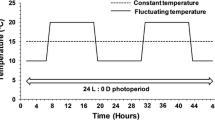
Abstract
To accurately predict species’ phenology under climate change, we need to gain a detailed mechanistic understanding of how different environmental cues interact to produce the seasonal timing response. In the winter moth (Operophtera brumata), seasonal timing of egg hatching is strongly affected by ambient temperature and has been under strong climate change-induced selection over the past 25 years. However, it is unclear whether photoperiod received at the egg stage also influences timing of egg hatching. Here, we investigated the relative contribution of photoperiod and temperature in regulating winter moth egg development using two split-brood experiments. We experimentally shifted the photoperiod eggs received by 2–4 weeks compared to the actual calendar date and measured the timing of egg hatching, both at a constant temperature and in combination with two naturally changing temperature treatments – mimicking a cold and a warm year. We found an eight-fold larger effect of temperature compared to photoperiod on egg development time. Moreover, the very small photoperiod effects we found were outweighed by both between- and within-clutch variation in egg development time. Thus, we conclude that photoperiod received at the egg stage does likely not play a substantial role in regulating the seasonal timing of egg hatching in the winter moth. These insights into the regulatory mechanism of seasonal timing could have important implications for predicting insect climate change adaptation, as we might expect different targets of selection depending on the relative contribution of different environmental cues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The seasonal timing of a wide range of species is shifting in response to climate change (Parmesan and Yohe 2003; Root et al. 2003; Thackeray et al. 2010), largely in response to increasing temperatures (Cohen et al. 2018). In many cases, interacting species are shifting their phenology at different rates, with the resulting phenological mismatches between consumer and resource leading to natural selection on phenology and possibly negative consequences for population viability (Kharouba et al. 2018; Visser and Gienapp 2019). The relative importance of different environmental cues, such as temperature and photoperiod, to time key life history events is generally thought to play a role in determining a species’ seasonal timing shift (Chmura et al. 2019; Renner and Zohner 2018). However, to accurately predict species’ responses to climate change, we need to gain a detailed mechanistic understanding of how different environmental cues interact to produce the seasonal timing response (Chmura et al. 2019; McNamara et al. 2011).
In many species, the seasonal timing of life history events is under photoperiodic control, with additional environmental cues such as temperature used to fine-tune the response (Bradshaw and Holzapfel 2007). In insects, photoperiod similarly plays a major role in determining the timing of development, particularly in regulating dormancy responses (Danks 1987; Denlinger 2002). While photoperiod is primarily involved in the induction of diapause in insects (Denlinger 2002), it can similarly act as a cue for diapause maintenance (Tauber and Tauber 1976) and diapause termination (Brunnarius and Dumortier 1984; Koštál et al. 2017). For example, in the European corn borer (Ostrinia nubilalis) and Asian corn borer (Ostrinia furnacalis), larvae that have entered diapause remain sensitive to photoperiod throughout autumn and early winter, with day length in combination with temperature regulating the duration of diapause (McLeod and Beck 1963; Yang et al. 1950; van Dis et al. 2021). Eggs become transparent only very close to hatching, which is when they might start responding to photoperiod. Indeed, winter moth egg hatching has previously been observed to follow a circadian rhythm (Embree 1970) similar to other insects (Saunders 2002) and responding to photoperiod only at the end of egg development might also explain the small 1.4–2.5 days delay we observed for some of the delayed photoperiod treatments.
Compared to the overriding eight-fold larger temperature effect on egg development time (for arguably a smaller treatment difference of on average 1.36 °C between warm and cold treatments compared to a day length difference of 2 h in mid-March between photoperiod treatments), we would argue that photoperiod received at the egg stage does not play a substantial role in regulating the seasonal timing of egg hatching. In fact, the small photoperiod effects we found were outweighed by both between- and within-clutch variation in egg development time (Fig.S3). Nevertheless, photoperiod received at a different life stage might still play a role, as previous work on the winter moth has found indications of maternal effects of photoperiod (Salis et al. 2017). The first stage of insect embryogenesis critically depends on the maternally set-up environment in the egg (Irvine 2020) and dormancy responses are often maternally regulated in insects (Mousseau and Dingle 1991). For example, egg diapause is maternally induced in the silk moth, B. mori (Kogure 1933). In the winter moth, previous work indicated that photoperiod received by the mother can carry over into the next generation: mothers that received an early season photoperiod as caterpillars laid eggs that took less time to develop and vice versa, with an effect size of up to 10 days depending on the temperature treatment the eggs received (Salis et al. 2017). This trans-generational photoperiod response might be linked to the nutritional status of the mother (i.e. whether she was timed well to budburst as a caterpillar (van Asch et al. 2010), but the causal mechanism behind this maternal effect remains unclear.
Because climate change affects ambient temperature but not photoperiod, the relative importance of temperature and photoperiod as cues has important implications for climate change adaptation. Importantly, phenological traits mostly regulated by temperature are expected to immediately shift under climate change and such temperature-only controls of phenology might be common in moth species (e.g. in at least 34% of 112 analysed Finnish species, (Valtonen et al. 2011). While development rate is temperature dependent in all insect species (Nedved 2009), it might be that insects with obligate diapause (i.e. where diapause does not need to be induced by environmental cues) are more likely to have temperature-only controls of phenology, while species with facultative diapause often also rely on photoperiod as a cue to regulate dormancy (Denlinger 2002). Indeed, photoperiod regulation of diapause induction, maintenance, and termination has mostly been reported for facultative diapausers (e.g. Brunnarius and Dumortier 1984; Wang et al. 2009; Yang et al. 2014), while studies in obligate diapausers tend to focus on the effect of temperature only (e.g. Doherty et al. 2018; Gray et al. 2001). But to properly test this pattern, more experiments investigating the effect of photoperiod on the phenology of obligate diapausers are needed. In addition, it is important to identify where in the life cycle climate change-induced selection acts in order to understand which environmental cues are important for adaptation. This importance is illustrated by the few examples we have of insects evolving under climate change (Merilä and Hendry 2014): so far, genetic changes to the photoperiodic response were involved in pre-dormancy adaptations – e.g. the pitcher plant mosquito, Wyeomyia smithii (Bradshaw and Holzapfel 2001), and speckled wood butterfly, Pararge aegeria (Nielsen et al. 2023), where the photoperiodic response genetically changed to take advantage of the longer growing season. In contrast, genetic adaptation in the winter moth changed post-dormancy seasonal timing, involving changes to the temperature response (van Asch et al. 2007, 2013).
Conclusion
Seasonal timing shifts are one of the most ubiquitous responses to climate change across taxa (Parmesan and Yohe 2003; Root et al. 2003; Thackeray et al. 2010). Elucidating which environmental cues regulate these timing responses is a crucial step in determining how populations can adapt to climate change. We conclude that temperature has an overriding role compared to photoperiod in regulating the seasonal timing of winter moth egg hatching. These relative contributions of temperature and photoperiod could have important implications for climate change adaptation. So far, we know of only few species that have evolved under climate change (Catullo et al. 2019; Merilä and Hendry 2014), but selection often seems to target seasonal timing responses (Bradshaw and Holzapfel 2008; Visser and Gienapp 2019). Future work should take care in determining both the specific selection pressure that climate change exerts on the seasonal timing trait as well as its underlying mechanism, as we might expect different targets of selection depending on the relative contribution of different environmental cues.
Data availability
All data and scripts used for analysis are available in the Dryad digital repository https://doi.org/https://doi.org/10.5061/dryad.2v6wwpzvp (van Dis et al. 2024). Analysis scripts can also be found on GitHub: https://github.com/NEvanDis/WM_photoperiod.
References
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67:1–48
Bradshaw WE, Holzapfel CM (2001) Genetic shift in photoperiodic response correlated with global warming. Proc Natl Acad Sci 98(25):14509–14511. https://doi.org/10.1073/pnas.241391498
Bradshaw WE, Holzapfel CM (2006) Evolutionary response to rapid climate change. Science 312(5779):1477–1478. https://doi.org/10.1126/science.1127000
Bradshaw WE, Holzapfel CM (2007) Evolution of animal photoperiodism. Annu Rev Ecol Evol Syst 38:1–25. https://doi.org/10.1146/annurev.ecolsys.37.091305.110115
Bradshaw WE, Holzapfel CM (2008) Genetic response to rapid climate change: it’s seasonal timing that matters. Mol Ecol 17(1):157–166. https://doi.org/10.1111/j.1365-294X.2007.03509.x
Brunnarius J, Dumortier B (1984) Existence of a light-sensitive phase in the photoperiodic termination of diapause in Pieris brassicae L. (Insecta:Lepidoptera) and comparison with diapause induction. J Comp Physiol A 155(2):161–169. https://doi.org/10.1007/BF00612634
Catullo RA, Llewelyn J, Phillips BL, Moritz CC (2019) The potential for rapid evolution under anthropogenic climate change. Curr Biol 29(19):R996–R1007. https://doi.org/10.1016/j.cub.2019.08.028
Chmura HE, Kharouba HM, Ashander J, Ehlman SM, Rivest EB, Yang LH (2019) The mechanisms of phenology: the patterns and processes of phenological shifts. Ecol Monogr 89(1):e01337. https://doi.org/10.1002/ecm.1337
Cohen JM, Lajeunesse MJ, Rohr JR (2018) A global synthesis of animal phenological responses to climate change. Nat Clim Chang 8(3):224–228. https://doi.org/10.1038/s41558-018-0067-3
Danks HV (1987) Insect dormancy: an ecological perspective. Biological Survey of Canada (Terres- trial Arthropods)
Denlinger DL (2002) Regulation of diapause. Annu Rev Entomol 47:93–122
Doherty J, Guay J, Cloutier C (2018) Embryonic stage of obligatory diapause and effects of abiotic conditions on egg hatching in the balsam twig aphid, Mindarus Abietinus. Entomologia Exp Applicata 166(8):628–637. https://doi.org/10.1111/eea.12718
Embree DG (1970) The diurnal and seasonal pattern of hatching of winter moth eggs, Operophtera brumata (Geometridae: Lepidoptera). Can Entomol 102:759–768. https://doi.org/10.4039/Ent102759-6
Gaumont R (1950) Etudes embryologiques sur l’oeuf de cheimatobie Operopthera brumata L., Lepidoptère Geometridae. Annls Inst Natn Rech Agron Paris (C)(1), 253–273
Gray DR, Ravlin FW, Braine JA (2001) Diapause in the gypsy moth: a model of inhibition and development. J Insect Physiol 47: 173–184. www.elsevier.com/locate/**sphys
Irvine SQ (2020) Embryonic canalization and its limits—A view from temperature. J Exp Zool B Mol Dev Evol 334(2):128–144. https://doi.org/10.1002/jez.b.22930
Kharouba HM, Ehrlén J, Gelman A, Bolmgren K, Allen JM, Travers SE, Wolkovich EM (2018) Global shifts in the phenological synchrony of species interactions over recent decades. Proc Nat Acad Sci 115(20):5211–5216. https://doi.org/10.1073/pnas.1714511115
Kogure M (1933) The influence of light and temperature on certain characters of the silkworm, Bombyx Mori. J Fac Agric Kyushu Univ 4(1):1–93. https://doi.org/10.5109/22568
Koštál V, Štětina T, Poupardin R, Korbelová J, Bruce AW (2017) Conceptual framework of the eco-physiological phases of insect diapause development justified by transcriptomic profiling. PNAS 114(32):8532–8537. https://doi.org/10.1073/pnas.1707281114
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmertest package: tests in linear mixed effects models. J Statist Soft. https://doi.org/10.18637/jss.v082.i13
Lenth RV (2023) Emmeans: estimated marginal means, aka Least-Squares Means. R package version 1.8.8. https://github.com/rvlenth/emmeans
McLeod DGR, Beck SD (1963) Photoperiodic termination of diapause in an insect. Biol Bull 124(1):84–96
McNamara JM, Barta Z, Klaassen M, Bauer S (2011) Cues and the optimal timing of activities under environmental changes. Ecol Lett 14(12):1183–1190. https://doi.org/10.1111/j.1461-0248.2011.01686.x
Merilä J, Hendry AP (2014) Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol Appl 7(1):1–14. https://doi.org/10.1111/eva.12137
Mousseau TA, Dingle H (1991) Maternal effects in insect life histories. Annu Rev Entomol 36(136):511–534. https://doi.org/10.1146/annurev.ento.36.1.511
Nedved O (2009) Temperature, effects on development and growth. Encyclopedia of insects. Elsevier, Amsterdam, pp 990–993. https://doi.org/10.1016/B978-0-12-374144-8.00261-7
Nielsen ME, Nylin S, Wiklund C, Gotthard K (2023) Evolution of butterfly seasonal plasticity driven by climate change varies across life stages. Ecol Lett 26(9):1548–1558. https://doi.org/10.1111/ele.14280
Niimi T, Yamashita O, Yaginuma T (1993) A cold-inducible Bombyx gene encoding a protein similar to mammalian sorbitol dehydrogenase. Eur J Biochem 213(3):1125–1131. https://doi.org/10.1111/j.1432-1033.1993.tb17862.x
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change. Nature 421:37–42
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org/
Renner SS, Zohner CM (2018) Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annu Rev Ecol Evol Syst 49:165–182. https://doi.org/10.1146/annurev-ecolsys-110617-062535
Root T, Price J, Hall K, Schneider S (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60. https://doi.org/10.1038/nature01309.1
Salis L, van den Hoorn E, Beersma DGM, Hut RA, Visser ME (2017) Photoperiodic cues regulate phenological carry-over effects in an herbivorous insect. Funct Ecol 32:171–180. https://doi.org/10.1111/1365-2435.12953
Saunders DS (2002) Insect clocks, 3rd edn. Elsevier Science B.V, Amsterdam. https://doi.org/10.1016/B978-0-444-50407-4.X5000-9
Shingleton AW, Sisk GC, Stern DL (2003) Diapause in the pea aphid (Acyrthosiphon pisum) is a slowing but not a cessation of development. BMC Dev Biol. https://doi.org/10.1186/1471-213X-3-7
Tauber MJ, Tauber CA (1976) Insect seasonality: diapause maintenance, termination, and postdiapause development. Annu Rev Entomol 21: 81–107. www.annualreviews.org
Tenow O, Nilssen AC, Bylund H, Pettersson R, Battisti A, Bohn U, Caroulle F, Ciornei C, Csóka G, Delb H, De Prins W, Glavendekić M, Gninenko YI, Hrašovec B, Matošević D, Meshkova V, Moraal L, Netoiu C, Pajares J, Utkina I (2013) Geometrid outbreak waves travel across Europe. J Anim Ecol 82(1):84–95. https://doi.org/10.1111/j.1365-2656.2012.02023.x
Thackeray SJ, Sparks TH, Frederiksen M, Burthe S, Bacon PJ, Bell JR, Botham MS, Brereton TM, Bright PW, Carvalho L, Clutton-Brock T, Dawson A, Edwards M, Elliott JM, Harrington R, Johns D, Jones ID, Jones JT, Leech DI, Wanless S (2010) Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Global Change Biol 16:3304–3313. https://doi.org/10.1111/j.1365-2486.2010.02165.x
Valtonen A, Ayres MP, Roininen H, Pöyry J, Leinonen R (2011) Environmental controls on the phenology of moths: predicting plasticity and constraint under climate change. Oecologia 165(1):237–248. https://doi.org/10.1007/s00442-010-1789-8
van Asch M, van Tienderen PH, Holleman LJM, Visser ME (2007) Predicting adaptation of phenology in response to climate change, an insect herbivore example. Glob Change Biol 13(8):1596–1604. https://doi.org/10.1111/j.1365-2486.2007.01400.x
van Asch M, Julkunen-Tiito R, Visser ME (2010) Maternal effects in an insect herbivore as a mechanism to adapt to host plant phenology. Funct Ecol 24(5):1103–1109. https://doi.org/10.1111/j.1365-2435.2010.01734.x
van Asch M, Salis L, Holleman LJM, van Lith B, Visser ME (2013) Evolutionary response of the egg hatching date of a herbivorous insect under climate change. Nat Clim Chang 3(3):244–248. https://doi.org/10.1038/nclimate1717
van Dis NE, van der Zee M, Hut RA, Wertheim B, Visser ME (2021) Timing of increased temperature sensitivity coincides with nervous system development in winter moth embryos. J Exp Biol. https://doi.org/10.1242/jeb.242554
van Dis NE, Salis L, Visser ME (2024) Temperature has an overriding role compared to photoperiod in regulating the seasonal timing of winter moth egg hatching. Dryad Dataset. https://doi.org/10.5061/dryad.2v6wwpzvp
Varley GC, Gradwell GR, Hassell MP (1973) Insect population ecology. Blackwell Scientific Publications, Hoboken
Visser ME, Gienapp P (2019) Evolutionary and demographic consequences of phenological mismatches. Nat Ecol Evol 3:879–885. https://doi.org/10.1038/s41559-019-0880-8
Visser ME, Holleman JM (2001) Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc Royal Soc B Biol Sci 268(November):289–294. https://doi.org/10.1098/rspb.2000.1363
Wall C (1973) Embryonic development in two species of Chesias (Lepidoptera: Geometridae). J Zool Lond 169:65–84
Wang XP, Yang QS, Zhou XM, Xu S, Lei CL (2009) Effects of photoperiod and temperature on diapause induction and termination in the swallowtail, sericinus montelus. Physiol Entomol 34(2):158–162. https://doi.org/10.1111/j.1365-3032.2008.00668.x
Yang H, Tu X, **a Q, He H, Chen C, Xue F (2014) Photoperiodism of diapause induction and diapause termination in O strinia furnacalis. Entomol Exp Appl 153(1):34–46. https://doi.org/10.1111/eea.12226
Acknowledgements
The authors thank Gabriel Charvalakis for help with the lux measurements. They would also like to thank two anonymous reviewers for their constructive comments to improve the manuscript.
Funding
The authors received no funding for this study.
Author information
Authors and Affiliations
Contributions
LS and MEV designed the photoperiod experiment and LS performed the experiment; MEV designed and performed the photoperiod–temperature experiment; NEvD and LS analysed the data; NEvD and LS wrote the manuscript with input from MEV.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Communicated by Klaus Fischer.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Dis, N.E., Salis, L. & Visser, M.E. Temperature has an overriding role compared to photoperiod in regulating the seasonal timing of winter moth egg hatching. Oecologia 204, 743–750 (2024). https://doi.org/10.1007/s00442-024-05535-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-024-05535-w