Abstract
The growing need in the current market for innovative solutions to obtain lactose-free (L-F) milk is caused by the annual increase in the prevalence of lactose intolerance inside as well as the newborn, children, and adults. Various configurations of enzymes can yield two distinct L-F products: sweet (β-galactosidase) and unsweet (β-galactosidase and glucose oxidase) L-F milk. In addition, the reduction of sweetness through glucose decomposition should be performed in a one-pot mode with catalase to eliminate product inhibition caused by H2O2. Both L-F products enjoy popularity among a rapidly expanding group of consumers. Although enzyme immobilization techniques are well known in industrial processes, new carriers and economic strategies are still being searched. Polymeric carriers, due to the variety of functional groups and non-toxicity, are attractive propositions for individual and co-immobilization of food enzymes. In the presented work, two strategies (with free and immobilized enzymes; β-galactosidase NOLA, glucose oxidase from Aspergillus niger, and catalase from Serratia sp.) for obtaining sweet and unsweet L-F milk under low-temperature conditions were proposed. For free enzymes, achieving the critical assumption, lactose hydrolysis and glucose decomposition occurred after 1 and 4.3 h, respectively. The tested catalytic membranes were created on regenerated cellulose and polyamide. In both cases, the time required for lactose and glucose bioconversion was extended compared to free enzymes. However, these preparations could be reused for up to five (β-galactosidase) and ten cycles (glucose oxidase with catalase).
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
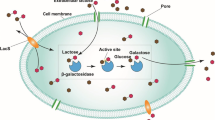
Lactose intolerance is a prevalent ailment affecting a significant portion of the global population. According to epidemiological data, up to 70% of the world’s population may be affected by this dysfunction [1]. Lactose intolerance is manifested by inadequate enzyme β-galactosidase (β-gal) required for lactose hydrolysis in the small intestine. Consequently, consumption of lactose-containing foods (both dairy and non-dairy products) by individuals with β-galactosidase deficiency results in gastrointestinal discomfort [2, 3]. One of the easiest ways to deal with lactose intolerance is its elimination from the daily diet by replacing traditional dairy with lactose-free (L-F) products. Nowadays, L-F-labeled dairy products are perceived as functional foods, substitutes for whole milk, and a low-cost dietary source of calcium with a wide range of availability [4, 5].
In recent years, there has been growing interest in finding technologies that provide nutritional value and functional, sensory, and quality properties of L-F dairy products [4]. Among the existing methods for removing lactose from food are membrane separation, fermentation, and enzymatic hydrolysis. The last one has gained high popularity in industrial technologies [4, 6]. It is the result of high specificity and relatively low cost of bioconversion (especially in the case of immobilized preparations) [7]. In the enzymatic approach, two strategies are proposed: batch, in which enzyme is added before pasteurization, and aseptic, in which the sterile enzyme is added to UHT milk before packaging [6].
Enzymatic lactose hydrolysis allows the production of two kinds of L-F milk: sweet and unsweet, Fig. 1. To get the first, the one-enzyme pathway with β-galactosidase is necessary. The higher sweetness of L-F milk is caused by the products of lactose hydrolysis, glucose, and galactose, both of which have a higher sweetness index than lactose. Therefore, L-F milk is gaining popularity in producing low-calorie dairy desserts without supplementary sweeteners [8]. Receiving unsweet L-F milk is more complicated and involves three enzymatic pathways [9]. Because the noticeable sweetness of L-F milk is mainly the result of glucose [10], its decomposition by glucose oxidase (GOX) is desirable [11]. Although catalase (CAT) does not directly participate in the decomposition of glucose, its presence, mainly in the one-pot mode, is necessary to maintain the high enzymatic activity of GOX inhibited by hydrogen peroxide. Unsweet L-F milk is dedicated to an increasingly more extensive group of consumers who negatively perceive the sweetness of L-F milk [12]. Furthermore, unsweet L-F milk, due to its similar taste to traditional milk, can be applied in savory dishes and compete with non-dairy milk alternatives [13].
Enzymatic pathways to obtain sweet and unsweet L-F milk. The red lines represent the step with product inhibition caused by H2O2, and the green lines show benefits related to the presence of catalase in the reaction mixture (decomposition of H2O2 and generation of O2, which acts as a substrate for GOX) (colour figure online)
Industrial biocatalysis is closely related to enzyme immobilization, hel** to overcome the limitation of free enzymes [14, 49, 50], but others offer a poorer immobilization efficiency by covalent bonding on the PDA coating membrane [51]. The satisfactory results of the covalent immobilization of catalase on polyamide have also been confirmed by literature reports [52, 53].
The proposed covalent immobilization of GOX and CAT onto RC membrane via hydroxyl groups exhibited lower efficiency compared to PA membrane. It is worth noting that the GOX bond involving glutaraldehyde, as highlighted in literature reports, is superior. This bond suggests the conversion of originally occurring hydroxyl groups of PMMA microspheres to aldehyde groups, a crucial step for efficient GOX immobilization [54]. Other references indicated the function of RC membrane solely as a separating layer for free GOX and CAT, allowing enzyme retention in the reactor space [55, 56].
It is essential to recognize the versatility of the two distinct unit processes—lactose hydrolysis for producing sweet L-F milk and glucose decomposition for obtaining L-F milk with a traditional taste. This versatility is a testament to the potential of using two different carriers for immobilization (RC membrane for NOLA and PA membrane for GOX and CAT), which should not be seen as an obstacle to future industrial processes.
The presented results indicate the high stability of catalytic membranes with co-immobilized enzymes onto polyamide. These results are notably intriguing since a polyamide membrane has not been widely discussed in the literature as a preferred carrier for the co-immobilization of GOX and CAT. Instead, the proposition of an ultrafiltration membrane composed of an acrylonitrile copolymer [57] or an ultrafiltration membrane made of polypropylene with a skin layer of RC can be found [58]. Polyamide materials, available in membranes, gels, and non-wovens, are widely acknowledged as popular carriers for enzyme immobilization. These materials gained popularity due to their porous structure, chemical and thermal resistance, resistance to biodegradation, and mechanical properties that enable them to withstand increased pressure in membrane flow bioreactors [59]. Some polyamide carriers require partial hydrolysis of the membrane surface to generate more reactive amine groups necessary for enzyme immobilization [60]. Examples of immobilized preparations created on polyamide (PA) and tailored for industrial applications include laccase bonded on functionalized polyamide 6,6 with an immobilization yield of 2% [60], β-xylosidase bonded on a microfiltration polyamide membrane with an immobilization yield of 20% [61], and laccase bonded on polyamide fabric hydrolyzed by bromelain with an immobilization yield of 68% [62].
The literature review focusing on carriers dedicated to GOX and CAT co-immobilization highlights the potential of the one-step strategy of co-immobilization onto polyamide membranes, which was presented in this work. Furthermore, the results suggest achieving a higher value of the bonded mass of enzymes in co-immobilized preparations, exceeding 0.83 mg to accomplish a bioconversion time close to free enzymes. This goal, whether for one-enzyme or two-enzyme immobilization, can be achieved, e.g., by increasing the surface area of the carrier.
The proposed strategy of immobilizing NOLA, GOX, and CAT via covalent binding on microfiltration membranes (RC and PA) was less effective than encapsulated preparations previously described by the authors [9, 27]. The lower activity of the catalytic membranes may be attributed to enzyme denaturation upon immobilization, changes in active-site conformation, and crowding of enzyme molecules on the carrier [45]. Despite the higher enzymatic activity of encapsulated preparations, their utilization in continuous processes, such as in packed bead reactors, may pose difficulties in maintaining a constant porosity of the deposit (alginate beads). Furthermore, the comparison of enzymes reused in subsequent cycles for encapsulated preparations and catalytic membranes with NOLA, GOX, and CAT [9, 27] indicated a decrease in enzymatic activity in each successive cycle, regardless of the form of the immobilized preparation, suggesting enzymes’ instability under process conditions.
Conclusion
The market potential for L-F products is still growing. Thanks to the enzymatic approach and immobilization technique, lactose hydrolysis can be recognized as an efficient and economical method for obtaining two types of L-F milk (sweet and unsweet) at low temperatures, similar to those used in storing and transporting raw milk. Catalytic membranes featuring individually immobilized enzymes and co-immobilized preparations are a prime illustration of multidisciplinary technology that aligns with the increasing demand for highly efficient biocatalysis in the dairy industry. However, creating catalytic membranes without the time and cost-consuming modification of membrane surface chemistry remains challenging. Fundamental limitations are associated with the low affinity of enzyme molecules to functional groups of the membrane and the risk of excessive stiffening of the spatial structure of the enzyme. Nevertheless, the stable connection of the enzyme with the carrier via covalent interactions enabled the catalytic membranes to be reused multiple times. In addition, the high mechanical stability of catalytic membranes allows their application in continuous industrial processes, offering significant opportunities for optimization at various stages. Nonetheless, the development of novel, cost-effective immobilization strategies that ensure high catalyst loading, elevated activity, and long-term stability of catalytic membranes remains a persistent challenge highlighting the need for ongoing research and innovation in this field.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Lee K, Erdle S, Mak R (2022) Lactose intolerance. Reference Module Food Sci. https://doi.org/10.1016/B978-0-323-96018-2.00031-6
Rocha JM, Guerra A (2020) On the valorization of lactose and its derivatives from cheese whey as a dairy industry by-product: an overview. Eur Food Res Technol. https://doi.org/10.1007/s00217-020-03580-2
Li A, Zheng J, Han X et al (2023) Health implication of lactose intolerance and updates on its dietary management. Int Dairy J. https://doi.org/10.1016/J.IDAIRYJ.2023.105608
Li A, Zheng J, Han X et al (2023) Advances in low-lactose/lactose-free dairy products and their production. Foods. https://doi.org/10.3390/FOODS12132553
Swiader K, Kulawiak M, Chen Y-P (2020) Types of lactose-free products and their availability on the Polishmarket. Food Eng 1:39–45
Dekker PJT, Koenders D, Bruins MJ (2019) Lactose-free dairy products: market developments, production, nutrition and health benefits. Nutrients. https://doi.org/10.3390/nu11030551
Qi T, Yang D, Chen X et al (2022) Rapid removal of lactose for low-lactose milk by ceramic membranes. Sep Purif Technol. https://doi.org/10.1016/J.SEPPUR.2022.120601
Abbasi S, Saeedabadian A (2015) Influences of lactose hydrolysis of milk and sugar reduction on some physical properties of ice cream. J Food Sci Technol. https://doi.org/10.1007/s13197-013-1011-1
Czyzewska K, Trusek A (2023) Critical parameters in an enzymatic way to obtain the unsweet lactose-free milk using catalase and glucose oxidase co-encapsulated into hydrogel with chemical cross-linking. Foods. https://doi.org/10.3390/FOODS12010113/S1
Qi X, Tester RF (2019) Fructose, galactose and glucose—In health and disease. Clin Nutr ESPEN. https://doi.org/10.1016/J.CLNESP.2019.07.004
Patent CN107475050B (2017) Method for reducing sweetness of sugar-containing beverage by usingbiological enzyme, https://patents.google.com/patent/CN107475050B/en
Rizzo PV, Harwood WS, Drake MA (2020) Consumer desires and perceptions of lactose-free milk. J Dairy Sci. https://doi.org/10.3168/JDS.2019-17940
Singh G, Vij R, Katoch S (2021) Comparison of dairy milk with vegan milk of different types available in India. Pharma Innov J 10:24–29
Yushkova ED, Nazarova EA, Matyuhina AV et al (2019) Application of immobilized enzymes in food industry. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.9b04385
**e J, Zhang Y, Simpson B (2022) Food enzymes immobilization: novel carriers, techniques and applications. Curr Opin Food Sci. https://doi.org/10.1016/J.COFS.2021.09.004
Rodrigues RC, Berenguer-Murcia Á, Carballares D et al (2021) Stabilization of enzymes via immobilization: multipoint covalent attachment and other stabilization strategies. Biotechnol Adv. https://doi.org/10.1016/J.BIOTECHADV.2021.107821
Yılmaz-Karaoğlu S, Gürel-Gökmen B, Tunali-Akbay T (2022) Lactose hydrolyzing activity of the lactase immobilized polycaprolactone and silk fibroin-based nanofiber and nitrocellulose membrane. Food Biosci. https://doi.org/10.1016/J.FBIO.2022.101828
Ansari SA, Satar R, Zaidi SK, Ahmad A (2014) Immobilization of Aspergillus oryzae β-galactosidase on cellulose acetate-polymethylmethacrylate membrane and its application in hydrolysis of lactose from milk and whey. Int Sch Res Notices. https://doi.org/10.1155/2014/163987
El-Masry MM, De Maio A, Martelli PL, et al (2001) Influence of the immobilization process on the activity of β-galactosidase bound to Nylon membranes grafted with glycidyl methacrylate: part 1. Isothermal behavior. J Mol Catal B Enzym. https://doi.org/10.1016/S1381-1177(01)00061-3
Jochems P, Satyawali Y, Diels L et al (2011) Enzyme immobilization on/in polymeric membranes: status, challenges and perspectives in biocatalytic membrane reactors (BMRs). Green Chem. https://doi.org/10.1039/c1gc15178a
Jeyaraman SN, Slaughter G (2021) Membranes, immobilization, and protective strategies for enzyme fuel cell stability. Curr Opin Electrochem. https://doi.org/10.1016/j.coelec.2021.100753
Trusek A (2005) A catalytic membrane for hydrolysis reaction carried out in the two-liquid phase system—Membrane preparation and characterisation, mathematical model of the process. J Mem Sci. https://doi.org/10.1016/jmemsci.2005.03.047
Bié J, Sepodes B, Fernandes PCB, Ribeiro MHL (2022) Enzyme immobilization and co-immobilization: main framework, advances and some applications. Processes. https://doi.org/10.3390/PR10030494
Hu D, Li R, Han Y et al (2023) Co-immobilization of PPL and GOx on DUT-5/PVDF hybrid membranes and catalytic activity in the cascade oxidation of glucose and styrene. New J Chem. https://doi.org/10.1039/D2NJ05293H
Ahmad R, Shanahan J, Rizaldo S et al (2020) Co-immobilization of an enzyme system on a metal-organic framework to produce a more effective biocatalyst. Catalysts. https://doi.org/10.3390/catal10050499
Arana-Peña S, Carballares D, Morellon-Sterlling R et al (2020) Enzyme co-immobilization: always the biocatalyst designers’ choice…or not? Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2020.107584
Czyzewska K, Trusek A (2021) Encapsulated NOLA™ Fit 5500 lactase: an economically beneficial way to obtain lactose-free milk at low temperature. Catalysts. https://doi.org/10.3390/CATAL11050527
Czyzewska K, Trusek-Holownia A, Dabrowa M et al (2019) A catalytic membrane used for H2O2 decomposition. Catal Today. https://doi.org/10.1016/J.CATTOD.2017.11.025
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem. https://doi.org/10.1016/s0021-9258(19)52451-6
Sutay Kocabaş D, Lyne J, Ustunol Z (2022) Hydrolytic enzymes in the dairy industry: applications, market and future perspectives. Trends Food Sci Technol. https://doi.org/10.1016/J.TIFS.2021.12.013
Czyżewska K, Trusek A (2018) Catalytic membrane with the recombinant catalase from psychrotolerant bacteria Serratia sp. in dairy applications. Desalination Water Treat. https://doi.org/10.5004/dwt.2018.22869
Pawlos M, Znamirowska A, Kluz M, et al (2020) Low-lactose fermented goat milks with Bifidobacterium animalis SSP. lactis BB-12. J Microbiol, Biotechnol Food Sci. https://doi.org/10.15414/JMBFS.2020.9.4.751-755
Sarmiento F, Peralta R, Blamey JM (2015) Cold and hot extremozymes: industrial relevance and current trends. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2015.00148
Zagorska J, Ciprovica I, Straumite E, Majore K (2020) Acceptance of low-sugar yoghurt among latvian teenagers. Agron Res. https://doi.org/10.15159/AR.20.111
Luzzi G, Steffens M, Clawin-Rädecker I et al (2020) Enhancing the sweetening power of lactose by enzymatic modification in the reformulation of dairy products. Int J Dairy Technol. https://doi.org/10.1111/1471-0307.12681
Tao Z, Raffel RA, Souid A, Goodisman J (2009) Kinetic studies on enzyme-catalyzed reactions: oxidation of glucose, decomposition of hydrogen peroxide and their combination. Biophysj. https://doi.org/10.1016/j.bpj.2008.11.071
Pečar D, Vasić-rački Đ, Presečki AV (2018) Immobilization of glucose oxidase on Eupergit C : impact of aeration, kinetic and operational stability studies of free and immobilized enzyme. Chem Biochem Eng Q. https://doi.org/10.15255/CABEQ.2018.1391
Karlsson MA, Langton M, Innings F et al (2019) Changes in stability and shelf-life of ultra-high temperature treated milk during long term storage at different temperatures. Heliyon. https://doi.org/10.1016/J.HELIYON.2019.E02431
GSFA Online Food Additive details for sodium hydroxide. https://www.fao.org/gsfaonline/additives/details.html?id=256&d-3586470-s=2&d-3586470-o=1&lang=
Phadungath C, Metzger LE (2011) Effect of sodium gluconate on the solubility of calcium lactate. J Dairy Sci. https://doi.org/10.3168/JDS.2011-4549
Qing W, Li X, Shao S et al (2019) Polymeric catalytically active membranes for reaction-separation coupling: a review. J Memb Sci. https://doi.org/10.1016/J.MEMSCI.2019.04.053
Trusek A, Wajsprych M, Noworyta A (2021) Low- and high-pressure membrane separation in the production of process water for coke quenching. Membranes. https://doi.org/10.3390/membranes11120937
Rautenbach R, Albrecht R (1989) Membrane processes. Wiley & Sons Ltd, UK.
Beddows CG, Mirauer RA, JGuthrie JT, et al (1979) The use of graft copolymers as enzyme supports. Polym Bull. https://doi.org/10.1007/BF00256275
Bhattacharyya D, Ganapathi S, Vishwanath S, et al (1996). Immobilized enzyme reactions on beads and membranes. In: Butterfield DA (eds) Biofunctional membranes. Springer, Boston, MA. https://doi.org/10.1007/978-1-4757-2521-6_8
Ansari SA, Satar R, Kashif Zaidi S, Ahmad A (2014) Immobilization of Aspergillus oryzae β -galactosidase on cellulose acetate-polymethylmethacrylate membrane and its application in hydrolysis of lactose from milk and whey. Int Sch Res Notices. https://doi.org/10.1155/2014/163987
Palai T, Singh AK, Bhattacharya PK (2014) Enzyme, β-galactosidase immobilized on membrane surface for galacto-oligosaccharides formation from lactose: kinetic study with feed flow under recirculation loop. Biochem Eng J. https://doi.org/10.1016/j.bej.2014.03.017
Bodnár M, Fazekas E, Nagy T et al (2023) Synthesis of Galacto-oligosaccharides in milk by using Bifidobacterium bifidum β-galactosidases (Saphera 2600L and Nola Fit 5500) immobilized on chitosan beads. Food Bioproc Tech. https://doi.org/10.1007/S11947-023-03222-X/TABLES/2
Pisoschi AM, Danet A (2006) Comparison between nylon-based and nitrocellulose-based potentiometric biosensors in glucose assessment. Analele UniversităŃii din Bucuresti—Chimie Anul XV 1:39–44
Vasileva N, Godjevargova T (2004) Study on the behaviour of glucose oxidase immobilized on microfiltration polyamide membrane. J Memb Sci. https://doi.org/10.1016/J.MEMSCI.2004.02.009
Zhang H, Luo J, Li S et al (2018) Biocatalytic membrane based on polydopamine coating: a platform for studying immobilization mechanisms. Langmuir. https://doi.org/10.1021/ACS.LANGMUIR.7B02860/ASSET/IMAGES/LARGE/LA-2017-02860Q_0009.JPEG
Kiehl K, Straube T, Opwis K, Gutmann JS (2015) Strategies for permanent immobilization of enzymes on textile carriers. Eng Life Sci. https://doi.org/10.1002/ELSC.201400148
Opwis K, Courth K, Gutmann JS, et al (2014) Various strategies for the immobilization of biocatalysts on textile carrier materials. https://doi.org/10.3303/CET1438038
Bulmuş V, Ayhan H, Pişkin E (1997) Modified PMMA monosize microbeads for glucose oxidase immobilization. Chem Eng J Biochem Eng J. https://doi.org/10.1016/S0923-0467(96)03156-9
Tao Z, Raffel RA, Souid A et al (2009) Kinetic studies on enzyme-catalyzed reactions: oxidation of glucose, decomposition of hydrogen peroxide and their combination. Biophys J. https://doi.org/10.1016/j.bpj.2008.11.071
Yotova LK, Ivanov IP (1997) Simultaneous immobilization of glucose oxidase and peroxidase to urea derivative of regenerated acetylcellulose granules. Appl Biochem Biotechnol. https://doi.org/10.1007/BF02787801
Godjevargova T, Dayal R, Marinov I (2004) Simultaneous covalent immobilization of glucose oxidase and catalase onto chemically modified acrylonitrile copolymer membranes. J Appl Polym Sci. https://doi.org/10.1002/APP.13617
Morthensen ST, Meyer AS, Jørgensen H, Pinelo M (2017) Significance of membrane bioreactor design on the biocatalytic performance of glucose oxidase and catalase: free vs. immobilized enzyme systems. Biochem Eng J. https://doi.org/10.1016/J.BEJ.2016.09.015
Vasileva N, Godjevargova TS (2004) Study on the behaviour of glucose oxidase immobilized on microfiltration polyamide membrane. J Membr Sci. https://doi.org/10.1016/j.memsci.2004.02.009
Silva C, Silva CJ, Zille A et al (2007) Laccase immobilization on enzymatically functionalized polyamide 6,6 fibres. Enzyme Microb Technol. https://doi.org/10.1016/j.enzmictec.2007.07.010
Delcheva G, Dobrev G, Pishtiyski I (2008) Performance of Aspergillus niger B 03 β-xylosidase immobilized on polyamide membrane support. J Mol Catal B Enzym. https://doi.org/10.1016/j.molcatb.2007.12.019
Song JE, Kim HR (2019) Effect of enzymatic hydrolysis on develo** support of polyamide woven fabric for enzyme immobilization. Text Res J. https://doi.org/10.1177/0040517518767148
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
KC: conceptualization, methodology, software, formal analysis, investigation, resources, writing—original draft preparation, visualization. AT: conceptualization, methodology, validation, data curation, writing—review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest regarding this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Czyżewska, K., Trusek, A. A catalytic membrane approach as a way to obtain sweet and unsweet lactose-free milk. Bioprocess Biosyst Eng 47, 919–929 (2024). https://doi.org/10.1007/s00449-024-03018-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-024-03018-z