Abstract
Direct monitoring of volatile organic compounds emitted from industrial sources as well as the monitoring of ambient levels thereof in the atmosphere play an important role in providing data for various legislative requirements. There are many volatile organic compounds emitted to the atmosphere, from biomass burning, power stations, and many other sources. Primary reference gas mixtures of volatile organic compounds containing six components, namely benzene, toluene, ethylbenzene, o-xylene, m-xylene, and p-xylene (BTEX) were prepared by gravimetric methods at nominal amount of substance fractions of 10 µmol/mol. The preparation was performed using the stainless steel (loop) method and direct liquid injection method using gas tight syringe connected to a dedicated syringe heater for comparison purposes. The results of the gravimetric values for both methods were within 1 % of the nominal amount of substance fraction. After the preparation, comparison was achieved by analysis of the BTEX gas mixtures using gas chromatography with flame ionisation detection (GC-FID) and showed comparability to within 2 % of the gravimetric values. The contributions to the uncertainty of measurement were from weighing stainless steel tubes, syringes, and gas cylinders, as well as purity data. The contribution to the uncertainty of measurement from the analysis by GC-FID were repeatability and reproducibility. The combined expanded uncertainty of measurement was between 1.09 % and 3.51 %. The larger deviation from the gravimetric values and associated uncertainty was observed for benzene due to its higher volatility compared to the other components. The BTEX reference gas mixtures prepared in this study are currently being used as a source of traceability in South Africa for air pollution monitoring purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Volatile organic compounds (VOCs) are known to be present in the atmosphere at nmol/mol down to pmol/mol amount fractions [1]. The VOCs of interest in this study are benzene, toluene, ethylbenzene, o-xylene, m-xylene, and p-xylene (BTEX), which are similar in structure and present similar physiochemical characteristics with a distinctive odour. The BTEX compounds are components of chemical products, such as fuel and paint, and they serve as solvents for many other applications.
The sources of air pollutants are both natural and anthropogenic. Natural sources include volcanic eruptions, and biomass burning, while anthropogenic sources include power plants, chemical plants, transport vessels, mining activities, and many more. Once in the atmosphere, pollutants undergo many photochemical reactions with other constituents of the atmosphere, in which some may form secondary pollutants, such as ground level ozone [1] and aerosols [2]. There are many observed adverse effects to the environment due to air pollutants and their reaction products, such as the following:
-
Reduced visibility due to heavy fog which includes emitted particles,
-
Photochemical smog,
-
Acid rain due to the emissions of nitrogen and sulphur oxides,
-
And ozone depletion.
The human health effects associated with VOCs are respiratory, carcinogenic and neurotoxic [3]. They enter the human body through direct skin contact, and breathing of polluted environments.
The legislative framework in different countries controls the monitoring of BTEX at ambient levels. In South Africa, benzene is in the list of National Ambient Air Quality Standards with other priority air pollutants such as sulphur dioxide (SO2), nitrogen dioxide (NO2), particulate matter (PM10), ozone (O3), lead (Pb) and carbon monoxide (CO) [4]. These pollutants are monitored in the ambient atmosphere across South Africa, are reported in the South African Air Quality Information System (SAAQIS), and serve as indicators of air pollution. Benzene, together with other pollutants such as the greenhouse gases and aerosols, are monitored in background atmospheric air by the South African Weather Service (SAWS) under a World Meteorological Organization—Global Atmosphere Watch Program (WMO-GAW) [5]. There are no measurements of benzene and other VOCs at the Cape Point (WMO-GAW) station due to lack of reference gas standards, among other challenges.
Air quality monitoring programmes require quality results, which is achieved by using reference gas standards that are traceable to the International System of Units (SI). The National Metrology Institute of South Africa (NMISA) is designated for such activities under the Measurement Units and Measurement Standard Act 18 of 2006 [6].
The aim of the study was to develop primary BTEX reference gas standards (PRGMs) containing multiple components in gas cylinders using the gravimetric method based on ISO 6142-1 [7]. These PRGMs are currently being used for calibration purposes in the priority ambient air pollution monitoring stations across South Africa.
Experimental
The BTEX reference gas mixtures were produced from high purity starting materials of benzene, toluene, ethylbenzene, o-xylene, m-xylene, and p-xylene, which were sourced from Sigma-Aldrich and nitrogen gas Built-in-Purifier (BIP™) sourced from Air Products Southern Africa. The manufacturer’s stated purity for BTEX components ranged between 99.1 % and 99.95 %, whilst that of nitrogen was grade 6.0.
The purity analysis of BTEX components was done using a HP-INNOWax column (60 m × 0.32 mm id × 0.5 µm df) on an Agilent 7890B GC-FID.
Using method 1, the gravimetric preparation was achieved by mixing of BTEX components into a glass vial. The BTEX components were then introduced into a 5 dm3 stainless steel cylinder using a transfer vessel (stainless steel loop). The connection of the loop was achieved by using the minimum dead volume (MDV) connector patented at National Physical Laboratory (NPL) in the UK [1]. After the addition of BTEX, nitrogen was added to complete the preparation of 10 µmol/mol PRGM. The required mass of each component was pre-calculated.
Using method 2, the gravimetric preparation was achieved by mixing of BTEX components into a glass vial. The BTEX components were then introduced into a 5 dm3 stainless steel cylinder using a one mL Hamilton gas tight syringe, followed by flushing with 200 kPa pure nitrogen.
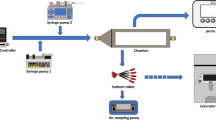
The cylinders were homogenised by rolling on the roller bench for a minimum of 2 h prior to analysis. The analysis was achieved by using gas chromatography with flame ionisation detection (either a Varian CP 3800 GC or an Agilent 7890B GC). Each of the gas chromatographs was connected to a 16 port stainless steel VICI multi position stream selector valve. The sample lines were constructed of Sulfinert® treated tubing with 1.57 mm outside diameter. Gas flow control was achieved by using a Bronkhorst mass flow controller set at 400 mL/min connected between the VICI multi stream selector valve and the sample loop in the gas valve box of the gas chromatograph. The sample loop used was 1 mL capacity Sulfinert® treated tubing. Four gas mixtures containing six components of benzene, toluene, ethylbenzene, m-xylene, p-xylene, and o-xylene (BTEX) at a nominal amount of substance fraction of 10 µmol/mol were prepared and analysed by gas chromatography with a flame ionisation detector using both method one and method two. The separation was accomplished by using a Restek Rxi624SilMS (60 m × 0.32 mm ID × 1.8 µm) column (method one) and Agilent DB-624 (60 m × 0.32 mm id 1.8 µm df) column (method two), where m,p-xylene eluted as one peak. The GC analytical conditions of method one and method two are summarised in Table 1. A single point calibration method was used since all the BTEX standards nominal amount of substance fraction was within 1% of the target 10 µmol/mol. Peak area drift equal or less than 1 % of a reference standard measured between analysed samples was used to monitor the detector behaviour during the analysis.
Results and discussion
Purity analysis results
The purity analysis results of BTEX and nitrogen are presented in Table 2. P-xylene had the highest number of impurities, followed by o-xylene and m-xylene. All BTEX components purity results had no significant impurities that would render the material unfit for purpose. The results of nitrogen BIP™ analysis showed that it did not contain BTEX impurities.
Analysis of BTEX gas mixtures
Analysis results of BTEX using method one
The verification results of method one for BTEX are presented in Table 3. Cylinder number M395461 was chosen randomly as a reference against cylinders M395412, M395469, and M395438. The analysis sequence followed was reference 1 > sample 1 > reference 1 > sample 2 > reference 1, etc., to ensure that the detector response was constantly monitored during the analysis. The detector drift was measured by the difference of peak area of a component of a reference at the beginning of analysis to a peak area of the same reference after each sample analysis. A difference of less than 1 % showed that the detector drift was within the accepted limit for long analysis methods such as those of BTEX. For benzene, the verification between the gravimetric and the verification values were − 2.69 %, for M395412, − 0.06 % for M395469 and − 2.26 % for M395438. For toluene, the verification between the gravimetric and the verification values were − 0.57 %, for M395412, − 0.55 % for M395469 and − 1.20 % for M395438. For ethylbenzene, the verification between the gravimetric and the verification values were − 0.15 %, for M395412, − 1.32 % for M395469 and − 0.16 % for M395438. For m,p-xylene, the verification between the gravimetric and the verification values were − 0.10 %, for M395412, -0.48 % for M395469 and − 0.00 % for M395438. For o-xylene, the verification between the gravimetric and the verification values were 2.02 %, for M395412, 0.62 % for M395469 and 126 % for M395438.
Analysis results of BTEX using method two
A set of gas mixtures was prepared using method two. Four gas mixtures were chosen randomly and analysed by gas chromatography with a flame ionisation detector. The chromatogram of the gas mixture used as a reference cylinder is shown in Fig. 1 including the elution order of components with their respective retention times. M,p-xylene eluted together as one component, therefore their respective amount of substance fractions were summed and their corresponding measurement uncertainties were combined quadratic following the GUM guideline[8]. The verification results are presented in Table 4. PRGM20005415 was chosen randomly as a reference to analyse which the other three cylinders were compared. The analysis was performed over five consecutive days.
The results of all BTEX components in all cylinders showed satisfactory consistency. The difference between the gravimetric and the verification values were less than 0,5%, with the highest difference being 0,29% for benzene in mixture PRGM50005422.
A graphical representation of six gas mixtures is shown in Fig. 2, excluding the two gas mixtures that were used as reference (M395461 and PRGM20005415). The blue diamonds represent the analysis value. The red squares represent the gravimetric values. The error bars represent the combined gravimetric and analytical standard uncertainty.
Amount of substance fractions for each BTEX component obtained for 10 µmol/mol BTEX gas mixtures prepared in six cylinders (M395412, M395469, M395438, PRGM30005374, PRGM70005690 and PRGM50005422). The blue diamonds represent the analysis value. The red squares represent the gravimetric values. The error bars represent the combined gravimetric and analytical standard uncertainty
Benzene showed the largest deviation from the gravimetric value compared to other components due to its higher volatility. The combined expanded uncertainty obtained ranged from 1% to 3,5%, which was found to be satisfactory.
The results suggest that method two displays better accuracy than method one, probably due to the simple operation of filling the components into the cylinder by syringe followed by flushing with 200 kPa of nitrogen. With method one, the transfer was achieved by drawing the components in the loop by cylinder vacuum. The method has critical steps that require care to ensure that the loss of components is minimal and accurately accounted for. In method two, the mixture of components in the vial was used to prepare four cylinders, while with method one each cylinder is prepared independently. The analysis results presented in this study are similar in accuracy to those reported in other literature [9,10,11] at similar nominal amount of substance fraction of 10 µmol/mol of BTEX primary reference gas standards.
Contribution to measurement uncertainty
The uncertainty of measurement was quantified following the GUM guidelines. The uncertainty of measurement of gas mixtures has two components which are the uncertainty of the gravimetric preparation and the uncertainty of analysis. The amount of substance fraction of each component of a gas mixture is given by model Eq. (1).
where y(b) is the amount-of-substance fraction of component k in the prepared mixture, x(b,j) is the amount-of-substance fraction of component k in parent gas or liquid j, m(j) is the mass added of parent gas or liquid j, x(i,j) is the amount-of-substance fraction of component i in parent gas or liquid j, M(i) is the molar mass of component i, q is the number of components in the mixture, and r is the number of parent gases or liquids. It also follows that the molar mass M(i) of the of gas components is- given by model Eq. (2),
Where \({A}_{r}(E)\) denotes the atomic weight of element E and \({V}_{E}\) the atomic composition coefficient of element E in the molecular formula [12].
The results of a typical purity table data of 10 µmol/mol BTEX gas mixture are shown in Table 5. Using benzene as an example to explain uncertainty contributions associated with gravimetric preparation. The amount-of-substance fraction of y(b), is 10.26 µmol/mol and its associated uncertainty u(y(b,)) is 0.062 µmol/mol. The amount of substance fraction of benzene from its purity data in Table 2, is represented by x(b,i) (0.9999 mol/mol) and the corresponding uncertainty u(x(b,j)) (0.000058 mol/mol).
The uncertainty of u(mj), the mass of benzene, was calculated from the mass difference before (mass of loop and benzene) and after (mass of empty loop) it was added into the cylinder. The experimental standard deviation of the mean (ESDM) calculated represents the uncertainty.
The component x(i,j), is the amount-of-substance fraction of other components and its associated uncertainty is u(x(i,j)). In the benzene purity data in Table 2, it is the value of- all other components present in the final gas mixture, each obtained from the purity table of their respective parent components. This analogy is used to compute the uncertainty of all components and their impurities and is presented in the uncertainty column in Table 2.
The uncertainty of u(Mi), the molar mass of a component, is calculated from the IUPAC molar mass data, where the molar mass of each compound was computed based on the elemental composition of the molecule (carbon and hydrogen), including their stoichiometric values [12].
For gas chromatography, the uncertainty calculation was based on the peak areas obtained during the analysis of the sample and reference cylinders and the gravimetric uncertainty of the reference gas mixture used to verify the prepared gas mixtures. The analytical combined uncertainty is represented by verification column in Table 3 and Table 4.
Conclusion
Primary reference gas mixtures of BTEX were successfully produced using two methods that are similar in principle, however differ in some critical steps. In method one, the introduction of components into the cylinder is achieved via vacuum, whereas in method two it was achieved by heating of a syringe containing BTEX components, followed by injection into the cylinder and flushing of the line with nitrogen gas. The analysis results showed that method two produce better accuracy than method one, the difference between the gravimetric values and the verification values are smaller than observed in method 1. This is attributed critical steps in method 1 that may contribute to the error in the weighing of the transfer vessel, small amount of liquid components may remain in the void volume of the valves during transfer and be lost to the atmosphere before weighing, leading to error in the amounts transferred. Benzene showed the largest deviation from the gravimetric value compared to other components due to its higher volatility. The combined expanded uncertainty obtained ranged from 1 % to 3.5 %, which was found to be satisfactory.
The BTEX reference gas mixtures produced in this study play an important role in contributing to quality data produced by air pollution monitoring networks in priority areas in South Africa.
References
Grenfell RJP, Milton MJT, Harling AM et al (2010) Standard mixtures of ambient volatile organic compounds in synthetic and whole air with stable reference values. J Geophys Res Atmos 115:1–16. https://doi.org/10.1029/2009JD012933
Englert J, Claude A, Demichelis A et al (2017) Preparation and analysis of zero gases for the measurement of trace VOCs in air monitoring. Atmos Meas Tech Discuss. https://doi.org/10.5194/amt-2017-412
U.S. EPA (2017) Hazardous Air Pollutants: Sources and Exposure. https://www.epa.gov/haps. Accessed 25 Jul 2021
Government Gazette No. 32816 (2009) National environmental management: air quality act: national ambient air quality standards
WMO-GAW (1995) GAW Report No. 111 WMO-BMBF Workshop on VOCEstablishment of a “World Calibration/InstrumentIntercomparison Facility for VOC” to Serve the WMO GlobalAtmosphere Watch (GAW) Programme
Government Gazette (2007) Measurement and Standard Act 18 of 2006)
ISO 6142-1 (2015) Gas anlysis- Preparation of calibration gas mixtures — Part 1: Gravimetric method for Class I
JCGM 100 (2008) Uncertainty of measurement—Part 3: Guide to the expression of uncertainty in measurement (GUM:1995)
Rhoderick GC, Yen JH (2006) Development of a NIST standard reference material containing thirty volatile organic compounds at 5 nmol/mol in nitrogen. Anal Chem 78:3125–3132. https://doi.org/10.1021/ac052136r
Rhoderick GC, Miller WR (1990) Multipoint calibration of a gas chromatograph using cryogenic preconcentration of a single gas standard containing volatile organic compounds. Anal Chem 62:810–815. https://doi.org/10.1021/ac00207a008
Rhoderick GC, Zielinski WL (1988) Preparation of accurate multicomponent gas standards of volatile toxic organic compounds in the low-parts-per-billion range. Anal Chem 60:2454–2460. https://doi.org/10.1021/ac00173a004
Van Der Veen AMH, Meija J, Possolo A, Hibbert DB (2021) Interpretation and use of standard atomic weights (IUPAC Technical Report). Pure Appl Chem 93:629–646. https://doi.org/10.1515/pac-2017-1002
Funding
Open access funding provided by Mintek.
Author information
Authors and Affiliations
Contributions
Napo Ntsasa and James Tshilongo, wrote the research proposal and design the experimental work, participated in the experimental work and review of the results. They also drafted the Manuscript. Nompumelelo Leshabane, Mudalo Jozela and Goitsemang Lekoto executed all the experimental work and added experimental section, reviewed data for this publication Patricia Forbes and James Tshilongo, supervisory role and editorial changes, guidance on experimental approach and approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ntsasa, N., Tshilongo, J., Leshabane, N. et al. Development of benzene, toluene, ethyl benzene, m-xylene, p-xylene and o-xylene (BTEX) gas reference standards for air pollution monitoring in South Africa. Accred Qual Assur 28, 271–278 (2023). https://doi.org/10.1007/s00769-023-01555-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-023-01555-w