisms in lung adenocarcinoma. Cancer Immunol Immunother 71(11):2645–2661
Komohara Y, Fujiwara Y, Ohnishi K, Takeya M (2016) Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev 99:180–185
Matsubara E, Komohara Y, Shinchi Y et al (2021) CD163-positive cancer cells are a predictor of a worse clinical course in lung adenocarcinoma. Pathol Int 71(10):666–673
Matsubara E, Komohara Y, Esumi S et al (2022) SPP1 derived from macrophages is associated with a worse clinical course and chemo-resistance in lung adenocarcinoma. Cancers 14(18):4374
Y, Kanda. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Kovaleva OV, Rashidova MA, Samoilova DV, Podlesnaya PA, Mochalnikova VV, Gratchev A (2020) Immunosuppressive phenotype of esophagus tumors stroma. Anal Cell Pathol 2020:5424780
Wang Y, Du J, Gao Z, Sun H, Mei M, Wang Y, Ren Y, Zhou X. Evolving landscape of PD-L2: bring new light to checkpoint immunotherapy. Br J Cancer. 2022; Online ahead of print.
Zerdes I, Matikas A, Bergh J, Rassidakis GZ, Foukakis T (2018) Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: biology and clinical correlations. Oncogene 37(34):4639–4661
Matsusaka K, Fujiwara Y, Pan C et al (2021) α1-acid glycoprotein enhances the immunosuppressive and protumor functions of tumor-associated macrophages. Cancer Res 81(17):4545–4559
Umezu D, Okada N, Sakoda Y, Adachi K, Ojima T, Yamaue H, Eto M, Tamada K (2019) Inhibitory functions of PD-L1 and PD-L2 in the regulation of anti-tumor immunity in murine tumor microenvironment. Cancer Immunol Immunother 68(2):201–211
Yonemitsu K, Pan C, Fujiwara Y, Miyasato Y, Shiota T, Yano H, Hosaka S, Tamada K, Yamamoto Y, Komohara K (2022) GM-CSF derived from the inflammatory microenvironment potentially enhanced PD-L1 expression on tumor-associated macrophages in human breast cancer. Sci Rep 12(1):12007
Li A, Wu W, Deng S et al (2022) Expression of programmed death ligand-2 is associated with prognosis in nasopharyngeal carcinoma microenvironment. J Cancer 13(15):3606–3614
Davidson C, Taggart D, Sims AH, Lonergan DW, Canel M, Serrels A (2022) FAK promotes stromal PD-L2 expression associated with poor survival in pancreatic cancer. Br J Cancer 127(10):1893–1905
Komohara Y, Fujiwara Y, Ohnishi K, Takeya M (2016) Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev 99(Pt B):180–185
Cassetta L, Pollard JW (2018) Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 17(12):887–904
Komohara Y, Kurotaki D, Tsukamoto H et al (2023) Involvement of protumor macrophages in breast cancer progression and characterization of macrophage phenotypes. Cancer Sci 114(6):2220–2229
Acknowledgements
We thank Mr. Takenobu Nakagawa for the technical assistance.
Funding
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Nos. 16H05162 and 20H03459).
Author information
Authors and Affiliations
Contributions
EM: Data curation, Formal Analysis, Investigation, Software, Writing-original draft; YS: Investigation, Software, Visualization; YF: Conceptualization, Methodology, Validation; MS: Supervision; YK: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing-review & editing. All authors read and approved the final version of the manuscript to be submitted.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the Institutional Review Board of Kumamoto University (#1174), and conducted in accordance with the Declaration of Helsinki. Human macrophages were obtained from healthy donors in accordance with protocols approved by the Kumamoto University Hospital Review Board (approval No. 1169. No animal studies or registry/registration of this study was performed.
Consent to participate
Patient consent for inclusion in this study was waived by the Institutional Review Board of Kumamoto University (#2059) because the CSS and PFS data were obtained from previous reports [10, 15, 16]. Although all of the retrospective patient data were automatically included in the study, the patients were given the opportunity to refuse participation by opting out.
Consent for publication
Informed consent for publication was obtained from the relevant participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsubara, E., Shinchi, Y., Komohara, Y. et al. PD-L2 overexpression on tumor-associated macrophages is one of the predictors for better prognosis in lung adenocarcinoma. Med Mol Morphol 56, 250–256 (2023). https://doi.org/10.1007/s00795-023-00361-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-023-00361-0