Abstract
Purpose
To evaluate longitudinal changes in optical coherence tomography angiography (OCTA) metrics in children and adolescents with type 1 diabetes (T1D).
Methods
This prospective observational cohort study included thirty-two eyes from thirty T1D children with no history of diabetic retinopathy (DR) who were followed up for 4 years. Participants underwent OCTA examinations at baseline and during follow-up. Quantitative OCTA metrics were measured using a customized MATLAB algorithm. Generalized mixed-effect models were used to determine their relationship with DR development. Systemic parameters and OCTA metrics were screened using least absolute shrinkage and selection operator to identify predictors for visual function.
Results
Over the 4-year period, seven of the included eyes developed DR, and most OCTA metrics decreased with diabetes duration. Higher peripapillary and parafoveal nasal quadrant vessel area density (VAD) in the superficial capillary plexus (SCP) and vessel skeleton density (VSD) in both the SCP and the deep capillary plexus (DCP) were associated with a lower risk of DR in T1D. Parafoveal DCP VSD and VAD in the temporal and inferior quadrants were anticorrelated with changes in best corrected visual acuity.
Conclusions
OCTA metrics dynamically change over the duration of diabetes and can be used as biomarkers to improve the risk evaluation of DR development and visual function in T1D children and adolescents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes (T1D) is a chronic health condition characterized by autoimmune destruction of beta cells in the pancreatic islets, resulting in an absolute insulin deficiency. Globally, T1D affects over 8.4 million people, with 1.5 million of them aged younger than 20 years in 2021 [1]. Despite significant progress in the management of T1D, modifying the risk of long-term complications remains a primary concern, especially for pediatric-onset patients. Diabetic retinopathy (DR) is one of the most common complications of T1D and the leading cause of visual impairment [2]. To address this, the International Society for Pediatric and Adolescent Diabetes recommends annual screening for retinopathy by slit lamp or fundus photography, starting at age 11 and for those with 2 to 5 years of diabetes duration [3]. Adding a layer of complexity to the disease prevention paradigm, in vivo studies have suggested that retinal neurodegeneration and microvascular regression may precede clinically detectable retinopathy signs in fundus images [4]. Furthermore, established risk factors (such as diabetes duration and glycated hemoglobin [HbA1c]) have proven insufficient in predicting the development of DR [5].
Advancements in optical coherence tomography angiography (OCTA) technology have enabled noninvasive assessment of retinal microvasculature and capillary perfusion. In addition to offering a three-dimensional structural visualization, OCTA excels in precise quantification of vessel features by tracking red blood cell motion. With the increased availability of OCTA, cumulative studies have established a strong association between DR and OCTA-derived metrics. For example, Tan and colleagues demonstrated the remarkable accuracy of wide-field OCTA (12 × 12-mm field) in grading the severity of nonproliferative DR (NPDR) [6]. Takase et al. suggested that changes in the size and circularity of the foveal avascular zone (FAZ) could be observed before DR development, potentially serving as indicators for early detection of DR [7]. Additionally, a prospective study by Cheung et al. reported that the FAZ area, vessel density, and fractal dimension of the deep capillary plexus (DCP) can predict DR progression [8]. These findings provide robust support for the application of OCTA in evaluating DR risk.
There is imperative need for longitudinal evidence demonstrating the capacity of quantifiable OCTA metrics to reveal invaluable insights into vascular health and disease in juvenile T1D patients. In this study, we leveraged a prospective observational study to unravel the alterations in retinal vasculature characteristics among T1D children and adolescents over a span of 4 years. We also explored the possible correlation of OCTA metrics with systemic factors, delving deeper into their predictive capacity for the onset of DR and vision loss. Our study offers a novel and longitudinal perspective on retinal imaging traits as prognostic factors, shedding light on early microvascular abnormalities in the retina and their role in predicting visual impairment in pediatric patients with T1D.
Methods
Participants
This 4-year longitudinal cohort study was conducted as a part of the SCADE project (Shanghai Children and Adolescent Diabetes Eye study, ClinicalTrials.gov identifier: NCT 03587948) [9]. In brief, the SCADE is a prospective cohort study spanning from January 2018 to 2028. The overarching aim of this project is to recruit 300 children with T1D in Children's Hospital of Fudan University in Shanghai as the case group, along with 300 healthy children without diabetes as the control group. To guide disease screening of children and adolescents with diabetes is the primary determination of the SCADE study. It also seeks for providing comprehensive, continuous, and dynamic information to diabetic eye disease management.
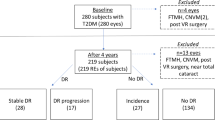
A total of 30 children and adolescents who finished the year-four visit were included in this longitudinal study, additional 64 individuals who participated in the 2023 visit were designated for the cross-sectional validation study. Main inclusion criteria were (1) age younger than 20 years, in accordance with the World Health Organization’s definition of adolescence [10]; (2) a confirmed diagnosis of T1D following the American Diabetes Association criteria [11]; and (3) a compliance with all examinations at baseline and during follow-up. Those who met any of the following criteria were excluded: (1) the presence of eye diseases that may interfere with OCTA examinations, such as inherited retinopathy, high myopia, keratitis, scleritis, and cataracts; (2) a history of systemic diseases other than T1D, such as hypertension and systemic lupus erythematosus; and (3) eyes with recent intraocular surgeries, laser treatment or intravitreal therapy within the preceding 6 months.
The present study adhered to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Shanghai General Hospital of Shanghai Jiao Tong University and the Children's Hospital of Fudan University in Shanghai (approval number, 2016KY005 and LS No. 01 (2018)). All participants provided written informed consent.
Data collection
At baseline and follow-up visits, parents of all participants completed a questionnaire providing demographic information, including sex, birth date, history of T1D and diabetic complications. Anthropometric measurements (height, weight, and blood pressure) were recorded for all subjects, and ophthalmic examinations were conducted by trained examiners using standardized protocols and instruments. These examinations included (1) slit lamp examination (SL130; Zeiss, Germany), (2) digital nonmydriatic fundus photography (AFC-210; NIDEK, Tokyo, Japan), (3) intraocular pressure (IOP) measurement (NT-530P; NIDEK), (4) IOL Master 700 assessments (Carl Zeiss Meditec, Dublin, CA, USA), (5) best-corrected visual acuity (BCVA) measured using the international standard LogMAR visual acuity chart after adequate pupil dilation (1% tropicamide, 3 drops in total, one at a time, at 5-min intervals) [12], (6) refractive power measured by optometry (KR-8900; Topcon, Tokyo, Japan), and (7) OCTA (CIRRUS HD-OCT Model 5000; Carl Zeiss Meditec) scanning a 6 × 6-mm acquisition protocol centered at the macula and the optic disc in sequence [13]. To ensure the quality of the OCTA examinations, eye motion artifacts were minimized by the CIRRUS eye-tracking algorithm during image acquisition. Poor-quality images (defined as a signal strength index < 6/10) were retaken immediately unless subjects were unable to cooperate with continued examination.
For the definition of DR development, retinal fundus photographs were taken using a nonmydriatic camera after pharmacologic pupil dilation. Two photographs of each eye were taken at each visit, one centered at the optic disc and the other centered at the fovea. Both baseline and follow-up photographs were assessed using the Early Treatment of Diabetic Retinopathy Study severity grading scale by the masked grader.
OCTA image analysis
The original OCTA images were automatically segmented by the built-in software into the superficial capillary plexus (SCP) and the DCP. The examiner carefully scrolled the B-scans to maintain accuracy and eliminate major errors. Following this, quality control was carried out by masked graders in a standardized environment. Images with significant artifacts, such as blur, defocus, extensive eye motion, and fixation problems, were excluded from subsequent analysis (N = 6). In our quantitative OCTA analysis, a customized MATLAB algorithm (The MathWorks Inc), as previously detailed by Wang et al. [14], was used to yield measurements. The reliability and repeatability of the measurements have been reported by Zhao and colleagues [15]. The following metrics were computed: (1) vessel area density (VAD), which quantifies the total area of perfused vasculature per unit area in a region of measurement; (2) vessel skeleton density (VSD), which denotes the total length of vessels containing particles with velocities ranging between 0.3 and 3 mm/sec; (3) FAZ size. Additionally, VAD and VSD were assessed in four quadrants surrounding either the macular fovea or optic disc. To adjust the ocular magnification from axial eye growth, we manually calculated scaling factors for these metrics using a previously described formula [2). Histologically, the retinal vascular system is categorized into four distinct strata: (1) radial peripapillary capillaries encircling the optic nerve within the retinal nerve fiber layer (RNFL), (2) SCP located at the retinal ganglion cell layer (RGCL) and the superficial segment of the inner plexiform layer, (3) intermediate capillary plexuses (ICP) situated at the inner boundary of the inner plexiform layer and the superficial portion of the inner nuclear layer, and (4) DCP positioned at the outer border of the inner nuclear layer. The fovea of the macula is devoid of the RNFL, RGCL and inner plexiform layer corresponding to the SCP, and the thickness of the RNFL increases as it approaches the optic disc [19, 20]. These anatomical features explain to some extent the disparities in perfusion defect segmentation between images centered on the macula and the optic disc. Furthermore, OCTA metrics in different quadrants demonstrated varying degrees of change. Specifically, we identified a notable decline in the temporal and nasal quadrants of VAD and VSD over 4 years, in contrast to the relatively stable values observed in the superior and inferior quadrants (Table 2). A prior study by our colleagues unveiled longitudinal alternations in retinal thickness among T1D children and adolescents [21], indicating a reduction in peripapillary RGCL thickness in the temporal sector. Additionally, Cao et al. reported that peripapillary RNFL thinning was significant in the nasal quadrant of the retina in preclinical DR patients compared to normal controls [22]. It is thus reasonable to posit that the variations in vessel density across quadrants are inherently linked to structural changes in RNFL and RGCL thickness, with the onset of these changes seemingly differing from one quadrant to another over the course of the disease.
This study provides essential insights into the application of OCTA for predicting the development of DR in T1D children and adolescents, a context that may diverge from observations in adults with type 2 diabetes (T2D). Prior research tended to conclude that OCTA-derived retinal microvascular abnormalities were evident in the earlier stages of DR in T2D patients. For instance, Yoon et al. reported that the decreasing slope of parafoveal VSD in the DCP was not significant until the severe NPDR stage in T1D, whereas in T2D, deterioration of VSD could be found even in the early stage of DR [23]. A meta-analysis also indicated that diabetic patients without DR showed an enlarged FAZ area and reduced VAD in both the SCP and the DCP compared to healthy controls, whereas most of these differences became insignificant in T1D [24]. In contrast, our results suggested that early abnormalities in the retinal blood flow can be observed in T1D children and adolescents before the onset of DR. By employing a generalized mixed-effect model on the longitudinal data, we improved the efficiency of identifying risk factors, particularly given the challenges of handling an unbalanced dataset with limited patient numbers. Our investigation yielded valuable results after adjusting for factors influencing OCTA metrics (Fig. 2c,d; Supplementary Table 2). For example, we identified multiple DCP metrics as prognostic factors for DR, consistent with prior research emphasizing the association between DCP metrics and DR progression [25, 26]. This implies that early disturbances within the retinal microenvironment may affect DCP earlier than other regions. Moreover, our findings revealed that higher VSD in different areas and layers was linked to a lower risk of DR. This observation can be elucidated by the heightened sensitivity of VSD in capturing perfusion changes at the capillary level compared to VAD [14]. Taken together, these results not only corroborate existing evidence but also extend our knowledge of relationships between retinal blood flow and DR in children and adolescents with T1D.
It is also worth noting the comparison of the performance between peripapillary and parafoveal OCTA metrics in the clinical assessment of pediatric patients. Our findings revealed that peripapillary metrics outperformed parafoveal parameters in discriminating young eyes with NPDR, as indicated by Fig. 2c and d. Although previous studies have predominantly centered on the diagnostic utility of peripapillary OCTA in the context of glaucoma [27, 28], there is a growing body of research focusing on the optic disc vascular network in diabetic populations [29]. Unlike the macula, where blood supply is mainly attributed to smaller-diameter capillaries, the overall perfusion of the disc region is considerably influenced by larger-diameter vessels, primarily venules [30]. Therefore, our result presents an opportunity to position peripapillary metrics as a valuable predictor of abnormalities in large-vessel perfusion that can manifest at the very early stages of NPDR. However, it is imperative to approach the interpretation of these results with caution. The smaller sample size for peripapillary OCTA data analysis raises concerns about more potential for overfitting than the parafoveal dataset. Whether large-vessel perfusion dysfunction occurs earlier than previously considered remains undecided, pending validation through functional experiments.
Moreover, our findings reveal a remarkable association between DCP OCTA metrics and BCVA, suggesting that the identification of perfusion deficits in the parafoveal DCP holds clinical value for predicting vision loss in children and adolescents with T1D (Supplementary Table 4). Diabetic macular ischemia, defined as enlargement or irregularity of the FAZ and capillary dropout in the parafoveal area, is also a common comorbidity in diabetic patients. Both DR and diabetic macular ischemia can adversely affect visual function. It is generally postulated that the length of the photoreceptor layer disruption, disorganization of the retinal inner layers, and the FAZ circularity are independent determinants of visual acuity [19]. Consistent with previous research, our findings suggest that in diabetic eyes, visual impairment is more closely associated with alterations in the DCP than with the SCP [31, 32]. Decreased vessel density in the DCP is sufficient to cause visual impairment by affecting blood supply to the photoreceptors. Histologic evidence further supports the notion that the DCP may be more susceptible to ischemia-induced endothelial damage, which could play a pivotal role in modulating visual function in patients with T1D through neurovascular coupling. Additionally, we demonstrated positive correlations between BMI, AL and BCVA. Previous studies in healthy adults have also reported a connection between a higher BMI and visual impairment, implying potential links between hyperlipidemia and an increased risk of visual impairment [33]. For the effects of AL on visual function, mechanisms proposed to explain involve elongation of the eyeball resulting in the simultaneous stretching of the retina and a reduction in blood vessel width, ultimately leading to decreased overall blood flow [34].
Several confounding variables can introduce bias in quantitative OCTA analysis. First, age can cause divergent trends in retinal vasculature in childhood and adulthood. Despite most studies on healthy adults have concluded a decrease in retinal perfusion and vessel density with normal aging [35, 36], a recent study on 333 healthy Chinese children from 4 to 16 years old suggested that the retinal microvasculature continues to develop, resulting in increased VAD and VSD with age [37]. In our study of T1D children and adolescents, we observed a significantly decreasing trend of retinal vessel density with age based on linear models, suggesting that the deteriorating effect of prolonged hyperglycemia on retinal perfusion appears to outweigh the physiological retinal development. Additionally, factors such as pupil size, media opacities, and ocular pathologies can significantly impact OCTA image acquisition, potentially introducing bias [19]. To address these concerns, we administrated a standard pharmacological pupil dilation regimen to all subjects and meticulously examined the most recent slit-lamp results and medical records to exclude patients with these conditions. Moreover, most T1D patients adopt insulin therapy that potentially affects blood flow in the retina. Peng et al. reported that insulin-intensive treatment in diabetic patients can cause an instant reduction in VSD in the macular DCP and optic disc areas for 6 months compared to those treated with oral anti-hyperglycemic agents [38]. Nevertheless, the precise impact of long-term medication on retinal blood flow remains a subject of ongoing research, necessitating careful control of insulin use (including dose and frequency) in future large-scale investigations.
Furthermore, methods for OCTA image visualization and quantification may yield ambiguous results, potentially leading to misinterpretation. In our study, we used a MATLAB-based algorithm to generate quantitative metrics, consistent with our previous work for obtaining comparable observations [9]. MATLAB-based algorithms have been extensively used by researchers and demonstrated its accuracy and repeatability in recent years [6]. However, certain challenges persist, particularly regarding the DCP, which is often plagued with artefacts and projection of the SCP. Additionally, distinguishing the third vascular plexus ICP from the DCP can be difficult due to the layer segmentation border of current devices [39]. Caution is thus required when interpreting the effect of T1D on various capillary plexus. Of note, we did not observe any statistically meaningful associations between FAZ size and the onset of DR, which contradicts previous evidence highlighting the prognostic value of FAZ in DR [7]. Our FAZ area calculation depended on manually delineating the FAZ detected on OCTA, which may cause inaccuracies covering true alterations. While deep learning models have made significant advancements in the field of quantifying OCTA metrics, offering improved discrimination between non-perfusion and image artifacts, their development largely relies on extensive and diverse datasets for training [40]. For now, the acquisition of such datasets for OCTA, especially for the relatively small population of T1D children and adolescents, poses challenges, limiting the robustness of these models.
We acknowledge several limitations in the present study. First, the small sample size, especially the limited numbers of eyes with NPDR, restricts the generalizability of our results. Due to the COVID-19 outbreak, most participants from our SCADE cohort failed to attend annual visit. Therefore, the interpretation of these associations should be approached cautiously, and future replications in larger, independent cohorts are urgently needed. Second, we did not enroll sex- and age-matched healthy children as controls, resulting in inadequate knowledge of the physiological alterations in retinal vasculature during developmental stages. Third, the reproducibility of the measurements among different platforms was not tested. The variability of different OCTA devices may cause variations in the analysis when the same eye is imaged using different systems. Specifically, algorithms to detect flow in different platforms, artifact handling, and segmentation strategies can be sources of heterogeneity [41, 42]. Finally, DR can manifest as changes in the peripheral retinal vascular network at an early stage [6, 43]. The 6 × 6-mm scanning protocol we used in the study may be insufficient to detect distant peripheral vasculature and perfusion deficits. In recent years, widefield and ultrawidefield OCT systems have emerged that potentially address this technical limitation [44]. However, there is no consensus on whether these novel techniques are superior to commercially available devices in distinguishing patients at different stages of DR.
In conclusion, our results highlight the importance of monitoring OCTA metrics through repeated examinations and close follow-up in the clinical screening of retinopathy in T1D children and adolescents. A prominent decreasing trend in retinal perfusion and vessel density is most likely to serve as a meaningful biomarker of DR development. These findings provide a broader and more dynamic insight into the structural and functional alterations in the retinal vasculature, which may aid in designing effective prevention strategies to reduce the risk of DR.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Gregory GA, Robinson TIG, Linklater SE et al (2022) Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol 10(10):741–760
Bebu I, Braffett BH, de Boer IH et al (2023) Relationships between the cumulative incidences of long-term complications in type 1 diabetes: the DCCT/EDIC study. Diabetes Care 46(2):361–368
Donaghue KC, Marcovecchio ML, Wadwa RP et al (2018) ISPAD clinical practice consensus guidelines: microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes 19(27):262–274
Sohn EH, van Dijk HW, Jiao C et al (2016) Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A 113(19):E2655–E2664
Lachin JM, Genuth S, Nathan DM et al (2008) Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trialdrevisited. Diabetes 57:995e1001
Tan B, Chua J, Lin E, et al (2020) Quantitative microvascular analysis with wide-field optical coherence tomography angiography in eyes with diabetic retinopathy [published correction appears in JAMA Netw Open. 2020 Jun 1;3(6):e2010994]. JAMA Netw Open 3(1):e1919469.
Takase N, Nozaki M, Kato A et al (2015) Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina 35:2377–2383
Sun Z, Tang F, Wong R et al (2019) OCT angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema: a prospective study [published correction appears in Ophthalmology. 2020 Dec;127(12):1777]. Ophthalmology 126(12):1675–1684.
Li T, Jia Y, Wang S et al (2019) Retinal microvascular abnormalities in children with type 1 diabetes mellitus without visual impairment or diabetic retinopathy. Invest Ophthalmol Vis Sci 60(4):990–998
WHO (1977) Health needs of adolescents. World Health Organization, Geneva
American Diabetes Association (2015) Diagnosis and classification of diabetes mellitus. Diabetes Care 38:S8–S16
Lim J, Chia A, Saffari SE, Handa S (2019) Factors affecting pupil reactivity after cycloplegia in Asian children. Asia Pac J Ophthalmol (Phila) 8(4):304–307
Biswas S, Jhanji V, Leung CK (2016) Prevalence of glaucoma in myopic corneal refractive surgery candidates in Hong Kong China. J Refract Surg 32(5):298–304
Chu Z, Lin J, Gao C et al (2016) Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J Biomed Opt 21(6):66008
Zhao Q, Yang WL, Wang XN et al (2018) Repeatability and reproducibility of quantitative assessment of the retinal microvasculature using optical coherence tomography angiography based on optical microangiography. Biomed Environ Sci 31(6):407–412
Dai Y, **n C, Zhang Q et al (2021) Impact of ocular magnification on retinal and choriocapillaris blood flow quantification in myopia with swept-source optical coherence tomography angiography. Quant Imaging Med Surg 11(3):948–956
Yang B, Ye C, Yu M, Liu S, Lam DS, Leung CK (2012) Optic disc imaging with spectral-domain optical coherence tomography: variability and agreement study with Heidelberg retinal tomograph. Ophthalmology 119(9):1852–1857
Linderman R, Salmon AE, Strampe M, Russillo M, Khan J, Carroll J (2017) Assessing the accuracy of foveal avascular zone measurements using optical coherence tomography angiography: segmentation and scaling [published correction appears in Transl Vis Sci Technol. 2017 Aug 15;6(4):17]. Transl Vis Sci Technol 6(3):16
Cheung CMG, Fawzi A, Teo KY et al (2022) Diabetic macular ischaemia- a new therapeutic target? Prog Retin Eye Res 89:101033
Hayreh SS et al. The central artery OF the retina. Its role IN the blood supply OF the optic nerve. Br J Ophthalmol 47(1963):651–663
Yu Q, **ao Y, Lin Q et al (2022) Two-year longitudinal study on changes in thickness of the retinal nerve fiber layer and ganglion cell layer in children with type 1 diabetes mellitus without visual impairment or diabetic retinopathy. Curr Eye Res 47(8):1218–1225
Cao D, Yang D, Yu H et al (2019) Optic nerve head perfusion changes preceding peripapillary retinal nerve fibre layer thinning in preclinical diabetic retinopathy. Clin Exp Ophthalmol 47(2):219–225
Um T, Seo EJ, Kim YJ, Yoon YH (2020) Optical coherence tomography angiography findings of type 1 diabetic patients with diabetic retinopathy, in comparison with type 2 patients. Graefes Arch Clin Exp Ophthalmol 258(2):281–288
Zhang B, Chou Y, Zhao X, Yang J, Chen Y (2021) Early detection of microvascular impairments with optical coherence tomography angiography in diabetic patients without clinical retinopathy: a meta-analysis. Am J Ophthalmol 222:226–237
Nesper PL, Roberts PK, Onishi AC et al (2017) Quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Investig Ophthalmol Vis Sci 58:307–315
Tang FY, Chan EO, Sun Z et al (2020) Clinically relevant factors associated with quantitative optical coherence tomography angiography metrics in deep capillary plexus in patients with diabetes. Eye Vis (Lond) 7:7
Richter GM, Sylvester B, Chu Z et al (2018) Peripapillary microvasculature in the retinal nerve fiber layer in glaucoma by optical coherence tomography angiography: focal structural and functional correlations and diagnostic performance. Clin Ophthalmol 12:2285–2296
Richter GM, Chang R, Situ B et al (2018) Diagnostic performance of macular versus peripapillary vessel parameters by optical coherence tomography angiography for glaucoma. Transl Vis Sci Technol 7(6):21
Yuan M, Wang W, Kang S et al (2022) Peripapillary microvasculature predicts the incidence and development of diabetic retinopathy: an SS-OCTA study. Am J Ophthalmol 243:19–27
Ikram MK, Ong YT, Cheung CY, Wong TY (2013) Retinal vascular caliber measurements: clinical significance, current knowledge and future perspectives. Ophthalmologica 229(3):125–136
Ong JX, Konopek N, Fukuyama H, Fawzi AA (2023) Deep capillary nonperfusion on OCT angiography predicts complications in eyes with referable nonproliferative diabetic retinopathy. Ophthalmol Retin 7(1):14–23
Yang D, Tang Z, Ran A et al (2023) Assessment of parafoveal diabetic macular ischemia on optical coherence tomography angiography images to predict diabetic retinal disease progression and visual acuity deterioration. JAMA Ophthalmol 141(7):641–649
Wong PW, Lau JK, Choy BN et al (2020) Sociodemographic, behavioral, and medical risk factors associated with visual impairment among older adults: a community-based pilot survey in Southern District of Hong Kong. BMC Ophthalmol 20(1):372
Wang X, Kong X, Jiang C, Li M, Yu J, Sun X (2016) Is the peripapillary retinal perfusion related to myopia in healthy eyes? A prospective comparative study. BMJ Open 6(3):e010791
Iafe NA, Phasukkijwatana N, Chen X, Sarraf D (2016) Retinal capillary density and foveal avascular zone area are age-dependent: quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 57(13):5780–5787
Garrity ST, Iafe NA, Phasukkijwatana N, Chen X, Sarraf D (2017) Quantitative analysis of three distinct retinal capillary plexuses in healthy eyes using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 58(12):5548–5555
Li S, Yang X, Li M et al (2020) Developmental changes in retinal microvasculature in children: a quantitative analysis using optical coherence tomography angiography. Am J Ophthalmol 219:231–239
Yang N, Li MX, Peng XY (2022) Effects of intensive insulin therapy on the retinal microvasculature in patients with type 2 diabetes mellitus: a prospective observational study. BMC Ophthalmol 22(1):187
Spaide RF (2015) Volume-rendered optical coherence tomography of diabetic retinopathy pilot study. Am J Ophthalmol 160(6):1200–1210
Guo Y, Hormel TT, **ong H et al (2019) Development and validation of a deep learning algorithm for distinguishing the nonperfusion area from signal reduction artifacts on OCT angiography. Biomed Opt Express 10(7):3257–3268
Spaide RF, Fujimoto JG, Waheed NK et al (2018) Optical coherence tomography angiography. Prog Retin Eye Res 64:1–55
Sacconi R, Borrelli E, Querques G (2018) Reproducibility of vessel density, fractal dimension, and foveal avascular zone using 7 different optical coherence tomography angiography devices. Am J Ophthalmol 192:252–253
Wang XN, Cai X, Li SW, Li T, Long D, Wu Q (2022) Wide-field swept-source OCTA in the assessment of retinal microvasculature in early-stage diabetic retinopathy. BMC Ophthalmol 22(1):473
Niederleithner M, de Sisternes L, Stino H et al (2023) Ultra-widefield OCT angiography. IEEE Trans Med Imaging 42(4):1009–1020
Acknowledgements
Not applicable.
Funding
This study was supported by Chinese National key research and development program (Project number 2021YFC2702100), and Shanghai engineering research center of precise diagnosis and treatment of eye diseases, Shanghai, China (Project No. 19DZ2250100).
Author information
Authors and Affiliations
Contributions
QL and YX designed the study. Data collection was conducted by XQ, LC, SC, QA, TY, YW. XQ, YX, LC analyzed and interpreted the data. XQ drafted the manuscript. HZ and CY supervised the study and revised the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
This study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of the Shanghai General Hospital affiliated with Shanghai Jiao Tong University and the Children's Hospital of Fudan University in Shanghai (approval number, 2016KY005 and LS No. 01 (2018)).
Informed consent
Informed consent was acquired from legal guardians of children and adolescents included in this study.
Consent for publication
Not applicable.
Additional information
Managed By Massimo Federici .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, X., **ao, Y., Cui, L. et al. Evaluation of optical coherence tomography angiography metrics in children and adolescents with type 1 diabetes: 4-year longitudinal study. Acta Diabetol (2024). https://doi.org/10.1007/s00592-024-02291-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00592-024-02291-4