Summary
Ultrasound (US) guidance for implantation of cardiac implantable electronic devices (CIED) is currently not routine practice. This article sought to review published data on the use of ultrasound in each of the major surgical steps involved in implantation of CIEDs, including achieving anesthesia, obtaining venous access and implantation of leads. A literature review was performed, revealing a total of 20 peer-reviewed studies that assessed US guidance for CIED implantation; 3 of these were randomized trials while the remainder were mostly feasibility studies. The available data suggest that ultrasound can be useful in guiding implantation of CIEDs, with a trend towards less complication rates; however, more high-quality studies that compare US guidance to traditional techniques in CIED implantation are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
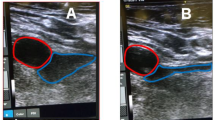
Cardiac implantable electronic devices (CIEDs) is a term that encompasses a number of devices that provide treatment for potentially fatal cardiac arrythmia. The CIEDs include devices such as pacemakers, implantable cardioverter defibrillators (ICD), and cardiac resynchronization therapy (CRT). Most CIEDs are implanted under the skin and are connected to the heart via wires (leads) travelling through large veins in the thorax to the heart delivering electrical impulses that regulate the heart’s rhythm. The CIED implantation typically involves active sedation and local anesthesia or rarely general anesthesia, percutaneous cannulation of axillary or a more central vein, guidance of lead(s) through the vein into the right heart chambers with implantation into the endocardium, and finally lead(s) being connected to pulse generator which itself is implanted in a subcutaneous pocket [1]. Figs. 1, 2, 3 and 4 were taken from our group at LHSC and highlight the key steps in implantation of CIEDs using ultrasound.
Pacemakers are implanted transvenously using either single (right ventricle), dual (right atrium and ventricle), or triple lead systems. The CRT is a triple lead pacing technology that improves cardiac function in patients with reduced ejection fraction heart failure with a wide QRS of > 130 ms. In addition to right atrial and right ventricular leads of conventional pacemakers, CRT requires a left ventricle lead that is usually traversed through the coronary sinus with the aid of fluoroscopy. There also exist leadless pacemakers that are implanted directly into the endocardium; their implantation requires the same approach for anesthesia and venous access but subvert the need for leads [2].
The ICDs can be implanted either transvenously or subcutaneously (S-ICDs). S‑ICDs eliminate the need for venous access as the lead is traversed subcutaneously and placed adjacent to the sternum [3]. By virtue of their subcutaneous lead placement, S‑ICDs have the disadvantage of requiring higher energy for defibrillation (with larger generator size) and an inability to provide antitachycardia pacing. An alternative to S‑ICDs was developed called extravascular ICD (EV-ICD) for which the lead was placed substernally to overcome limitations of S‑ICDs [4]. Nevertheless, S‑ICDs and EV-ICDs are mainly defibrillators and cannot provide extended back-up pacing. Theoretically, S‑ICDs and EV-ICDs minimize the risks associated with venous access including endocarditis, pneumothorax and cardiac perforations, although results from meta-analyses have shown mixed results [5,6,7]. They are often used in patients who have a high risk for complications or in whom vascular assess is not possible [8].
Regardless of transvenous or extravascular approach for implantation of CIEDs, ultrasound (US) can be effectively utilized for most major surgical steps outlined above, including achieving anesthesia in the form of nerve blocks, obtaining venous access, and implantation of lead(s), which are reviewed below. Other implantable devices in cardiology, such as implantable loop recorders which do not provide treatment are not covered in this review.
Methods
Studies were searched using PubMed and OVID Medline. The search strategy for OVID Medline used a combination of the following terms: cardiac pacing, defibrillator, pacemaker, cardiac resynchronization therapy, leadless, ultrasound. The search yielded 235 articles which were then screened for original research and for relevance to the topic of this review article, namely utilization of ultrasound for anesthesia, vein cannulation or optimal lead placement of CIEDs. The resulting 20 publications were included in this review article.
Figures 1, 2, 3 and 4 were captured during implantation of CIEDs in patients at the London Health Science Center (LHSC). Informed consent was obtained to utilize the ultrasound images for demonstration purposes.
US-guided anesthesia administration
Most CIEDs are currently implanted using procedural sedation with subcutaneous injection and infiltration of local anesthetic agents. This typically requires patients to fast to minimize intraprocedural aspiration risk. Furthermore, achieving analgesia can be challenging, requiring escalating doses of local anesthetic agents and sedatives which can impair wound healing and negatively impact hemodynamics [9].
Alternatively, US-guided nerve blocks can be used to achieve anesthesia. For instance, pectoral nerve blocks (PECS) involve using US guidance to administer local anesthetic agents in the fascia between the pectoralis major and minor muscles. Outside of CIED implantation, the perioperative use of US-guided nerve blocks in chest wall surgery has demonstrated a reduction in doses of local anesthetics and periprocedural narcotics [10,11,12].
There are some case reports and feasibility studies assessing US-guided nerve blocks in CIEDs. Antiperovitch et al. demonstrated that a cardioverter-defibrillator could be implanted with only US-guided pectoral and supraclavicular nerve blocks; the patient did not require any sedation or anesthetic, and was pain-free before and after the procedure [13]. A feasibility study by Boyzel et al. showed that only 4 of 36 patients required postprocedural analgesia following implantation of CIED with US-guided PECS nerve block [14]. Similarly, in a non-randomized trial of patients undergoing S‑ICD, Uran et al. showed that combined US-guided serratus anterior plane block and parasternal block required lower doses of local anesthetics and IV analgesia, and less procedural time compared with parasternal block alone or conventional local anesthesia and sedation [15]. In addition to the observed benefits of US-guided nerve blocks in these studies, theoretical benefits include less harmful analgesia to patients who have documented analgesia intolerance, are hemodynamically unstable, older, or those with neuromuscular diseases [16, 17]. Thus, US-guided nerve blocks for implantation of CIEDs is promising but requires more comparative trials to determine noninferiority, if not superiority.
US-guided venous access
Historically, blind (non-US-guided) subclavian vein cannulation was used to implant CIEDs as it was fast, easy to learn and offered high success rates [18, 19]; however, this approach is associated with higher rates of early (pneumothorax, hemothorax) and late (lead crush) complications [20]. To minimize complications, alternative approaches for vein cannulation were developed including a) fluoroscopy, whereby the axillary or a more proximal vein is cannulated using X‑rays with or without IV contrast for landmarking or b) cephalic vein cut down, whereby the cephalic vein is exposed by surgical dissection of the deltopectoral groove and then cannulated. The use of fluoroscopy has demonstrated higher success rates (~95% vs. 60–85%) and shorter procedure duration time [21]. Cephalic vein cut down reduces the risk of complications compared to subclavian vein cannulation, but no difference was found compared to axillary vein cannulation in meta-analyses [21,22,23].
US-guided venous access is common practice and is strongly recommended by the American Society of Anesthesiologists particularly for cannulation of internal jugular veins given superior outcomes [24]. At the time of this review, a number of retrospective and prospective (including two randomized controlled trials, RCT) have assessed US-guided axillary vein cannulation (AVC) for implantation of CIEDs (Table 1). Most of these studies showed noninferiority in success rates when comparing US-guided cannulation to traditional forms of cannulation with or without fluoroscopy. Of the two RCTs published, Tagliari et al. showed superiority of US guidance in achieving cannulation, although the comparison was with cephalic vein cut down, which is known to have inferior success rates; interestingly, the complications rates were similar despite cephalic vein cut down being considered the safest form of cannulation [25]. The other RCT published by Liccardo et al. compared US-guided to blind cannulation; this study showed noninferiority in success rates. The remaining comparative studies showed a nonsignificant trend towards less complication rates following US-guided cannulation.
Although US-guided cannulation for CIED implantation is becoming more commonplace, it is not yet practiced in the majority of centers or guideline-based. While the studies outlined in this review lend support for US-guided axillary cannulation for venous access in CIEDs, more studies, particularly randomized controlled trials are needed.
US-guided lead implantation, assessment and extraction
Although the number of published studies are few, transthoracic echocardiography (TTE) has been used for the placement of temporary transvenous pacing leads in various clinical settings (Table 2). To our knowledge, no large studies have thus far evaluated real-time US-guided placement of leads for permanent pacemakers. A case report of a full implant by TTE was reported recently with a good example of how the US can aid lead guidance past the tricuspid valve into the right ventricular septum [35]. A recently published case series of a single operator experience demonstrated safe use of ultrasound for the entire duration of single chamber CIED implants in a selected population [36]. However, Saba et al. demonstrated that speckle tracking echocardiography could be used to identify sites of last activation of myocardium and thus guide optimal implantation of left ventricle (LV) lead using fluoroscopy for CRT; in their 2013 RCT (n = 187), patients enrolled in the echocardiography-guided LV lead placement group had a significantly longer time to heart failure hospitalization or death compared to the routine fluoroscopy group (hazard ratio, HR 0.48, confidence interval, 95% CI 0.28–0.82, p = 0.006) [37].
Separately, lead assessment and extraction has been demonstrated with ultrasound guidance. Beaser et al. showed that using TTE among 60 patients, the degree of ultrasound-graded intravascular lead adherence correlated significantly with difficulty of lead extraction [43]. Transesophageal echocardiography (TEE) can also be used; during lead extraction of CIEDs, TEE can identify potential complications as well as guide management if complications arise [44, 45].
Conclusion
The use of ultrasound is a relatively recent technique to improve the deliverance of anesthesia in the form of nerve blocks, for cannulation of axillary vein, and for implantation of temporary CEID leads. The data suggest great potential for using ultrasound in CEID implantation and management. US-guidance might become more necessary as pacemaker techniques require more precision, for instance, in conductive system pacing where good visualization of the interventricular septum is beneficial during lead implantation. The dearth of literature in this field is likely multifactorial, including the difficulty in trial design (blinding, maintaining clinical equipoise) and perhaps the perception that fluoroscopy guidance is reliable and good enough; however, use of fluoroscopy can result in high expenses associated with using fluoroscopy machines and protecting medical professionals from potential musculoskeletal injuries due to extended periods of wearing radiation protection gear. More studies are actively recruiting to evaluate the feasibility of US guidance CEID implantation such as the RADICAL USE study (NCT04858698). Further studies should be undertaken to discern the comparative effectiveness of US-guided versus traditional CEID implantation alongside surgeon training to get familiar with US views that will assist in the procedures.
References
Kotsakou M, et al. Pacemaker insertion. Ann Transl Med. 2015;3:1–8.
Reddy VY, et al. Percutaneous implantation of an entirely Intracardiac leadless pacemaker. N Engl J Med. 2015;373:1125–35.
Bardy GH, et al. An entirely subcutaneous implantable cardioverter—defibrillator. N Engl J Med. 2010;363:36–44.
Friedman P, et al. Efficacy and safety of an extravascular implantable cardioverter—defibrillator. N Engl J Med. 2022;387:1292–302.
Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35:1186–94.
Rordorf R, et al. Subcutaneous versus transvenous implantable defibrillator: An updated meta-analysis. Heart Rhythm. 2021;18:382–91.
Knops RE, et al. Subcutaneous or Transvenous Defibrillator Therapy. N Engl J Med. 2020;383:526–36.
Thompson AE, et al. The development of the extravascular defibrillator with substernal lead placement: A new Frontier for device-based treatment of sudden cardiac arrest. J Cardiovasc Electrophysiol. 2022;33:1085.
Bentov I, Reed MJ. Anesthesia, microcirculation, and wound repair in aging. Anesthesiology. 2014;120:760–72.
Bashandy GMN, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery: a randomized clinical trial. Reg Anesth Pain Med. 2015;40:68–74.
Wahba SS, Kamal SM. Thoracic paravertebral block versus pectoral nerve block for analgesia after breast surgery. Egypt J Anaesth. 2014;30:129–35.
Blanco R. The ‘pecs block’: a novel technique for providing analgesia after breast surgery. Anaesthesia. 2011;66:847–8.
Antiperovitch P, Mokhtar AT, Mian M, Yee R, Khan HR. A Novel Nerve Block Technique for a Patient Undergoing Cardiac Device Implantation. Jacc Case Rep. 2022;4. https://doi.org/10.1016/j.jaccas.2022.08.028.
Bozyel S, Yalnız A, Aksu T, Guler TE, Genez S. Ultrasound-guided combined pectoral nerve block and axillary venipuncture for the implantation of cardiac implantable electronic devices. Pace—pacing Clin Electrophysiol. 2019; https://doi.org/10.1111/pace.13725.
Uran C, et al. Ultrasound-guided serratus anterior plane block combined with parasternal block in subcutaneous implantable cardioverter defibrillator implantation: Results of a pilot study. Pacing Clin Electrophysiol. 2020;43:705–12.
Looi KL, et al. Conscious sedation and analgesia use in cardiac device implantation. Int J Cardiol. 2013;168:561–3.
Tobias JD, Leder M. Procedural sedation: A review of sedative agents, monitoring, and management of complications. Saudi J Anaesth. 2011;5. https://doi.org/10.4103/1658-354X.87270.
Kessinger JM, Holter AR, Geha AS. Implantation of permanent transvenous pacemaker via subclavian vein. Arch Surg. 1982;117:1105–7.
Littleford PO, Parsonnet V, Spector SD. Method for the rapid and atraumatic insertion of permanent endocardial pacemaker electrodes through the subclavian vein. Am J Cardiol. 1979;43:980–2.
Lau EW. Upper body venous access for transvenous lead placement—Review of existent techniques. Pace—pacing Clin Electrophysiol. 2007;30:901–9.
Calkins H, et al. Prospective randomized comparison of the safety and effectiveness of placement of endocardial pacemaker and defibrillator leads using the extrathoracic subclavian vein guided by contrast venography versus the cephalic approach. Pacing Clin Electrophysiol. 2001;24:456–64.
Atti V, et al. Subclavian and axillary vein access versus cephalic vein cutdown for cardiac implantable electronic device implantation: a meta-analysis. JACC Clin Electrophysiol. 2020;6:661–71.
Benz AP, Vamos M, Erath JW, Hohnloser SH. Cephalic vs. subclavian lead implantation in cardiac implantable electronic devices: a systematic review and meta-analysis. Europace. 2019;21:121–9.
Practice Guidelines for Central Venous Access 2020: An Updated Report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2020;132:8–43.
Tagliari AP, et al. Axillary vein puncture guided by ultrasound vs cephalic vein dissection in pacemaker and defibrillator implant: A multicenter randomized clinical trial. Heart Rhythm. 2020;17:1554–60.
Liccardo M, Nocerino P, Gaia S, Ciardiello C. Efficacy of ultrasound-guided axillary/subclavian venous approaches for pacemaker and defibrillator lead implantation: a randomized study. J Interv Cardiac Electrophysiol. 2018;51:153–60.
Chandler JK, et al. Ultrasound guided axillary vein access: An alternative approach to venous access for cardiac device implantation. J Cardiovasc Electrophysiol. 2020;32:458–65.
Migliore F, et al. Axillary vein access for permanent pacemaker and implantable cardioverter defibrillator implantation: Fluoroscopy compared to ultrasound. Pacing Clin Electrophysiol. 2020;43:566–72.
Lin J, et al. Feasibility of ultrasound-guided vascular access during cardiac implantable device placement. J Interv Cardiac Electrophysiol. 2017;50:105–9.
Deluca G, et al. Ultrasound-guided venipuncture for implantation of cardiac implantable electronic devices: A single-center, retrospective study. Pacing Clin Electrophysiol. 2020;43:713–9.
Ahmed AS, et al. Predictors of successful ultrasound-guided lead implantation. Pacing Clin Electrophysiol. 2020;43:217–22.
Clark BC, Janson CM, Nappo L, Pass RH. Ultrasound-guided axillary venous access for pediatric and adult congenital lead implantation. Pace—pacing Clin Electrophysiol. 2019;42:166–70.
Esmaiel A, Hassan J, Blenkhorn F, Mardigyan V. The use of ultrasound to improve axillary vein access and minimize complications during pacemaker implantation. Pacing Clin Electrophysiol. 2016;39:478–82.
Nash A, Burrell CJ, Ring NJ, Marshall AJ. Evaluation of an ultrasonically guided venepuncture technique for the placement of permanent pacing electrodes. Pace—pacing Clin Electrophysiol. 1998;21:452–5.
Khan HR. A first-in-human complete insertion of single-chamber cardiac pacemaker using ultrasound. Jacc Case Rep. 2022;4:101528.
Khan HR, Moustafa AT, Triemstra S, et al. Vascular and Cardiac Ultrasound as the Primary Imaging Tool to safely deliver pacing leads while implanting Single Chamber Permanent Pacemakers: A single operator experience in a tertiary cardiac centre. Heart Rhythm. 2023; https://doi.org/10.1016/j.hrthm.2023.05.032.
Saba S, et al. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy results of the speckle tracking assisted resynchronization therapy for electrode region trial. Circ Heart Fail. 2013;6:427–34.
Ferri LA, et al. Emergent transvenous cardiac pacing using ultrasound guidance: a prospective study versus the standard fluoroscopy-guided procedure. Eur Heart J Acute Cardiovasc Care. 2016;5:125–9.
Pinneri F, et al. Echocardiography-guided versus fluoroscopy-guided temporary pacing in the emergency setting: An observational study. J Cardiovasc Med. 2013;14:242–6.
Sjaus A, Fayad A. The use of subcostal echocardiographic views to guide the insertion of a right ventricular temporary transvenous pacemaker—description of the technique. J Cardiothorac Vasc Anesth. 2019;33:2797–803.
Aguilera PA, Durham BA, Riley DC. Emergency transvenous cardiac pacing placement using ultrasound guidance. Ann Emerg Med. 2000;36:224–7.
Jesus I, Pereira S, Camacho A, Leiria G. Echocardiography-guided temporary implantation of electrode catheters: an alternative with reliable results even during prolonged use. Rev Port Cardiol. 1992;11:655–8.
Beaser AD, et al. Characterization of lead adherence using Intravascular ultrasound to assess difficulty of transvenous lead extraction. Circ Arrhythm Electrophysiol. 2020; https://doi.org/10.1161/CIRCEP.119.007726.
Oestreich BA, et al. Use of transesophageal echocardiography to improve the safety of transvenous lead extraction. JACC Clin Electrophysiol. 2015;1:442–8.
Burkett DA, Runciman M, Jone PN, Collins KK. Transesophageal three-dimensional echocardiographic guidance for pacemaker lead extraction. Pacing Clin Electrophysiol. 2021;44:641–50.
Funding
The publication costs of this article were supported by the Academic Medical Organization of Southwestern Ontario (AMOSO) Innovation Fund grant INN21-013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Mian and H.R. Khan declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained for using the ultrasound images used in this review article. Consent for publication: for this type of article consent for publication is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mian, M., Khan, H.R. Ultrasound utilization for implantation of cardiac implantable electronic devices. Wien Klin Wochenschr 135, 712–718 (2023). https://doi.org/10.1007/s00508-023-02215-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-023-02215-2