Abstract
Introduction
Colorectal cancer (CRC) creates elevated self-management demands and unmet support needs post-discharge. Follow-up care through eHealth post-primary surgery may be an effective means of supporting patients’ needs. This integrative review describes the evidence regarding eHealth interventions post-hospital discharge focusing on delivery mode, user-interface and content, patient intervention adherence, impact on patient-reported outcomes and experiences of eHealth.
Methods
A university librarian performed literature searches in 2021 using four databases. After screening 1149 records, the authors read 30 full-text papers and included and extracted data from 26 papers. Two authors analysed the extracted data using the ‘framework synthesis approach’.
Results
The 26 papers were published between 2012 and 2022. The eHealth interventions were mainly delivered by telephone with the assistance of healthcare professionals, combined with text messages or video conferencing. The user interfaces included websites, applications and physical activity (PA) trackers. The interventions comprised the monitoring of symptoms or health behaviours, patient information, education and counselling. Evidence showed a better psychological state and improved PA. Patients reported high satisfaction with eHealth. However, patient adherence was inadequately reported.
Conclusions
eHealth interventions may positively impact CRC patients’ anxiety and PA regardless of the user interface. Patients prefer technology combined with a human element.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Colorectal cancer (CRC) is the third most common cancer globally [1]. Cancer stages I–III (i.e. nonmetastatic disease) dominate among CRC cases, with curative surgery being the cornerstone of treatment [2]. Patients with CRC are prone to comorbidities [3]. The impact of surgery, in combination with comorbidities, is found to be highest in the first year following surgery [4]. Most CRC patients are currently managed within enhanced recovery schemes [5], including early discharge to home post-surgery, when physiological functions such as oral intake of nutrients or bowel functions may not be fully restored [6]. About half of anastomotic leakages after bowel resection occur after discharge from hospital, with serious consequences for the patient [7]. Consequently, the period of transition from hospital to home may represent a vulnerable time, prone to issues that can contribute to readmission. Readmission rates for CRC range from 9 to 25% [8] and are deemed markers of quality of care [9].
Following discharge, many CRC patients may struggle with navigating the healthcare system and adopting recommended self-management behaviours. The self-management of CRC includes monitoring health, accessing health information [10] and initiating health behaviour changes, such as exercising more [11]. Moreover, CRC patients may struggle with self-management tasks like finding medical information, monitoring health and interacting with healthcare services, which may result in physical and mental fatigue [10].
eHealth is defined as ‘the use of information and communication technologies (ICT) for health’ [12]. eHealth support deployed post-hospitalisation may promote self-management among people with severe conditions [13]. However, further insight is needed into how a more seamless eHealth service during the transition from inpatient to outpatient care may enable patients to obtain adequate self-management support, feel safe and recover well [14].
There is some evidence that eHealth can support cancer survivors in the self-management of treatment side effects and complications and increase their quality of life (QOL) [15]. Recent reviews of eHealth in the context of CRC populations are sparse. In an overview of reviews on telemedicine (e.g. eHealth) in post-treatment cancer survivorship, none of the 29 included systematic reviews focused on CRC patients only [16]. A systematic review aiming to study eHealth support directed at CRC survivors’ follow-up needs upon discharge from the hospital addressed the interventions’ service content, outcomes and software infrastructure [17]. The findings demonstrated that eHealth was useful for CRC survivors in supporting physiological, psychological and cognitive needs and enabling better symptom management and QOL [17]. Nevertheless, there is a knowledge gap concerning technology acceptance and how patients adhere to eHealth interventions. Adherence is defined as ‘the extent to which a person’s behaviour corresponds with agreed recommendations from a healthcare provider’ [18] (p. 3), but little is known about how eHealth may promote adherence to recommended CRC self-care [19].
This study aimed to (1) explore the user interface, content and delivery mode of CRC eHealth interventions following discharge after surgery, (2) investigate patient adherence to the interventions, (3) establish intervention effects on patient-reported outcome measures (PROMs) and (4) describe patients’ experiences of eHealth follow-up interventions.
Methods
The study was conducted according to Whittemore and Knafl’s five-step framework for integrative reviews [20] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [21].
Step 1: literature search
Comprehensive literature searches were performed by a university librarian in October 2021 using the Embase, Medline, CINAHL and Cochrane Library databases, as well as by manually searching reference lists. The search terms, limitations and search results are displayed in Table 1.
Step 2: study selection
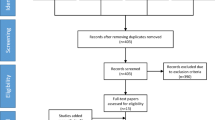
Endnote™ Version X9 [22] was used to manage the generated records from Search no. 3 (Table 1). After removing duplicates (N=471), a blinded screening of 1373 titles and abstracts was performed using the web application Rayyan [232] and a priori inclusion and exclusion criteria (Table 2). Following the blinded screening, a comparison of the decisions showed discrepancies for 37 records (4.5%), resolved through discussions among the authors. Fifty-nine full-text articles were distributed among the authors and assessed for final inclusion, with conflicting opinions being resolved through discussions among the authors. The results of the study selection process are displayed in a PRISMA flow chart [23].
Step 3: data extraction
To achieve consistency in data extraction, an extraction tool was constructed, including publication identifiers, study design, study context and participants, eHealth program, program adherence and patient outcomes and experiences. Any inconsistencies among co-authors were resolved via the assessment of a second reviewer.
Step 4: critical assessment of articles
The authors used the mixed methods appraisal tool (MMAT) [24] in teams of two to establish the risk of bias in the included studies. Here, the MMAT checklists for randomised controlled trials (RCTs) and non-randomised, descriptive, qualitative and mixed methods were used. Each study was assigned an overall quality score, varying from 25% when one criterion was met to 100% when all criteria were met. The MMAT was used as a summarising tool, with methodological quality considered according to the design of each study. The MMAT score was not used for exclusion decisions [24]. Studies were not excluded based on methodological quality. The strength of evidence was summarised as part of the review’s limitations.
Step 5: data synthesis
To analyse and synthesise data, the ‘framework synthesis approach’ was used, which includes five analytical stages: familiarisation with the data content, identification of themes, indexing, charting and map** and interpretation [25]. Data from the extraction table allowed the authors to familiarise themselves with the findings. Coding of the data was performed by one author according to key issues, concepts and themes, namely the outcomes and practices of eHealth follow-up programs, including content, delivery mode and user interface, patient adherence, impact of eHealth interventions and patient experience. The synthesis of the findings was then reviewed by a second author and finally examined by the co-authors.
Results
After the full-text assessment of the 30 records, four were excluded based on the eligibility criteria resulting in a total of 26 included papers [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] (Fig. 1).
Risk of bias
Among the 12 RCTs, two achieved full scores (7/7 points) [30, 51], four achieved 6/7 points [35, 42, 46, 49], five scored 5/7 points [38, 40, 43, 47, 48] and one scored 4/7 points [28]. Across the MMAT domains, seven of 12 RCTs did not report the blinding of outcome assessors. Nearly all the non-randomised studies scored 5/6 points, while one achieved 4/6 points [26]. Here, the less reported item referred to administration of the intervention as intended (5/8 studies). Only one of the three descriptive achieved the full score of 7 points [45] as the authors of the other two did not report on sample representativeness, risk of non-response bias and appropriate statistical analysis [33, 44]. The only mixed-methods study scored 5/7 points as it lacked reporting on sample representativeness and an adequate rationale for using a mixed-methods design to address the research question [36]. Both qualitative studies [31, 50] demonstrated good methodological quality, scoring 7/7 points.
Overview of study characteristics
The studies were published between 2012 and 2022 (Table 3), and most were of European origin. Three studies were performed by the same Swedish research team [31,32,33], while three Dutch studies involved the same eHealth application (i.e. Oncokompas) [47,48,49]. Fourteen studies applied RCT or quasi-experimental study methods, seven used observational designs, two were qualitative, two used mixed methods, and one used a case-study design. The study populations ranged from 1 to 756 participants (median number of participants, n=118). In one study, presented in two publications, the CRC patient population accounted for 25% of the participants [47, 48]. All studies recruited adult CRC patients (18–81 years of age). Median age, based on 21 of 26 studies that provided information on mean or median age was 65 years of age [26,27,28,29,30,31,32,33,34,35,36,37,38,39, 41,42,43,44, 46,47,48,49, 51]. In four studies, most of the participants were female [29, 30, 35, 41, 42]. Only two studies addressed the importance of a diverse sample as to provide eHealth services to demographically (e.g. education and income) and geographically (e.g. rural areas) diverse groups [42, 46]. In all the studies, patients were enrolled during the post-operative care trajectory. In eight studies, patients received the eHealth intervention during adjuvant chemotherapy [26, 28,29,30,31,32,33, 38, 39].
Results of data analysis
eHealth interventions’ delivery mode, user interface and content
The modes of eHealth intervention delivery included telephone (n=14) [26, 28, 61] that depends on motivational support from healthcare professionals [62]. We found that eHealth interventions containing information, monitoring and real-time communication from healthcare professionals improved CRC patients’ engagement in PA. The monitoring of behaviour is the cornerstone of a health behaviour change and is often associated with a positive result [63]. In addition, eHealth may facilitate participation for cancer patients who lack access to or cannot conveniently access PA programs in their community [64].
Strengths and limitations
This review clearly describes the methods and outlines the process of data identification and selection as well as steps to synthesise the results from individual studies and evaluate the evidence, all of which create a robust and meaningful review. The inclusion of studies with different study designs enabled a more comprehensive approach to meeting the study aims. On the other hand, even though we employed a rigorous literature search overseen by a highly experienced librarian and used a digital sorting tool for the screening of records, relevant records may have been missed. We did not exclude inadequately reported studies as doing so would not affect the findings in any meaningful way [64].
Conclusion
In this review, we identified 26 studies of eHealth interventions following the discharge of patients from the hospital after curative surgery for CRC. eHealth interventions upon hospital discharge can offer support during a critical period. This review demonstrated that eHealth interventions were mainly telephone-based, delivering education, counselling or support and monitoring symptoms or health behaviours. However, there was a lack of focus on CRC patients’ adherence to eHealth. More research is needed on adherence to eHealth programs and its relationship with the implementation of eHealth in CRC populations.
eHealth follow-up may mitigate anxiety and depression in CRC patients, while the proof of its impact on other psychological morbidities or QOL is less clear. We also did not find strong evidence of the ameliorating effects of eHealth programs regarding the side effects of cancer treatment. eHealth interventions may have a positive influence on CRC patients’ PA behaviours regardless of the user interface, but the combination of technology and human interaction appears important. In general, remote, digital follow-up was experienced as positive, accessible and usable and as an improvement to healthcare services delivery.
This review can inform future intervention research on discharge planning in CRC care. In addition, it may support clinicians working towards ensuring the uneventful and swift recovery of CRC patients. Furthermore, the findings may have value in the development of eHealth services for other cancer patient populations.
References
World Health Organization. Cancer. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 20 Feb 2023
Peterson CY, Blank J, Ludwig KA (2018) Colorectal cancer in elderly patients: considerations in treatment and management. In: Rosenthal R, Zenilman M, Katlic M (eds) Principles and Practice of Geriatric Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-47771-8_59
Boakye D, Walter V, Jansen L, Martens UM, Chang-Claude J, Hoffmeister M, Brenner H (2020) Magnitude of the age-advancement effect of comorbidities in colorectal cancer prognosis. J Natl Compr Canc Netw 18(1):59–68. https://doi.org/10.6004/jnccn.2019.7346
Osseis M, Esposito F, Lim C, Doussot A, Lahat E, Fuentes L, Moussallem T, Salloum C, Azoulay D (2018) Impact of postoperative complications on long-term survival following surgery for T4 colorectal cancer. BMC Surg 18(1):87. https://doi.org/10.1186/s12893-018-0419-y
Forsmo HM, Erichsen C, Rasdal A, Tvinnereim JM, Körner H, Pfeffer F (2018) Randomized controlled trial of extended perioperative counseling in enhanced recovery after colorectal surgery. Dis. Colon Rectum 61(6):724–732. https://doi.org/10.1097/DCR.0000000000001007
Hoang CM, Davids JS, Maykel JA, Flahive JM, Sturrock PR, Alavi K (2020) Not all discharge settings are created equal: thirty-day readmission risk after elective colorectal surgery. Dis Colon Rectum 63(9):1302–1309. https://doi.org/10.1097/DCR.0000000000001727
Jutesten H, Draus J, Frey J, Neovius G, Lindmark G, Buchwald P, Lydrup ML (2018) Late leakage after anterior resection: a defunctioning stoma alters the clinical course of anastomotic leakage. Colorec Dis. 20(2):150–159. https://doi.org/10.1111/codi.13914
Damle RN, Alavi K (2016) Risk factors for 30-d readmission after colorectal surgery: a systematic review. J Surg Res 200(1):200–207. https://doi.org/10.1016/j.jss.2015.06.052
Ang CW, Seretis C, Wanigasooriya K, Mahadik Y, Singh J, Chapman MAS (2015) The most frequent cause of 90-day unplanned hospital readmission following colorectal cancer resection is chemotherapy complications. Colorectal Dis 17(9):779–786. https://doi.org/10.1111/codi.12945
Husebø AML, Dalen I, Richardson A, Bru E, Søreide JA (2021) Factors influencing treatment burden in colorectal cancer patients undergoing curative surgery: a cross-sectional study. European J Cancer Care 30(5):e13437. https://doi.org/10.1111/ecc.13437
Sweeney-Magee M, Moustaqim-Barrette A, Gotay C, Dummer T (2020) A systematic mixed studies review of health behaviour change interventions in colorectal cancer survivors. JAdv Nurs 76(8):1936–1948. https://doi.org/10.1111/jan.14389
Global Observatory for eHealth (2022) World Health Organization. Available from: https://www.who.int/observatories/global-observatory-for-ehealth#:~:text=eHealth%20is%20the%20use%20of,an%20eHealth%20strategy%20for%20WHO. Accessed 21 Feb 2022
Gee PM, Greenwood DA, Paterniti DA, Ward D, Miller LMS (2015) The eHealth enhanced chronic care model: a theory derivation approach. J Med Internet Res 17(4):e86. https://doi.org/10.2196/jmir.4067
Storm M, Siemsen IMD, Laugaland K, Dyrstad DN, Aase K (2014) Quality in transitional care of the elderly: key challenges and relevant improvement measures. Int J Integr Care 14:e013–e013. https://doi.org/10.5334/ijic.1194
Escriva Boulley G, Leroy T, Bernetière C, Paquienseguy F, Desfriches-Doria O, Préau M (2018) Digital health interventions to help living with cancer: a systematic review of participants’ engagement and psychosocial effects. Psycho-Oncology 27(12):2677–2686. https://doi.org/10.1002/pon.4867
Chan RJ, Crichton M, Crawford-Williams F, Agbejule OA, Yu K, Hart NH, de Abreu AF, Ashbury FD, Eng L, Fitch M, Jain H, Jefford M, Klemanski D, Koczwara B, Loh K, Prasad M, Rugo H, Soto-Perez-de-Celis E, van den Hurk C, Chan A (2021) The efficacy, challenges, and facilitators of telemedicine in post-treatment cancer survivorship care: an overview of systematic reviews. Ann Oncol 32(12):1552–1570. https://doi.org/10.1016/j.annonc.2021.09.001
Ayyoubzadeh S, Kalhori S, Shirkhoda M (2020) Supporting colorectal cancer survivors using eHealth: a systematic review and framework suggestion. Supp Care Cancer 28:3543–3555. https://doi.org/10.1007/s00520-020-05372-6
World Health Organization (2003) Adherence to long-term therapies: evidence for action. World Health Organization, Geneva
O’Malley DM, Davis SN, Devine KA, Sullivan B, Bator A, Clemow L, Ferrante JM, Findley PA, Miller SM, Hudson SV (2020) Development and usability testing of the e-EXCELS tool to guide cancer survivorship follow-up care. Psycho-Oncol 29(1):123–131. https://doi.org/10.1002/pon.5222
Whittemore R, Knafl K (2005) The integrative review: updated methodology. J Adv Nurs 52(5):546–553. https://doi.org/10.1111/j.1365-2648.2005.03621.x
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Inter Med 151(4):264. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
The Endnote Team (2013) EndNote (Version 20) [softwear] Clarivate, Philadelphia, PA Available from: https://endnote.com/?language=en
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, Gagnon M-P, Griffiths F, Nicolau B, O’Cathain A, Rousseau M-C, Vedel I, Pluye P (2018) The mixed methods appraisal tool (MMAT) version 2018 for information professionals and researchers. Educ Inform 34:285–291. https://doi.org/10.3233/EFI-180221
Ritchie J, Spencer L (2002) Qualitative data analysis for applied policy research. In: Analyzing qualitative data. Routledge, pp 187–208
Avci IA, Altay B, Cavusoglu F, Cal A, Mumcu N, Eren DC, Oz O, Altin A, Karaoglanoglu O, Buberci A (2020) Evaluation of the efficacy of the three-component health care management program HEWCOT in colorectal cancer patients receiving chemotherapy. J Cancer Educ 35(2):274–283. https://doi.org/10.1007/s13187-018-1461-2
Barsom EZ, Jansen M, Tanis PJ, van de Ven AW, Blussé van Oud-Alblas M, Buskens C, J... & Schijven MP. (2021) Video consultation during follow up care: effect on quality of care and patient-and provider attitude in patients with colorectal cancer. Surg Endosc 35:1278–1287. https://doi.org/10.1007/s00464-020-07499-3
Beaver K, Campbell M, Williamson S, Procter D, Sheridan J, Heath J, Susnerwala S (2012) An exploratory randomized controlled trial comparing telephone and hospital follow-up after treatment for colorectal cancer. Colorectal Dis 14(10):1201–1209. https://doi.org/10.1111/j.1463-1318.2012.02936.x
Cheong IY, An SY, Cha WC, Rha MY, Kim ST, Chang DK, Hwang JH (2018) Efficacy of mobile health care application and wearable device in improvement of physical performance in colorectal cancer patients undergoing chemotherapy. Clin Colorectal Cancer 17(2):e353–e362. https://doi.org/10.1016/j.clcc.2018.02.002
Dong X, Sun G, Zhan J, Liu F, Ma S, Li P, Zhang C, Zhang H, **ng C, Liu Y (2019) Telephone-based reminiscence therapy for colorectal cancer patients undergoing postoperative chemotherapy complicated with depression: a three-arm randomised controlled trial. Support Care Cancer 27(8):2761–2769. https://doi.org/10.1007/s00520-018-4566-6
Drott J, Vilhelmsson M, Kjellgren K, Berterö C (2016) Experiences with a self-reported mobile phone-based system among patients with colorectal cancer: a qualitative Study. JMIR Mhealth Uhealth 4(2):e66. https://doi.org/10.2196/mhealth.5426
Drott J, Fomichov V, Starkhammar H, Börjeson S, Kjellgren K, Berterö C (2019) Oxaliplatin-induced neurotoxic side effects and their impact on daily activities: a longitudinal study among patients with colorectal cancer. Cancer Nurs 42(6). https://doi.org/10.1097/NCC.0000000000000674
Drott J, Fomichov V, Börjeson S, Berterö C (2020) Sense of coherence and health-related quality of life in patients with neurotoxicity after cancer chemotherapy: assessment from a real-time mobile phone–based system. Psycho-Oncol 29(1):107–113. https://doi.org/10.1002/pon.5243
Döking S, SS-v K, Thewes B, AMJ B, JAE C, Prins JB (2021) Combined face-to-face and online cognitive-behavioral therapy for high distress of colorectal cancer survivors: a case study. Cogn Behav Pract 28(1):107–123. https://doi.org/10.1016/j.cbpra.2020.06.008
Golsteijn RHJ, Bolman C, Volders E, Peels DA, de Vries H, Lechner L (2018) Short-term efficacy of a computer-tailored physical activity intervention for prostate and colorectal cancer patients and survivors: a randomized controlled trial. Int J Behav Nutr Phy 15(1):106. https://doi.org/10.1186/s12966-018-0734-9
Grimmett C, Simon A, Lawson V, Wardle J (2015) Diet and physical activity intervention in colorectal cancer survivors: a feasibility study. Eur J Oncol Nurs 19(1):1–6. https://doi.org/10.1016/j.ejon.2014.08.006
Kim B-Y, Park K-J, Ryoo S-B (2018) Effects of a mobile educational program for colorectal cancer patients undergoing the enhanced recovery after surgery. Open Nurs J 12:142–154. https://doi.org/10.2174/1874434601812010142
Li J, Liu X (2019) Incremental patient care program decreases anxiety, reduces depression and improves the quality of life in patients with colorectal cancer receiving adjuvant chemotherapy. Exp Ther Med 18(4):2789–2798. https://doi.org/10.3892/etm.2019.7877
Lin W-L, Sun J-L, Chang S-C, Wu P-H, Tsai T-C, Huang W-T, Tsao C-J (2014) Development and application of telephone counseling services for care of patients with colorectal cancer. Asia Pac J Cancer Prev 15(2):969–973. https://doi.org/10.7314/APJCP.2014.15.2.969
Lynch BM, Courneya KS, Sethi P, Patrao TA, Hawkes AL (2014) A randomized controlled trial of a multiple health behavior change intervention delivered to colorectal cancer survivors: effects on sedentary behavior. Cancer 120(17):2665–2672. https://doi.org/10.1002/cncr.28773
Mancini R, Bartolo M, Pattaro G, Ioni L, Picconi T, Pernazza G (2022) The role of telemedicine in the postoperative home monitoring after robotic colo-rectal cancer surgery: a preliminary single center experience. Updat Surg 74(1):171–178. https://doi.org/10.1007/s13304-021-01132-1
Mayer DK, Landucci G, Awoyinka L, Atwood AK, Carmack CL, Demark-Wahnefried W, McTavish F, Gustafson DH (2018) SurvivorCHESS to increase physical activity in colon cancer survivors: can we get them moving? J Cancer Surv 12(1):82–94. https://doi.org/10.1007/s11764-017-0647-7
Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N (2013) Home-based physical activity intervention for colorectal cancer survivors. Psycho-Onc 22(1):54–64. https://doi.org/10.1002/pon.2047
Qaderi SM, Swartjes H, Vromen H, Bremers AJ, Custers JA, de Wilt JH (2021) Acceptability, quality of life and cost overview of a remote follow-up plan for patients with colorectal cancer. Eur J Surg Oncol 47(7):1637–1644. https://doi.org/10.1016/j.ejso.2020.12.018 Get rights and content
Soh JY, Cha WC, Chang DK, Hwang JH, Kim K, Rha M, Kwon H (2018) Development and validation of a multidisciplinary mobile care system for patients with advanced gastrointestinal cancer: interventional observation study. Jmir Mhealth Uhealth 6(5):e9363. https://doi.org/10.1002/pon.2047
Van Blarigan EL, Chan H, Van Loon K, Kenfield SA, Chan JM, Mitchell E, Zhang L, Paciorek A, Joseph G, Laffan A (2019) Self-monitoring and reminder text messages to increase physical activity in colorectal cancer survivors (Smart Pace): a pilot randomized controlled trial. BMC Cancer 19(1):1–9. https://doi.org/10.1186/s12885-019-5427-5
Van der Hout A, Holtmaat K, Jansen F, Lissenberg-Witte BI, van Uden-Kraan CF, Nieuwenhuijzen G, Hardillo J, Baatenburg de Jong RJ, Tiren-Verbeet N, Sommeijer D (2021) The eHealth self-management application ‘Oncokompas’ that supports cancer survivors to improve health-related quality of life and reduce symptoms: which groups benefit most? Acta Oncol 60(4):403–411. https://doi.org/10.1080/0284186X.2020.1851764
Van Der Hout A, van Uden-Kraan CF, Holtmaat K, Jansen F, Lissenberg-Witte BI, Nieuwenhuijzen GA, Hardillo JA, De Jong RJB, Tiren-Verbeet NL, Sommeijer DW (2020) Role of eHealth application Oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: a randomised, controlled trial. Lancet Oncol 21(1):80–94. https://doi.org/10.1016/S1470-2045(19)30675-8
Vos JA, Duineveld LA, Wieldraaijer T, Wind J, Busschers WB, Sert E, Tanis PJ, Verdonck-de Leeuw IM, van Weert HC, van Asselt KM (2021) Effect of general practitioner-led versus surgeon-led colon cancer survivorship care, with or without eHealth support, on quality of life (I CARE): an interim analysis of 1-year results of a randomised, controlled trial. Lancet Oncol 22(8):1175–1187. https://doi.org/10.1016/S1470-2045(21)00273-4
Williamson S, Chalmers K, Beaver K (2015) Patient experiences of nurse-led telephone follow-up following treatment for colorectal cancer. Eur J Oncol Nurs 19(3):237–243. https://doi.org/10.1016/j.ejon.2014.11.006
Young JM, Butow PN, Walsh J, Durcinoska I, Dobbins TA, Rodwell L, Harrison JD, White K, Gilmore A, Hodge B (2013) Multicenter randomized trial of centralized nurse-led telephone-based care coordination to improve outcomes after surgical resection for colorectal cancer: the CONNECT intervention. J Clin Oncol 31(28):3585–3591. https://doi.org/10.1200/JCO.2012.48.1036
World Health Organization (2018). mHealth-use of appropriate digital technologies for public health. Available from: https://apps.who.int/iris/bitstream/handle/10665/274134/B142_20-en.pdf?sequence=1&isAllowed=y. Accessed 10 October 2023
den Bakker CM, Huirne JA, Schaafsma FG, de Geus C, Bonjer HJ, Anema JR (2019) Electronic health program to empower patients in returning to normal activities after colorectal surgical procedures: mixed-methods process evaluation alongside a randomized controlled trial. J Med Internet Res 21(1):e10674. https://doi.org/10.2196/10674
Linnan L, Steckler A (2002) Process evaluation for public health interventions and research. Jossey-Bass/Wiley
Howarth, J (2023). How many people own smartphones (2023-2028). Available from: https://explodingtopics.com/blog/smartphone-stats Assessed: 10 October 2023
Davis FD (1989) Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quarterly 13(3):319–340. https://doi.org/10.2307/249008
Jakob R, Harperink S, Rudolf AM et al (2022) Factors Influencing Adherence to mHealth Apps for Prevention or Management of Noncommunicable Diseases: Systematic Review. J Med Internet Res 24(5):e35371. https://doi.org/10.2196/35371
Penedo FJ, Dahn JR (2005) Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatr 18(2):189–193
Bergerot CD, Philip EJ, Bergerot PG, Siddiq N, Tinianov S, Lustberg M (2022) Fear of cancer recurrence or progression: what is it and what can we do about it? Am Soc Clin Oncol Edu Book 42:1–10. https://doi.org/10.1200/EDBK_100031
Lim CYS, Laidsaar-Powell RC, Young JM, Kao SC, Zhang Y, Butow P (2021) Colorectal cancer survivorship: a systematic review and thematic synthesis of qualitative research. Eur J Cancer Care 30(4):e13421. https://doi.org/10.1111/ecc.13421
Bluethmann SM, Basen-Engquist K, Vernon SW, Cox M, Gabriel KP, Stansberry SA, Carmack CL, Blalock JA, Demark-Wahnefried W (2015) Gras** the ‘teachable moment’: time since diagnosis, symptom burden and health behaviors in breast, colorectal and prostate cancer survivors. Psycho-Oncol 24(10):1250–1257. https://doi.org/10.1002/pon.3857
Husebo AM, Dyrstad SM, Soreide JA, Bru E (2013) Predicting exercise adherence in cancer patients and survivors: a systematic review and meta-analysis of motivational and behavioural factors. J Clin Nurs 22(1-2):4–21. https://doi.org/10.1111/j.1365-2702.2012.04322.x
van Achterberg T, Huisman-de Waal GG, Ketelaar NA, Oostendorp RA, Jacobs JE, Wollersheim HC (2011) How to promote healthy behaviours in patients? An overview of evidence for behaviour change techniques. Health Promot Int 26(2):148–162. https://doi.org/10.1093/heapro/daq050
Tate DF, Lyons EJ, Valle CG (2015) High-tech tools for exercise motivation: use and role of technologies such as the internet, mobile applications, social media, and video games. Diabetes Spectr 28(1):45–54. https://doi.org/10.2337/diaspect.28.1.4
Funding
Open access funding provided by University of Stavanger & Stavanger University Hospital This work was supported by the Norwegian Research Council grant number [301472] and the University of Stavanger, Norway. Alison Richardson is funded by the National Institute of Health and Care Research (NIHR) ARC Wessex.
Author information
Authors and Affiliations
Contributions
The manuscript was drafted by Anne Marie Lunde Husebø with extensive contributions from Bjørg Karlsen and Oda Karin Nordfonn. All authors contributed to the study design, data collection and analysis. All authors participated in the review, revision and approval of the final version of the manuscript before publication.
Corresponding author
Ethics declarations
Ethics approval
Not applicable since no new data from humans or animals is collected.
Conflict of interest
The authors declare no competing interests.
Research involving human participants and/or animals
Not applicable since no new data from humans or animals is collected.
Informed consent
Not applicable since no new data from humans or animals is collected.
Disclaimer
The views expressed are those of the author and not necessarily the NHS, NIHR, or Department of Health and Social Care.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Husebø, A.L.M., Søreide, J.A., Kørner, H. et al. eHealth interventions to support colorectal cancer patients’ self-management after discharge from surgery—an integrative literature review. Support Care Cancer 32, 11 (2024). https://doi.org/10.1007/s00520-023-08191-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08191-7