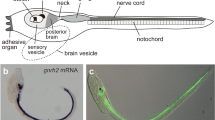
Abstract
Motilin (MLN) is a peptide hormone originally isolated from the mucosa of the porcine intestine. Its orthologs have been identified in various vertebrates. Although MLN regulates gastrointestinal motility in tetrapods from amphibians to mammals, recent studies indicate that MLN is not involved in the regulation of isolated intestinal motility in zebrafish, at least in vitro. To determine the unknown function of MLN in teleosts, we examined the expression of MLN and the MLN receptor (MLNR) at the cellular level in Japanese medaka (Oryzias latipes). Quantitative PCR revealed that mln mRNA was limitedly expressed in the gut, whereas mlnr mRNA was not detected in the gut but was expressed in the brain and kidney. By in situ hybridization and immunohistochemistry, mlnr mRNA was detected in the dopaminergic neurons of the area postrema in the brain and the noradrenaline-producing cells in the interrenal gland of the kidney. Furthermore, we observed efferent projections of mlnr-expressing dopaminergic neurons in the lobus vagi (XL) and nucleus motorius nervi vagi (NXm) of the medulla oblongata by establishing a transgenic medaka expressing the enhanced green fluorescence protein driven by the mlnr promoter. The expression of dopamine receptor mRNAs in the XL and cholinergic neurons in NXm was confirmed by in situ hybridization. These results indicate novel sites of MLN activity other than the gastrointestinal tract. MLN may exert central and peripheral actions through the regulation of catecholamine release in medaka.







Similar content being viewed by others
Data Availability
The data supporting the findings of present study are available from the corresponding author (MA) upon reasonable request.
References
Ando M, Ogawa M, Fukuda M (2013) A vagal nerve branch controls swallowing directly in the seawater eel. J Comp Physiol B 183(8):1015–1021
Apu AS, Mondal A, Kitazawa T, Takemi S, Sakai T, Sakata I (2016) Molecular cloning of motilin and mechanism of motilin-induced gastrointestinal motility in Japanese quail. Gen Comp Endocrinol 233:53–62
Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T (2007) Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol Motil 19(8):675–680
Bernier NJ, Perry SF (1997) Angiotensins stimulate catecholamine release from the chromaffin tissue of the rainbow trout. Am J Physiol 273(1 Pt 2):R49-57
Breves JP, Veillette PA, Specker JL (2009) Ghrelin in the summer flounder: immunolocalization to the gastric glands and action on plasma cortisol levels. Comp Biochem Physiol A Mol Integr Physiol 152(2):268–272
Broad J, Mukherjee S, Samadi M, Martin JE, Dukes GE, Sanger GJ (2012) Regional- and agonist-dependent facilitation of human neurogastrointestinal functions by motilin receptor agonists. Br J Pharmacol 167(4):763–774
Brown JC, Cook MA, Dryburgh JR (1972) Motilin, a gastric motor activity-stimulating polypeptide: final purification, amino acid composition, and C-terminal residues. Gastroenterology 62(3):401–404
Dasmahapatra AK, Tchounwou PB (2022) Histopathological evaluation of the interrenal gland (adrenal homolog) of Japanese medaka (Oryzias latipes) exposed to graphene oxide. Environ Toxicol 37(10):2460–2482
Dass NB, Hill J, Muir A, Testa T, Wise A, Sanger GJ (2003) The rabbit motilin receptor: molecular characterisation and pharmacology. Br J Pharmacol 140(5):948–954
De Clercq P, Depoortere I, Macielag M, Vandermeers A, Vandermeers-Piret MC, Peeters TL (1996) Isolation, sequence, and bioactivity of chicken motilin. Peptides 17(2):203–208
De Clercq P, Depoortere I, Peeters T (1997) Isolation and sequencing of the cDNA encoding the motilin precursor from sheep intestine. Gene 202(1–2):187–191
Delhanty PJ, van der Lely AJ (2011) Ghrelin and glucose homeostasis. Peptides 32(11):2309–2318
Delhanty PJ, van der Eerden BC, van Leeuwen JP (2014) Ghrelin and bone. BioFactors 40(1):41–48
Depoortere I, De Clercq P, Svoboda M, Bare L, Peeters TL (1997) Identification of motilin mRNA in the brain of man and rabbit. Conservation of polymorphism of the motilin gene across species. Peptides 18(10):1497–503
Fabbri E, Capuzzo A, Moon TW (1998) The role of circulating catecholamines in the regulation of fish metabolism: an overview. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 120(2):177–192
Feighner SD, Tan CP, McKee KK, Palyha OC, Hreniuk DL, Pong SS, Austin CP, Figueroa D, MacNeil D, Cascieri MA, Nargund R, Bakshi R, Abramovitz M, Stocco R, Kargman S, O’Neill G, Van Der Ploeg LH, Evans J, Patchett AA, Smith RG, Howard AD (1999) Receptor for motilin identified in the human gastrointestinal system. Science 284(5423):2184–2188
Finger TE (2008) Sorting food from stones: the vagal taste system in Goldfish, Carassius auratus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 194(2):135–143
Finger TE (2009) Evolution of gustatory reflex systems in the brainstems of fishes. Integr Zool 4(1):53–63
Fritsche R, Reid SG, Thomas S, Perry SF (1993) Serotonin-mediated release of catecholamines in the rainbow trout Oncorhynchus mykiss. J Exp Biol 178(1):191–204
Gfell B, Kloas W, Hanke W (1997) Neuroendocrine effects on adrenal hormone secretion in carp (Cyprinus carpio). Gen Comp Endocrinol 106(3):310–319
Goswami C, Shimada Y, Yoshimura M, Mondal A, Oda S, Tanaka T, Sakai T, Sakata I (2015a) Motilin stimulates gastric acid secretion in coordination with ghrelin in Suncus murinus. PLoS ONE 10(6):e0131554
Goswami C, Tanaka T, Jogahara T, Sakai T, Sakata I (2015b) Motilin stimulates pepsinogen secretion in Suncus murinus. Biochem Biophys Res Commun 462(3):263–268
Hamasaki S, Mukuda T, Kaidoh T, Yoshida M, Uematsu K (2016) Impact of dehydration on the forebrain preoptic recess walls in the mudskipper, Periophthalmus modestus: a possible locus for the center of thirst. J Comp Physiol B 186(7):891–905
He J, Irwin DM, Chen R, Zhang YP (2010) Stepwise loss of motilin and its specific receptor genes in rodents. J Mol Endocrinol 44(1):37–44
Huang J, Zhou H, Mahavadi S, Sriwai W, Lyall V, Murthy KS (2005) Signaling pathways mediating gastrointestinal smooth muscle contraction and MLC20 phosphorylation by motilin receptors. Am J Physiol Gastrointest Liver Physiol 288(1):G23-31
Huang Z, Depoortere I, De Clercq P, Peeters T (1999) Sequence and characterization of cDNA encoding the motilin precursor from chicken, dog, cow and horse. Evidence of mosaic evolution in prepromotilin. Gene 240(1):217–226
Ieki T, Okada S, Aihara Y, Ohmoto M, Abe K, Yasuoka A, Misaka T (2013) Transgenic labeling of higher order neuronal circuits linked to phospholipase C-β2-expressing taste bud cells in medaka fish. J Comp Neurol 521(8):1781–1802
Inatomi N, Sato F, Marui S, Itoh Z, Omura S (1996) Vagus-dependent and vagus-independent mechanisms of action of the erythromycin derivative EM574 and motilin in dogs. Jpn J Pharmacol 71(1):29–38
Ito S, Mukuda T, Ando M (2006) Catecholamines inhibit neuronal activity in the glossopharyngeal-vagal motor complex of the Japanese eel: significance for controlling swallowing water. J Exp Zool A Comp Exp Biol 305(6):499–506
Itoh Z (1997) Motilin and clinical application. Peptides 18(4):593–608
Itoh Z, Honda R, Hiwatashi K, Takeuchi S, Aizawa I, Takayanagi R, Couch EF (1976) Motilin-induced mechanical activity in the canine alimentary tract. Scand J Gastroenterol Suppl 39:93–110
Itoh Z, Takeuchi S, Aizawa I, Mori K, Taminato T, Seino Y, Imura H, Yanaihara N (1978) Changes in plasma motilin concentration and gastrointestinal contractile activity in conscious dogs. Am J Dig Dis 23(10):929–935
Katayama Y, Sakamoto T, Saito K, Tsuchimochi H, Kaiya H, Watanabe T, Pearson JT, Takei Y (2018) Drinking by amphibious fish: convergent evolution of thirst mechanisms during vertebrate terrestrialization. Sci Rep 8(1):625
Kishimoto I, Tokudome T, Hosoda H, Miyazato M, Kangawa K (2012) Ghrelin and cardiovascular diseases. J Cardiol 59(1):8–13
Kitazawa T, Kaiya H (2019) Regulation of gastrointestinal motility by motilin and ghrelin in vertebrates. Front Endocrinol 10:278
Kitazawa T, Kaiya H (2021) Motilin comparative study: structure, distribution, receptors, and gastrointestinal motility. Front Endocrinol (lausanne) 12:700884
Kitazawa T, Ichikawa S, Yokoyama T, Ishii A, Shuto K (1994) Stimulating action of KW-5139 (Leu13-motilin) on gastrointestinal motility in the rabbit. Br J Pharmacol 111(1):288–294
Kitazawa T, Shimazaki M, Kikuta A, Yaosaka N, Teraoka H, Kaiya H (2016) Effects of ghrelin and motilin on smooth muscle contractility of the isolated gastrointestinal tract from the bullfrog and Japanese fire belly newt. Gen Comp Endocrinol 232:51–59
Kitazawa T, Yoshida M, Teraoka H, Kaiya H (2017) Does motilin peptide regulate gastrointestinal motility of zebrafish? An in vitro study using isolated intestinal strips. Gen Comp Endocrinol 249:15–23
Kojima M, Kangawa K (2005) Ghrelin: structure and function. Physiol Rev 85(2):495–522
Konturek SJ, Dembinski A, Krol R, Wünsch E (1977) Effect of 13-NLE-motilin on gastric secretion, serum gastrin level and mucosal blood flow in dogs. J Physiol 264(3):665–672
Kozaka T, Fujii Y, Ando M (2003) Central effects of various ligands on drinking behavior in eels acclimated to seawater. J Exp Biol 206(Pt 4):687–692
Lin TC, Hsiao M (2017) Ghrelin and cancer progression. Biochim Biophys Acta Rev Cancer 1868(1):51–57
Liu Y, Li S, Huang X, Lu D, Liu X, Ko WH, Zhang Y, Cheng CH, Lin H (2013) Identification and characterization of a motilin-like peptide and its receptor in teleost. Gen Comp Endocrinol 186:85–93
Magee DF, Naruse S (1984) The role of motilin in periodic interdigestive pancreatic secretion in dogs. J Physiol 355:441–447
Matsuda K, Miura T, Kaiya H, Maruyama K, Shimakura S, Uchiyama M, Kangawa K, Shioda S (2006) Regulation of food intake by acyl and des-acyl ghrelins in the goldfish. Peptides 27(9):2321–2325
Matsumoto M, Takemi S, Sakai T, Sakata I (2022) Identification of motilin in Japanese fire bellied newt. Gen Comp Endocrinol 323–324:114031
Mihalache L, Gherasim A, Niţă O, Ungureanu MC, Pădureanu SS, Gavril RS, Arhire LI (2016) Effects of ghrelin in energy balance and body weight homeostasis. Hormones 15(2):186–196
Mondal A, Kawamoto Y, Yanaka T, Tsutsui C, Sakata I, Oda SI, Tanaka T, Sakai T (2011) Myenteric neural network activated by motilin in the stomach of Suncus murinus (house musk shrew). Neurogastroenterol Motil 23(12):1123–1131
Mondal A, **e Z, Miyano Y, Tsutsui C, Sakata I, Kawamoto Y, Aizawa S, Tanaka T, Oda S, Sakai T (2012) Coordination of motilin and ghrelin regulates the migrating motor complex of gastrointestinal motility in Suncus murinus. Am J Physiol Gastrointest Liver Physiol 302(10):G1207-1215
Montpetit CJ, Perry SF (2000) Vasoactive intestinal polypeptide- and pituitary adenylate cyclase activating polypeptide-mediated control of catecholamine release from chromaffin tissue in the rainbow trout. Oncorhynchus Mykiss J Endocrinol 166(3):705–714
Morita Y, Finger TE (1985) Topographic and laminar organization of the vagal gustatory system in the goldfish. Carassius Auratus J Comp Neurol 238(2):187–201
Mukuda T, Ando M (2010) Central regulation of the pharyngeal and upper esophageal reflexes during swallowing in the Japanese eel. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 196(2):111–122
Mukuda T, Matsunaga Y, Kawamoto K, Yamaguchi K, Ando M (2005) “Blood-contacting neurons” in the brain of the Japanese eel Anguilla japonica. J Exp Zool A Comp Exp Biol 303(5):366–376
Nobata S, Takei Y (2011) The area postrema in hindbrain is a central player for regulation of drinking behavior in Japanese eels. Am J Physiol Regul Integr Comp Physiol 300(6):R1569–R1577
Nobata S, Ando M, Takei Y (2013) Hormonal control of drinking behavior in teleost fishes; insights from studies using eels. Gen Comp Endocrinol 192:214–221
Ogawa A, Mochiki E, Yanai M, Morita H, Toyomasu Y, Ogata K, Ohno T, Asao T, Kuwano H (2012) Interdigestive migrating contractions are coregulated by ghrelin and motilin in conscious dogs. Am J Physiol Regul Integr Comp Physiol 302(2):R233-41
Ogino H, McConnell WB, Grainger RM (2006) High-throughput transgenesis in Xenopus using I-SceI meganuclease. Nat Protoc 1(4):1703–1710
Ohshiro H, Nonaka M, Ichikawa K (2008) Molecular identification and characterization of the dog motilin receptor. Regul Pept 146(1–3):80–87
Olsson C, Holbrook JD, Bompadre G, Jönsson E, Hoyle CH, Sanger GJ, Holmgren S, Andrews PL (2008) Identification of genes for the ghrelin and motilin receptors and a novel related gene in fish, and stimulation of intestinal motility in zebrafish (Danio rerio) by ghrelin and motilin. Gen Comp Endocrinol 55(1):217–216
Poitras P (1984) Motilin is a digestive hormone in the dog. Gastroenterology 87(4):909–913
Poitras P, Trudel L, Lahaie RG, Pomier-Layrargue G (1987) Motilin-like-immunoreactivity in intestine and brain of dog. Life Sci 40(14):1391–1395
Poitras P, Gagnon D, St-Pierre S (1992) N-terminal portion of motilin determines its biological activity. Biochem Biophys Res Commun 183(1):36–40
Reid SG, Perry SF (1995) Cholinoceptor-mediated control of catecholamine release from chromaffin cells in the American eel. Anguilla Rostrata J Comp Physiol B 165(6):464–470
Reid SG, Vijayan MM, Perry SF (1996) Modulation of catecholamine storage and release by the pituitary-interrenal axis in the rainbow trout. Oncorhynchus Mykiss J Comp Physiol B 165(8):665–676
Roche J, Hergalant S, Depp A, Ben Ammar I, Lafont AG, Policar T, Fontaine P, Milla S (2020) First identification of dopamine receptors in pikeperch, Sander lucioperca, during the pre-ovulatory period. Comp Biochem Physiol Part D Genomics Proteomics 36:100747
Rotllant J, Ruane NM, Dinis MT, Canario AV, Power DM (2006) Intra-adrenal interactions in fish: catecholamine stimulated cortisol release in sea bass (Dicentrarchus labraxL.). Comp Biochem Physiol A Mol Integr Physiol 143(3):375–81
Sanger GJ (2008) Motilin, ghrelin and related neuropeptides as targets for the treatment of GI diseases. Drug Discov Today 13(5–6):234–239
Sanger GJ, Holbrook JD, Andrews PL (2011) The translational value of rodent gastrointestinal functions: a cautionary tale. Trends Pharmacol Sci 32(7):402–409
Sato T, Nakamura Y, Shiimura Y, Ohgusu H, Kangawa K, Kojima M (2012) Structure, regulation and function of ghrelin. J Biochem 151(2):119–128
Satoh M, Sakai T, Koyama H, Shiba Y, Itoh Z (1995) Immunocytochemical localization of motilin-containing cells in the rabbit gastrointestinal tract. Peptides 16(5):883–887
Seino Y, Tanaka K, Takeda J, Takahashi H, Mitani T, Kurono M, Kayano T, Koh G, Fukumoto H, Yano H, Fujita J, Inagaki N, Yamada Y, Imura H (1987) Sequence of an intestinal cDNA encoding human motilin precursor. FEBS Lett 223(1):74–76
Sjölund K, Sandén G, Håkanson R, Sundler F (1983) Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology 85(5):1120–1130
Sugiyama M, Yamaki A, Furuya M, Inomata N, Minamitake Y, Ohsuye K, Kangawa K (2012) Ghrelin improves body weight loss and skeletal muscle catabolism associated with angiotensin II-induced cachexia in mice. Regul Pept 178(1–3):21–28
Suzuki H, Mochiki E, Haga N, Satoh M, Mizumoto A, Itoh Z (1998) Motilin controls cyclic release of insulin through vagal cholinergic muscarinic pathways in fasted dogs. Am J Physiol 274(1):G87-95
Suzuki H, Kuwano H, Mochiki E, Haga N, Shimura T, Nomoto K, Tanaka T, Mizumoto A, Itoh Z (2003) Effect of motilin on endogenous release of insulin in conscious dogs in the fed state. Dig Dis Sci 48(12):2263–2270
Suzuki A, Ishida Y, Aizawa S, Sakata I, Tsutsui C, Mondal A, Kanako K, Sakai T (2012) Molecular identification of GHS-R and GPR38 in Suncus murinus. Peptides 36(1):29–38
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evo 38(7):3022–3027
Tsukada T, Nobata S, Hyodo S, Takei Y (2007) Area postrema, a brain circumventricular organ, is the site of antidipsogenic action of circulating atrial natriuretic peptide in eels. J Exp Biol 210(Pt 22):3970–3978
Vantrappen G, Janssens J, Peeters TL, Bloom SR, Christofides ND, Hellemans J (1979) Motilin and the interdigestive migrating motor complex in man. Dig Dis Sci 24(7):497–500
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77(3):591–625
Yamamoto I, Kaiya H, Tsutsui C, Sakai T, Tsukada A, Miyazato M, Tanaka M (2008) Primary structure, tissue distribution, and biological activity of chicken motilin receptor. Gen Comp Endocrinol 156(3):509–514
Yoshimoto M, Albert JS, Sawai N, Shimizu M, Yamamoto N, Ito H (1998) elencephalic ascending gustatory system in a cichlid fish, Oreochromis (Tilapia) niloticus. J Comp Neurol 392(2):209–26
You CH, Chey WY, Lee KY (1980) Studies on plasma motilin concentration and interdigestive motility of the duodenum in humans. Gastroenterology 79(1):62–66
Zhang S, Teraoka H, Kaiya H, Kitazawa T (2021) Motilin- and ghrelin-induced contractions in isolated gastrointestinal strips from three species of frogs. Gen Comp Endocrinol 300:113649
Zhang S, Kaiya H, Kitazawa T (2023) Motilin is a regulator of gastric contraction in Japanese fire belly newts (Cynops pyrrhogaster), in vitro studies using isolated gastrointestinal strips of newts, rabbits, and chickens. Gen Comp Endocrinol 330:114140
Zheng J, Ariga H, Taniguchi H, Ludwig K, Takahashi T (2009) Ghrelin regulates gastric phase III-like contractions in freely moving conscious mice. Neurogastroenterol Motil 21(1):78–84
Zhou Y, Qi X, Wen H, Zhang K, Zhang X, Li J, Li Y, Fan H (2019) Identification, expression analysis, and functional characterization of motilin and its receptor in spotted sea bass (Lateolabrax maculatus). Gen Comp Endocrinol 277:38–48
Acknowledgements
We thank Ms. Sayaka Sunada of the laboratory in the University of Toyama for technical assistance. We also thank Enago (www.enago.jp) for English-language editing.
Funding
This study was partly supported by JSPS KAKENHI Grants (Numbers 20K15834 to MA, 20K06717 to NK, and 22H02659 to HK). Japan Society for the Promotion of Science, 20K15834, Morio Azuma, 20K06717, Norifumi Konno, NK,Hiroyuki Kaiya, 22H02659, Hiroyuki Kaiya.
Author information
Authors and Affiliations
Contributions
MA coordinated the project, wrote the manuscript, and performed in situ hybridization and immunohistochemistry; NK performed phylogenetically analysis, quantitative PCR, and establishment MLNR-EGFP transgenic medaka; IS supervised and validated the project; TK supervised and validated the project; HK performed measurement of intracellular Ca2+ mobilization. All authors read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were performed after receiving approval from the Institutional Animal Experiment Committee of Jichi Medical University (Approval number: 18023–01) and the University of Toyama (Approval number: G2019SCI-3, G2024SCI-03).
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure 1. Sequence and phylogenetic tree of motilin (MLN) precursors.
The amino acid sequences obtained from GenBank and a genome browser for vertebrate genomes (Ensemble) were edited using Biological Sequence Alignment Editor version 7.2 (BioEdit software) and the ClustalW algorithm (DNA Data Bank of Japan). Molecular phylogenetic relationships were analyzed using the maximum-likelihood method with MEGA11 software (Tamura et al., 2021). To estimate the reliability of the trees, bootstrap** of the data (1000 replicates) was performed. a Nucleotide sequences and deduced amino acid sequences of the MLN precursor identified in the Japanese medaka Oryzias latipes. The predicted signal peptide is underlined. The mature peptide is indicated with a gray background. The dibasic cleavage sites are indicated in the box. b The phylogenetic tree was constructed from the alignment of amino acid sequences of MLN precursors by MEGA11 using the maximum-likelihood method. Data were resampled with 1,000 bootstrap replicates. The accession numbers of the sequences used in this analysis are shown in Table S2. (TIF 26782 KB)
Supplementary Figure 2. Multiple alignment of deduced amino acid sequences of motilin receptor (MLNR) from human, chicken, clawed frog, zebrafish, and medaka.
Hydropathy analysis was performed using the Kyte–Doolittle algorithm to predict seven putative hydrophobic transmembrane regions characteristic of GPCRs. Computer analysis of the presence of glycosylation sites was performed using NetNGlyco1.0 software (https://services.healthtech.dtu.dk/services/NetNGlyc-1.0/). The deduced amino acid sequences were aligned using the ClustalW algorithm [asterisks, identical amino acid residues; open box, predicted transmembrane regions; black box, putative N-glycosylation sites; gray character, putative phosphorylation sites; hash, ERY motif; black triangle, NPxxY motif (where x represents any amino acid)]. The accession numbers of the sequences used in this analysis are shown in Table S2. (TIF 22060 KB)
Supplementary Figure 4. Construction of a vector for establishing MLNR transgenic medaka.
The DNA fragment of the 5′-upstream region of the medaka mlnr gene (amplified the 4,040 bp) and the ISpBSIISK+EGFP vector (Ogino et al., 2006) with medaka heat-shock protein 70 core promoter (hsp) were ligated using in-Fusion technology (TaKaRa Bio, Shiga, Japan) to generate the IS-medaka mlnr+EGFP plasmid. The injection solution with linear plasmid was injected into the cytoplasm of one-cell stage medaka embryos. (TIF 21209 KB)
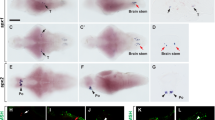
Supplementary Figure 5. Expression of catecholamine-synthesizing enzymes in the medaka area postrema and interrenal gland.
In situ hybridization was performed with a fluorescein-labeled antisense cRNA probe for catecholamine-synthesizing enzyme (a, f tyrosine hydroxylase 1, th1; b, g tyrosine hydroxylase 2, th2; c, h dopa decarboxylase, ddc;d, i dopamine beta-hydroxylase, dbh; e, j phenylethanolamine N-methyltransferase, pnmt) mRNAs to determine the types of catecholamines produced in the medaka area postrema and interrenal gland. Dopamine and noradrenaline were produced in the area postrema by the expression of th1, ddc, and dbh mRNAs. Noradrenaline and adrenaline were produced in the interrenal gland by the expression of th1,ddc, dbh, and pnmt mRNAs. Asterisks show the vein of the kidney. In situ hybridization with 4-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP,blue). Bars, 10 μm (a–e), 100 μm (f–j). (TIF 19121 KB)
Supplementary Figure 6. Expression of dopamine receptors in projection areas of MLNR-expressing neurons in the medulla oblongata.
In situ hybridization was performed with a DIG-labeled antisense cRNA probe for the D1-types (d1a,d1b, d5a, d5b, d6a) and the D2-types (d2a, d3,d4a, d4b, d3, d9) of the medaka dopamine receptor in the lobus vagi (XL) and nucleus motorius nervi vagi (NXm). The dopamine receptors of d1b and d2a were qualitatively major types in these areas compared with d5a, d3, and d8. The expression of d1a,d5b, d6a, d4a, d4b, and d9 mRNAs was not detected in these areas. In situ hybridization with NBT/BCIP (blue). Bars, 100 μm. (TIF 20428 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azuma, M., Konno, N., Sakata, I. et al. Molecular characterization and distribution of motilin and motilin receptor in the Japanese medaka Oryzias latipes. Cell Tissue Res (2024). https://doi.org/10.1007/s00441-024-03896-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00441-024-03896-5