Abstract
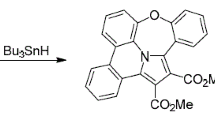
Pyrazolines are an important class of heterocyclic compounds with diverse applications. A novel bis-chalcone-derived fused ring pyrazoline has been obtained by the reaction of bis-chalcone with hydrazine hydrate in acetic acid solvent having a catalytic amount of HCl and characterized by IR, 1H and 13C NMR, GC–MS, and finally by X-ray single crystal analysis. Interestingly, the in situ post-cyclization substitution pattern on pyrazoline ring nitrogen is entirely different from the previously reported related pyrazoline derivatives. The new compound crystallizes out from its ethanolic solution, which has monoclinic crystal lattice with P21/c space group, a = 6.1001(4) Å, b = 38.602(2) Å, c = 7.7332(5) Å, α = 90°, β = 113.039(3)°, γ = 90°, V = 1675.73(19) Å3, Z = 4, crystal size = 0.38 × 0.30 × 0.18 mm3, and R int = 0.043.
Graphical abstract




Similar content being viewed by others
References
Elinson MN, Nasybullin RF, Ryzhkov FV, Zaimovskaya TA, Nikishin GI (2015) Monatsh Chem 146:631
Albuquerque HMT, Santos CMM, Cavaleiro JAS, Silva AMS (2014) Curr Org Chem 18:2750
Seebacher W, Michl G, Belaj F, Brun R, Saf R, Weis R (2003) Tetrahedron 59:2811
Tong X, Chen R, Zhang TT, Han Y, Tang WJ, Liu XH (2015) Bioorg Med Chem 23:515
Montoya A, Quiroga J, Abonia R, Derita M, Sortino M, Ornelas A, Zacchino S, Insuasty B (2016) Molecules 21:969
Kaplancikli ZA, Ozdemir A, Turan-Zitouni G, Altintop MD, Can OD (2010) Eur J Med Chem 45:4383
Bhandari S, Tripathi AC, Saraf SK (2013) Med Chem Res 22:5290
Jadhav SY, Shirame SP, Kulkarni SD, Patil SB, Pasale SK, Bhosale RB (2013) Bioorg Med Chem Lett 23:2575
Evranos-Aksoz B, Onurdag FK, Ozgacar SO (2015) Z Naturforsch C 70:183
Elmeligie S, Khalil NA, Ahmed EM, Emam SH, Zaitone SAB (2016) Biol Pharm Bull 39:1611
Bruno O, Bondavalli F, Ranise A, Schenone P, Losasso C, Cilenti L, Matera C, Marmo E (1990) Farmaco 45:147
Harikrishna N, Isloor AM, Ananda K, Obaid A, Fun HK (2016) New J Chem 40:73
Ahn JH, Kim HM, Jung SH, Kang SK, Kim KR, Rhee SD, Yang SD, Cheon HG, Kim SS (2004) Bioorg Med Chem Lett 14:4461
Amr AE, AL-Omar MA, Abdalla MM (2016) Lat Am J Pharm 35:950
Lee Y, Kim BS, Ahn S, Koh D, Lee YH, Shin SY, Lim Y (2016) Bioorg Chem 68:166
Havrylyuk D, Zimenkovsky B, Vasylenko O, Lesyk R (2013) J Heterocycl Chem 50:E55
Mete E, Comez B, Gul HI, Gulcin I, Supuran CT (2016) J Enz Inhib Med Chem 31:1
Szukalski A, Haupa K, Miniewicz A, Mysliwiec J (2015) J Phys Chem C 119:10007
Szukalski A, Miniewicz A, Haupa K, Przybyl B, Janczak J, Sobolewski AL, Mysliwiec J (2016) J Phys Chem C 120:14813
Afsah EM, Kandeel EEDM, Khalifa MM, Hammouda WM (2007) Z Naturforsch B 62:540
Levai A (2002) J Heterocycl Chem 39:1
Abbas A, Flores-Holguin N, Naseer MM (2015) New J Chem 39:4359
Abbas A, Hussain S, Hafeez N, Naseer MM (2014) Spectrochim Acta A 133:182
Abbas A, Hussain S, Hafeez N, Hasan A, Naseer MM (2014) Spectrochim Acta A 127:32
Abbas A, Nazir H, Naseer MM, Bolte M, Hussain S, Hafeez N, Hasan A (2014) Spectrochim Acta A 120:176
Abbas A, Naseer MM (2014) Acta Chim Slov 61:792
Anam F, Abbas A, Lo KM, Zia-ur-Rehman, Hameed S, Naseer MM (2014) New J Chem 38:5617
Song J, Zhao PS, Zhang WG (2010) Bull Korean Chem Soc 31:1875
Asiri AM, Khan SA (2012) J Heterocycl Chem 49:1434
Asiri AM, Khan SA (2011) Molecules 16:523
Azam MA, Gomathy S, Alam F, Suresh B (2008) Indian J Heterocycl Chem 18:157
Hadi S, Rilyanti M (2010) Orient J Chem 26:775
Hadi S, Afriyani H, Anggraini WD, Qudus HI, Suhartati T (2015) Asian J Chem 27:1509
Naseer MM, Hameed S (2012) Cryst Eng Comm 14:4247
Ahmad M, Pervez H, Hadda TB, Toupet L, Naseer MM (2014) Tetrahedron Lett 55:5400
Sheldrick GM (2008) Acta Crystallogr A 64:112
Acknowledgements
We are grateful to HEC and QAU for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ashraf, S., Hameed, S., Tahir, M.N. et al. Synthesis and crystal structure of bis-chalcone-derived fused-ring pyrazoline having an unusual substitution pattern. Monatsh Chem 148, 1871–1875 (2017). https://doi.org/10.1007/s00706-017-1995-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-1995-8