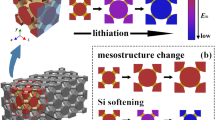
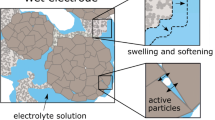
Abstract
Compared with a large number of researches on the stress analysis of lithiation active particles and electrode plates, the study on structural stability of the electrode composites during the service of lithium-ion batteries (LIBs) is bare. In order to account for the large deformation that occurs during the axial force loading, the models of geometrically nonlinear deformation are proposed, and the effects of the drying process, chemo-mechanical coupling and concentration-dependent material properties on the stability are investigated. The imperfect interface properties of the collector/active layer are modeled by a cohesive zone model (CZM), which originates from different drying conditions. As a result, the competition mechanism of the interface failure and buckling instability of the laminated electrode structure is formulated on the basis of our experimental observations and analytical modeling. Moreover, the effects of drying rate, geometric conditions, and charge/discharge rates on the stability of the electrode structure are systematically examined. Our findings can provide valuable insights for designing next-generation mechanically stable electrode composites.








Similar content being viewed by others
References
Zhang, Y.Y., Wang, C.M., Challamel, N.: Bending, buckling, and vibration of micro/nanobeams by hybrid nonlocal beam model. J. Eng. Mech-ASCE. 136(5), 562–574 (2010). https://doi.org/10.1061/(Asce)Em.1943-7889.0000107
Grygorowicz, M., Magnucki, K., Malinowski, M.: Elastic buckling of a sandwich beam with variable mechanical properties of the core. Thin Wall. Struct. 87, 127–132 (2015). https://doi.org/10.1016/j.tws.2014.11.014
Lai, W.J., Ali, M.Y., Pan, J.: Mechanical behavior of representative volume elements of lithium-ion battery cells under compressive loading conditions. J. Power Sources 245, 609–623 (2014). https://doi.org/10.1016/j.jpowsour.2013.06.134
Zhang, Y., Zhan, S., Zhang, K., Zheng, B., Lyu, L.: Buckling behavior of a wire-like electrode with a concentration-dependent elastic modulus based on a deformed configuration. Eur. J. Mech. A/Solid. 85, 104111 (2021). https://doi.org/10.1016/j.euromechsol.2020.104111
Zhu, Z., Hu, H., He, Y., Tao, B.: Buckling analysis and control of layered electrode structure at finite deformation. Compos. Struct. 204, 822–830 (2018). https://doi.org/10.1016/j.compstruct.2018.08.020
Bhandakkar, T.K., Johnson, H.T.: Diffusion induced stresses in buckling battery electrodes. J Mech. Phys. Solids. 60(6), 1103–1121 (2012). https://doi.org/10.1016/j.jmps.2012.02.012
Chakraborty, J., Please, C.P., Goriely, A., Chapman, S.J.: Combining mechanical and chemical effects in the deformation and failure of a cylindrical electrode particle in a Li-ion battery. Int. J. Solids Struct. 54, 66–81 (2015). https://doi.org/10.1016/j.ijsolstr.2014.11.006
Neogi, S., Chakraborty, J.: Size-dependent effects sensitively determine buckling of a cylindrical silicon electrode particle in a lithium-ion battery. J. Appl. Phys. (2018). https://doi.org/10.1063/1.5052236
Zhang, K., Li, Y., Wu, J.S., Zheng, B.L., Yang, F.Q.: Lithiation-induced buckling of wire-based electrodes in lithium-ion batteries: A phase-field model coupled with large deformation. Int. J. Solids Struct. 144, 289–300 (2018). https://doi.org/10.1016/j.ijsolstr.2018.05.014
Zhang, K., Li, Y., Zheng, B., Wu, G., Wu, J., Yang, F.: Large deformation analysis of diffusion-induced buckling of nanowires in lithium-ion batteries. Int. J. Solids Struct. 108, 230–243 (2017). https://doi.org/10.1016/j.ijsolstr.2016.12.020
**ng, H.Z., Liu, Y.L., Wang, B.: Mechano-electrochemical and buckling analysis of composition-gradient nanowires electrodes in lithium-ion battery. Acta. Mech. 230(12), 4145–4156 (2019). https://doi.org/10.1007/s00707-019-02486-9
Zhang, X.W., Sahraei, E., Wang, K.: Deformation and failure characteristics of four types of lithium-ion battery separators. J. Power Sources 327, 693–701 (2016). https://doi.org/10.1016/j.jpowsour.2016.07.078
Kisters, T., Sahraei, E., Wierzbicki, T.: Dynamic impact tests on lithium-ion cells. Int. J. Impact Eng. 108, 205–216 (2017). https://doi.org/10.1016/j.ijimpeng.2017.04.025
Dixon, B., Mason, A., Sahraei, E.: Effects of electrolyte, loading rate and location of indentation on mechanical integrity of Li-ion pouch cells. J. Power Sources 396, 412–420 (2018). https://doi.org/10.1016/j.jpowsour.2018.06.042
**a, Y., Wierzbicki, T., Sahraei, E., Zhang, X.W.: Damage of cells and battery packs due to ground impact. J. Power Sources 267, 78–97 (2014). https://doi.org/10.1016/j.jpowsour.2014.05.078
Zhu, J., Li, W., Wierzbicki, T., **a, Y., Harding, J.: Deformation and failure of lithium-ion batteries treated as a discrete layered structure. Int. J. Plast. 121, 293–311 (2019). https://doi.org/10.1016/j.ijplas.2019.06.011
Zhu, J., Zhang, X.W., Sahraei, E., Wierzbicki, T.: Deformation and failure mechanisms of 18650 battery cells under axial compression. J. Power Sources 336, 332–340 (2016). https://doi.org/10.1016/j.jpowsour.2016.10.064
Lai, W.J., Ali, M.Y., Pan, J.: Mechanical behavior of representative volume elements of lithium-ion battery modules under various loading conditions. J. Power Sources 248, 789–808 (2014). https://doi.org/10.1016/j.jpowsour.2013.09.128
Amodeo, C.M., Ali, M.Y., Pan, J.: Computational models for simulations of lithium-ion battery modules under quasi-static and dynamic constrained compression tests. Int. J. Crashworthiness. 22(1), 1–14 (2016). https://doi.org/10.1080/13588265.2016.1213489
Ali, M.Y., Lai, W.J., Pan, J.: Computational models for simulations of lithium-ion battery cells under constrained compression tests. J. Power Sources 242, 325–340 (2013). https://doi.org/10.1016/j.jpowsour.2013.05.022
Ali, M.Y., Lai, W.J., Pan, J.: Computational models for simulation of a lithium-ion battery module specimen under punch indentation. J. Power Sources 273, 448–459 (2015). https://doi.org/10.1016/j.jpowsour.2014.09.072
Zhu, Z.Q., He, Y.L., Hu, H.J.: Role of heterogeneous inactive component distribution induced by drying process on the mechanical integrity of composite electrode during electrochemical operation. J. Phys. D Appl. Phys. 54(5), 055503 (2021). https://doi.org/10.1088/1361-6463/abc043
Westphal, B.G., Kwade, A.: Critical electrode properties and drying conditions causing component segregation in graphitic anodes for lithium-ion batteries. J. Energy Storage. 18, 509–517 (2018). https://doi.org/10.1016/j.est.2018.06.009
Baunach, M., Jaiser, S., Schmelzle, S., Nirschl, H., Scharfer, P., Schabel, W.: Delamination behavior of lithium-ion battery anodes: Influence of drying temperature during electrode processing. Drying Technol. 34(4), 462–473 (2015). https://doi.org/10.1080/07373937.2015.1060497
Lim, S., Kim, S., Ahn, K.H., Lee, S.J.: Stress development of Li-ion battery anode slurries during the drying process. Ind. Eng. Chem. Res. 54(23), 6146–6155 (2015). https://doi.org/10.1021/acs.iecr.5b00878
Shi, D.H., **ao, X.R., Huang, X.S., Kia, H.: Modeling stresses in the separator of a pouch lithium-ion cell. J. Power Sources 196(19), 8129–8139 (2011). https://doi.org/10.1016/j.jpowsour.2011.05.026
Sethuraman, V.A., Van Winkle, N., Abraham, D.P., Bower, A.F., Guduru, P.R.: Real-time stress measurements in lithium-ion battery negative-electrodes. J. Power Sources 206, 334–342 (2012). https://doi.org/10.1016/j.jpowsour.2012.01.036
Zhang, J.Q., Lu, B., Song, Y.C., Ji, X.: Diffusion induced stress in layered Li-ion battery electrode plates. J. Power Sources 209, 220–227 (2012). https://doi.org/10.1016/j.jpowsour.2012.02.104
Bohn, E., Eckl, T., Kamlah, M., McMeeking, R.: A model for lithium diffusion and stress generation in an intercalation storage particle with phase change. J. Electrochem. Soc. 160(10), A1638–A1652 (2013). https://doi.org/10.1149/2.011310jes
Purkayastha, R., McMeeking, R.: A parameter study of intercalation of lithium into storage particles in a lithium-ion battery. Comp. Mater. Sci. 80, 2–14 (2013). https://doi.org/10.1016/j.commatsci.2012.11.050
Haftbaradaran, H., Song, J., Curtin, W.A., Gao, H.: Continuum and atomistic models of strongly coupled diffusion, stress, and solute concentration. J. Power Sources 196(1), 361–370 (2011). https://doi.org/10.1016/j.jpowsour.2010.06.080
Park, J., Seo, J.H., Plett, G., Lu, W., Sastry, A.M.: Numerical simulation of the effect of the dissolution of limn2o4 particles on Li-ion battery performance. Electrochem. Solid State Lett. 14(2), A14–A18 (2011). https://doi.org/10.1149/1.3516619
Takahashi, K., Srinivasan, V.: Examination of graphite particle cracking as a failure mode in lithium-ion batteries: A model-experimental study. J. Electrochem. Soc. 162(4), A635–A645 (2015). https://doi.org/10.1149/2.0281504jes
Guo, Z., Zhang, T., Hu, H., Song, Y., Zhang, J.: Effects of hydrostatic stress and concentration-dependent elastic modulus on diffusion-induced stresses in cylindrical Li-ion batteries. J. Appl. Mech. 81 (2014). https://doi.org/10.1115/1.4025271
Keil, P., Jossen, A.: Charging protocols for lithium-ion batteries and their impact on cycle life—an experimental study with different 18650 high-power cells. J. Energy Storage. 6, 125–141 (2016). https://doi.org/10.1016/j.est.2016.02.005
Huang, P.Y., Liu, C., Guo, Z.S., Feng, J.M.: Analytical model and experimental verification of the interfacial peeling strength of electrodes. Exp. Mech. 61(2), 321–330 (2021). https://doi.org/10.1007/s11340-020-00651-z
Benzeggagh, M.L., Kenane, M.: Measurement of mixed-mode delamination fracture toughness of unidirectional glass/epoxy composites with mixed-mode bending apparatus. Compos. Sci. Technol. 56(4), 439–449 (1996). https://doi.org/10.1016/0266-3538(96)00005-X
Zhu, J., Feng, J., Guo, Z.: Mechanical properties of commercial copper current-collector foils. RSC Adv. 4(101), 57671–57678 (2014). https://doi.org/10.1039/c4ra07675c
Doyle, M., Newman, J., Gozdz, A.S., Schmutz, C.N., Tarascon, J.M.: Comparison of modeling predictions with experimental data from plastic lithium ion cells. J. Electrochem. Soc. 143(6), 1890–1903 (1996). https://doi.org/10.1149/1.1836921
Font, F., Protas, B., Richardson, G., Foster, J.M.: Binder migration during drying of lithium-ion battery electrodes: Modelling and comparison to experiment. J. Power Sources 393, 177–185 (2018). https://doi.org/10.1016/j.jpowsour.2018.04.097
Zaghib, K., Shim, J., Guerfi, A., Charest, P., Striebel, K.A.: Effect of carbon source as additives in LiFePO4 as positive electrode for lithium-ion batteries. Electrochem. Solid-State Lett. 8(4), A207–A210 (2005). https://doi.org/10.1149/1.1865652
Susarla, N., Ahmed, S., Dees, D.W.: Modeling and analysis of solvent removal during Li-ion battery electrode drying. J. Power Sources 378, 660–670 (2018). https://doi.org/10.1016/j.powsour.2018.01.007
Muller, M., Pfaffmann, L., Jaiser, S., Baunach, M., Trouillet, V., Scheiba, F., et al.: Investigation of binder distribution in graphite anodes for lithium-ion batteries. J. Power Sources 340, 1–5 (2017). https://doi.org/10.1016/j.jpowsour.2016.11.051
**e, H.M., Kang, Y.L., Song, H.B., Zhang, Q.: Real-time measurements and experimental analysis of material softening and total stresses of Si-composite electrode. J. Power Sources 424, 100–107 (2019). https://doi.org/10.1016/j.jpowsour.2019.03.107
Sahraei, E., Hill, R., Wierzbicki, T.: Calibration and finite element simulation of pouch lithium-ion batteries for mechanical integrity. J. Power Sources 201, 307–321 (2012). https://doi.org/10.1016/j.jpowsour.2011.10.094
Kermani, G., Keshavarzi, M.M., Sahraei, E.: Deformation of lithium-ion batteries under axial loading: analytical model and representative volume element. Energy Rep. 7, 2849–2861 (2021). https://doi.org/10.1016/j.egyr.2021.05.015
Wattanasakulpong, N., Prusty, B.G., Kelly, D.W.: Thermal buckling and elastic vibration of third-order shear deformable functionally graded beams. Int. J. Mech. Sci. 53(9), 734–743 (2011). https://doi.org/10.1016/j.ijmecsci.2011.06.005
Qi, Y., Guo, H., Hector, L.G., Timmons, A.: Threefold increase in the Young’s modulus of graphite negative electrode during lithium intercalation. J. Electrochem. Soc. 157(5), A558–A566 (2010). https://doi.org/10.1149/1.3327913
de Vasconcelos, L.S., Sharma, N., Xu, R., Zhao, K.: In-situ nanoindentation measurement of local mechanical behavior of a Li-ion battery cathode in liquid electrolyte. Exp. Mech. 59(3), 337–347 (2018). https://doi.org/10.1007/s11340-018-00451-6
Jaiser, S., Kumberg, J., Klaver, J., Urai, J.L., Schabel, W., Schmatz, J., et al.: Microstructure formation of lithium-ion battery electrodes during drying—an ex-situ study using cryogenic broad ion beam slope-cutting and scanning electron microscopy (cryo-bib-SEM). J. Power Sources 345, 97–107 (2017). https://doi.org/10.1016/j.jpowsour.2017.01.117
Jones, E.M.C., Silberstein, M.N., White, S.R., Sottos, N.R.: In situ measurements of strains in composite battery electrodes during electrochemical cycling. Exp. Mech. 54(6), 971–985 (2014). https://doi.org/10.1007/s11340-014-9873-3
Whitney JM, McCullough RL. Micromechanical Materials Modeling. Technomic (1990)
Acknowledgements
Zuoquan Zhu gratefully acknowledges the National Natural Science Foundation of China (Grant No. 11872235). **g Wan acknowledges the National Natural Science Foundation of China (Grant No.12102396 ) and China Postdoctoral Science Foundation (Grant No. 2021M692921).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A
Appendix A
In order to obtain the effective modulus of electrode composites, it is essential to evaluate the volume fractions of different components under various drying conditions. The electrode slurry with a thickness h after drying time t is \(h = h_{0} - \int_{0}^{t} \gamma {\text{d}}t\), where \(\gamma\) is the drying rate, and \(h_{0}\) is the initial thickness. According to the work of Font et al. [40], the concentration of inactive components \(C_{{{\text{ICs}}}}\) can be defined by a convective-diffusive equation,
where \(V_{{{\text{GM}}}}\) is the volume fraction of graphite material (GM) (i.e., active components). Note that GM is stabilized and can be regarded as uniformly distributed in the electrode slurry according to the previous work [50]. Therefore, \(V_{{{\text{GM}}}}\) can be calculated by its initial value \(V_{{{\text{GM}}}}^{0}\) as \(V_{{{\text{GM}}}} = V_{{{\text{GM}}}}^{0} h_{0} /h\). As reported in Ref. [40], the volume fraction of GM at the beginning and end of drying was 0.28 and 0.5, respectively. Here,\(D_{{{\text{eff}}}} = D_{{{\text{ICs}}}} (1 - V_{{{\text{GM}}}} )^{3/2}\) is the effective diffusivity of the tortuous electrode. \(D_{{{\text{ICs}}}}\) is the diffusivity of ICs in the solvent. In this equation, \(F_{{\text{l}}}\) denotes the liquid volume fraction flux and is given by \(F_{{\text{l}}} = - z{\text{d}}V_{{{\text{GM}}}} /{\text{d}}t\). Meanwhile, the drying process model satisfies the boundary conditions
Combining Eqs. (21), (22), we obtain the volume fractions of components in this electrode composites as
where \(h_{{{\text{end}}}}\) and \(C_{{{\text{ICs}}}}^{{{\text{end}}}}\) are the electrode thickness and concentration of ICs of dried composites, and \(h_{{\text{a}}} = h_{{{\text{end}}}}\). \(M_{{{\text{ICs}}}}\) and \(\rho_{{{\text{ICs}}}}\) are the molar mass and density of ICs, and \(\rho_{{{\text{ICs}}}}\) can be calculated by the density of binder and conductive additive. Moreover, the effective mechanical properties of electrode composites can be estimated by the combination of open cell theory and the S-combining rules [51]and written by
where \(E_{{{\text{ICs}}}}\) is elastic modulus of ICs, which is equal to 2.5 GPa [49]. \(\xi_{{{\text{lk}}}}\), \(\xi_{\lg }\), \(\chi_{{\text{k}}}\), \(\chi_{{\text{g}}}\), \(\Psi_{{\text{k}}}\), and \(\Psi_{{\text{g}}}\) are related to the material properties of active and inactive components and written by
where \(v_{{{\text{PICs}}}}\) is the Poisson’s ratio of the ICs porous matrix, and is equal to 1/3. \(\Gamma = \left( {2\lambda - 1} \right)/\lambda\), \(\lambda\) represents the critical volume fraction and is assumed as 2/3 [52]. \(K_{{{\text{PICs}}}}\) and \(G_{{{\text{PICs}}}}\) are the corresponding bulk and shear moduli and can be given by
Considering the concentration-dependent property of graphite material, the bulk and shear modulus \(K_{{{\text{GM}}}}\) and \(G_{{{\text{GM}}}}\) in Eq. (25) can be obtained by the elastic modulus \(E_{{{\text{GM}}}}\), where \(E_{{{\text{GM}}}} = \left( {19.25 + 82.234 \times {c \mathord{\left/ {\vphantom {c {c_{\max } }}} \right. \kern-\nulldelimiterspace} {c_{\max } }}} \right){\text{GPa}}\) and 0\(v_{{{\text{GM}}}} = 0.26\) [48]. With Eqs. (21)–(26), we can obtain the effective elastic modulus and Poisson’s ratio of electrode composites and have \(E_{{\text{a}}} \left( z \right) = 9G_{{\text{a}}} K_{{\text{a}}} /(3K_{{\text{a}}} + G_{{\text{a}}} )\) and \(v_{{\text{a}}} = \left( {3K_{{\text{a}}} - 2G_{{\text{a}}} } \right)/(6K_{{\text{a}}} + 2G_{{\text{a}}} )\), respectively. Moreover, the line strain of the composite electrode layer due to lithiation can be calculated as
where \(\varepsilon_{{{\text{GM}}}}\) is the lithiation strain of a graphite particle and estimated as \({{\Omega c} \mathord{\left/ {\vphantom {{\Omega c} 3}} \right. \kern-\nulldelimiterspace} 3}\). \(\varepsilon_{{{\text{PICs}}}}\) is the strain of the porous matrix (i.e. binder, carbon black, and porosity). And \(\varepsilon_{{{\text{PICs}}}}\) is equal to zero because the porous matrix does not influence the lithiation reaction.
Rights and permissions
About this article
Cite this article
Zhu, Z., Wan, J., Wu, T. et al. Effect of electrode processing on the stability of electrode structure. Acta Mech 233, 2471–2484 (2022). https://doi.org/10.1007/s00707-022-03229-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00707-022-03229-z