Abstract
Schistosomiasis, a neglected tropical disease (NTD), is one of the most prevalent parasitoses in the World. Certain freshwater snail species are the intermediate host in the life cycle of schistosome species. Controlling snails employing molluscicides is an effective, quick, and convenient intervention strategy to prevent the spread of Schistosoma species in endemic regions. Advances have been made in develo** both synthetic molluscicides and molluscicides derived from plants. However, at present, the development of molluscicides is not adapted to the actual demand for snails and schistosoma controlling. We undertake a systematic review of exploitation and application of synthetic molluscicides and molluscicides derived from plants to combat intermediate host snails. The detailed molluscicidal activity, structure–activity relationship, structural feature, and possible mechanism of some molluscicides are also highlighted, which may afford an important reference for the design of new, more effective molluscicides with low environmental impact and realize the aim of controlling schistosome at transmission stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis, a neglected tropical disease (NTD), is one of the most important infectious parasitoses of humans and animals in the tropical and subtropical regions (Njoroge et al. 2014; Thétiot-Laurent et al. 2013). It is estimated that schistosomiasis could affect more than 200 million people in approximately 70 countries (WHO (World Health Organization) 2021, 2020). About 700 million people live in endemic area (Colley et al. 2014). Six species of the genus Schistosoma including S. japonicum, S. mansoni, S. haematobium, S. mekongi, S. malayensis, and S. intercalatum can cause disease in people (Gryseels et al. 2006). The life cycle of the parasite is complex with an intermediate host (Oncomelania snails), definitive host (humans and mammals), and seven lifecycle stages involving the adult worm, egg, miracidium, mother sporocyst, daughter sporocyst, cercariae, and schistosomulum (Colley et al. 2014; Guo et al. 2008, 2015). It is a great challenge to control schistosomiasis in undeveloped regions because of financial burden and environmental pressures. The current strategies for schistosomiasis control mainly rely on snail control, drug treatment (such as Praziquantel), advanced sanitation, and health education (Limpanont et al. 2020). Although the present method for the control of schistosomiasis is largely based on drug treatment, snail control using molluscicides still plays a crucial role (de Souza 1995; Yuan et al. 2005; Wang et al. 2009).
There are three major species of snails acting as the intermediate hosts: Oncomelania hupensis (O. hupensis) for S. japonicum, Biomphalaria species for S. mansoni, and Bulinus species for S. haematobium (Sturrock 1995). At present, three main strategies including biological control, ecological control, and molluscicidal control have been carried out for the control of schistosome snails (Lardans and Dissous 1998; McCullough et al. 1980). For biological control, some natural predators of snails such as aquatic birds, turtles, fish, crayfish, and insects have been considered as well as competitor snails (Madsen 1990; Ohmae et al. 2003; Pointier and Jourdane 2000; Li et al. 2016). Furthermore, some small creatures such as trematodes, leeches, nematodes, rotifers, and ostracods can attack or devour the snails (Younes et al. 2017). However, the above biological control experiments were often investigated in the laboratory; further field studies are essential in evaluating the control of the snails. The ecological control is also a useful approach including advanced sanitation, agricultural and hydrological exploitation and management (Dong et al. 2018; Liang et al. 2018). However, the potential risk factors are negligible including the limited natural resources, extreme climate changes, and severe financial burden (Lo et al. 2018).
Up to now, the application of molluscicides is the most effective intervention strategy for the control of snails in endemic regions (King et al. 2015). For example, chemical treatment using molluscicides was performed in an area of 78,308.26 hectare in China, and environmental improvements were only carried out in an area of 4738.37 hectare in 2018 (Zhang et al. 2019). Great efforts have been made to explore all kinds of molluscicidal substances including chemical and plant molluscicides (Perrett and Whitfield 1996; de Paula-Andrade et al. 2019). Herein, we undertake a systematic review of molluscicides, and their detailed molluscicidal activity, structure–activity relationship, and possible mechanism of some molluscicides are also highlighted.
Development of chemical molluscicides
Inorganic salts
Copper sulfate (CuSO 4 )
Copper sulfate (compound 1, Table 1, Tab. S1, see Supplementary materials) was evaluated as molluscicides to combat B. alexandrina snails in Egypt (Hoffman and Zakhary 1953). The mortality of snails could reach 100% within 2 weeks at 0.25 ppm. Copper sulfate is an inexpensive inorganic salt. However, the toxicity of the molluscicides on non-target organisms and secondary environmental pollution issues are not ignorable.
Calcium cyanamide
Calcium cyanamide (2, Table 1) was previously reported as an efficient molluscicide for O. hupensis and has low toxicity for fish and other aquatic animals (Wei et al. 2008). The mortality of snails was up to 100% after 2 days at 80 ppm in immersion experiments, which is not good for a molluscicide. In pesticide spraying experiments in the laboratory, the mortality of snails was also 100% after 1 day with the concentration of 30 g/m2. While in natural field conditions, the mortality of snails was 97.18% at 50 ppm. In the field spraying experiments, the mortalities of snails were 81.11% and 96.03% in the concentrations of 30 and 50 g/m2 in 15 days, respectively (Wei et al. 2008). Calcium cyanamides exhibited low toxicity to other aquatic animals at the effective concentration. Further molluscicidal mechanism investigation showed that calcium cyanamide could damage epithelial cells, hepatocytes, and muscle cells of snails (**a et al. 2010). However, for calcium cyanamide, the toxicological explorations are worth of further investigation for practical applications.
Organic molluscicides
Sodium 2,3,4,5,6-pentachlorophenolate (NaPCP) and its derivatives
Sodium 2,3,4,5,6-pentachlorophenolate (3, Table 1) was found to have molluscicidal activity (LC50 = 0.54 ppm, LC100 = 2.0 ppm, 48 h) in immersion experiments (Moon et al. 1958; Zhang et al. 2006). In field experiments, NaPCP was effective at dosages of 10–20 ppm (immersion method) and 5–10 g/m2 (spraying method). Furthermore, NaPCP could kill snail eggs, young snails, and adult snails (Zhang et al. 2006). However, NaPCP has toxicity against fish, aquatic animals, and mammals, along with the risk of teratogenicity, carcinogenesis, and mutagenicity (** et al. 2016). In addition, the technical products of NaPCP often contain highly toxic tetrachloro dibenzodioxines and dibenzofuranes, and that these agents can also arise from light exposure or heat (fire) of pentachlorophenol-contaminated objects. Subsequently, the molluscicidal activity of sodium 2,5-dichloro-4-bromophenol (4, Table 1) was explored for the control of O. nasophora in immersion experiments (Kajihara et al. 1979). The LC50 and LC90 values of sodium 2,5-dichloro-4-bromophenol were 0.54 ppm and 1.59 ppm. Additionally, this compound showed lower toxicity than NaPCP for carp, rainbow trout, and killifish.
N-Bromoacetamide and its derivatives
N-Bromoacetamide (5, Table 1) exhibited molluscicidal activity against adult snails, young snails, and snail eggs, and low toxicity to fish (Zhu et al. 1984). The LC50 and LC90 values were 0.64 ppm and 1.0 ppm in immersion experiments in 24 h, respectively. The mortality of snails exceeded 80% after 7 days at a surface concentration of 1 g/m2 in spraying tests (Zhu et al. 1984). In particular, N-bromoacetamide has the advantages of low-dosage, good solubility in water, low toxicity and non-mutagenicity, which may be an ideal molluscicidal candidate. Nicotinanilide (6, Table 1) was also found to have the same molluscicidal activity as N-bromoacetamide (Sukumaran et al. 2004). The LC50 values of nicotinanilide against different stages of snails were 0.23 ppm (immature snails), 0.77 ppm (young mature snails), and 0.59 ppm (adult snails) in 24 h, respectively. In field immersion experiments, nicotinanilide could kill 95% of the snails at the concentration of 1–2 ppm in 3 days. Nicotinanilide was also harmless to humans, animals, fish, and plant (Sukumaran et al. 2004). Disadvantages include strong irritation to human skin, low toxicity to snail eggs, and high cost, which seriously restrict the large-scale application of the molluscicide.
Dipterex
Dipterex (7, Table 1) is an important organic phosphorous pesticide. Dipterex displayed promising molluscicidal activity, and the mortality of snails was 96% at 10 ppm in 3 days (Xu et al. 2016). Huang’s group explored the molluscicidal activity of 4% niclosamide ethanolamine salt powder-granula (PG) derived from niclosamide ethanolamine salt and additives (You et al. 2016). Compared with 4% NESDP, PG showed lower mortalities of the snails (O. hupensis) in laboratory test. However, in field experiments, for PG, the mortalities of snails were 91.71% (1 day) and 92.91% (3 days), which were higher than that of 4% NESDP with the mortalities of snails of 71.09% (1 day) and 90.11% (3 days). 4% PG has the advantages of good adsorption and penetrability, which may be more suitable for field applications.
To solve the solubility of niclosamide, emulsifiable concentrate of 25% niclosamide (ECN) was further prepared from niclosamide, organic solvents, and emulsifiers (Dai et al. 2007). In immersion experiments, the LC50 values of ECN were 0.041 ppm (24 h), 0.032 ppm (48 h), and 0.029 ppm (72 h), respectively, significantly lower than the control groups of SCN and WPN. ECN has more chance to contact snails, resulting in better molluscicidal activities. However, the addition of solvents and emulsifiers causes higher cost of molluscicide and environmental pollution.
Three new polymeric controlled release formulations of niclosamide (B1, B2, and B3) against B. alexandrina were developed by Kenawy et al. (Kenawy and Rizk 2004). These polymeric formulations were prepared either by the chemical modifications of poly(glycidyl methacrylate) or by physical entrapment of the niclosamide in calcium alginate beads. In the immersion experiments, LC50 values were 0.098 ppm (B1), 1.09 ppm (B2), and 0.073 ppm (B3), respectively. For B3, the mortality of snails was still up to 100% after 10 days. The polymeric molluscicide (B3) showed the highest molluscicidal activity than B1 and B2.
Recently, Harras et al. reported a new polymeric molluscicide-attractant from niclosamide and L-glutamate by a controlled-release technology to control B. alexandrina (Kenawy et al. 2020). The alginate niclosamide-L-glutamate formulation had good affinity ability to snails. The polymeric formulations B3-4b-L-glutamate (0.3 ppm niclosamide and 75% L-glutamate) caused the aggregation of snails and showed 100% mortality after 5 days. The novel polymer-niclosamide-L-glutamate not only prolonged the validity and efficiency of the niclosamide, but also still was effective at low concentrations with increasing concentrations of the applied attractants, which could reduce the toxicity to the ecosystem.
Though excellent advances on novel niclosamide formulations have been accomplished, there is still a huge challenge to reduce the toxicity through the structure modification strategy.
Yuan et al. reported the sodium of niclosamide sodium quinoid-2′, 5-dichloro-4′-nitrosalicylanilide (13, Table 1) (LDS) obtained through the reaction of NaOH with niclosamide (Yuan et al. 2011). LDS showed better solubility in water and ethanol, and lower toxicity than that of niclosamide. The molluscicidal activities of 10% LDS and 50% WPN were further investigated (Yuan et al. 2011). In immersion experiments, the mortalities of snails for LDS and WPN were 100% and 96.7% at 0.4 ppm in 72 h, respectively. In spraying experiments, the mortalities of snails for LDS and WPN were up to 100% and 97.3%, respectively. In field immersion and spraying experiments, LDS and WPN showed the similar molluscicidal activities (Xu et al. 2007; Zhang et al. 2013). In addition, LDS exhibited climbing-inhibition effect against snails (O. hupensis), and the cost of LDS was also less than that of WPN (** of novel formulations and rational structural modification may be an important research area.
Pyridylphenylureas
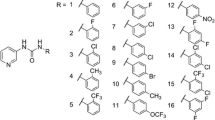
Wang et al. developed a series of pyridylphenylurea derivatives against B. straminea snails through the introduction of a urea functional group into the structure of nicotinanilide (Wang et al. 2018). For compounds 21 and 22 (Table 1), the mortalities against adult snails were all up to 100% at 1.0 ppm, and the LC50 values were 0.50 ppm and 0.51 ppm in immersion experiments, respectively (LC90 = 0.98 and 0.78 ppm). And the LC50 values against snail eggs were 0.05 ppm and 0.09 ppm, respectively (LC90 = 0.21 and 0.39 ppm). The acute lethal fish toxicity experimental results revealed that the safe dose of compound 22 was up to 5 ppm, which was higher than that of the effective molluscicidal dose (LC90, 0.78 ppm).
Furthermore, compound 21 (named PPU07) was made into the form of 25% PPU07 sulfate WP. The field molluscicidal activity and toxicity of 25% PPU07 sulfate WP were investigated against O. hupensis and local fish (Chen et al. 2019). In the spraying experiments, the mortality of snails was up to 95% at the concentration of 2.0 g/m2. When snails were exposed in the 2.0 g/m3 of the 25% PPU07 sulfate WP, the mortality reached 96%. In the spraying and immersion trials, the WHO-recommended molluscicidal concentrations of niclosamide were 1 g/m2 and 1 g/m3, respectively. Additionally, 25% PPU07 sulfate WP showed little toxicity to local fish and other aquatic organisms at the effective molluscicidal concentrations. PPU07 could be explored as promising molluscicide candidate for the control of snails.
Nitro-compounds
Abreu et al. explored the molluscicidal activity of nitroaromatic compounds against B. glabrata snails (de Abreu et al. 2001). Compounds 23–26 (Table 1) presented a significant bioactivity and the LC50 values of them (23, LC50 = 1.8 ppm; 24a, LC50 = 8.2 ppm; 24b, LC50 = 0.7 ppm; 25, LC50 = 7.6 ppm; 26, LC50 = 6.4 ppm) were lower than 10 ppm in 24 h. Further electrochemical tests revealed that the presence of the electro-active NO2 group in the molecular structures was crucial for the retention of molluscicidal activity. Bond et al. also discovered the molluscicidal activity of 4-(substituted phenoxy)-3,β-dinitrostyrenes against B. glabrata (Bond et al. 1969). The LC50 values of compounds 27a-d (Table 1) were 8.5, 12, 8.5, and 22 ppm, respectively. It is notable that β-nitrostyrene 28 (Table 1) displayed the best activity among the tested compounds (LC50 = 1.3 ppm).
Phenolic compounds
Lahlou et al. prepared some phenolic compounds for the control of Bulinus truncates snails (Lahlou 2004). Compounds 29–31 (Table 1) demonstrated good molluscicidal activity (29, LC50 = 8.89 ppm, LC90 = 16.82 ppm; 30, LC50 = 3.60 ppm, LC90 = 4.47 ppm; 31, LC50 = 7.71 ppm, LC90 = 14.14 ppm). The compound 30 was the most effective among the phenolic products.
Chalcones and its derivatives
Adewunmi et al. tested the molluscicidal activity of a serial of chalcones and chalcone epoxides (32, Table 1) against B. glabrata snails (Adewunmi et al. 1987). An appropriate hydrophile-lipophile balance in the structure of chalocones should facilitate the improvement of activity. For example, the mortality of compound 32c against snails was up to 100% at 10 ppm in 24 h; however, the activity of compound 32f declined dramatically (37.5% mortality) when the two OH groups were methylated. Furthermore, the epoxidation of the chalcones also resulted in the loss of molluscicidal activity. Nawwar et al. investigated the molluscicidal activity of several l-(hydroxy/substituted phenyl) propenones (33–35, Table 1) against B. alexandria snails (Nawwar et al. 1993). The activity of the prepared compounds was related to the conjugated system. Compounds 34a–c showed the best results among the synthetic products. Especially compound 34b afforded 100% mortality of the snails at 2 ppm in 24 h. When the furan moiety was removed, the molluscicidal activity exhibited negative results. Barsoum et al. evaluated the molluscicidal activity of bis[1-aryl-2-propen- 1-ones] (chalcones) (36a–h, Table 1) and bis[3-aryl-4,5-dihydro-1H-pyrazol-1-carboxaldehydes] (37a–h, Table 1) against B. alexandrina snails (Barsoum et al. 2006). Most of the synthesized products showed moderate bioactivity at 20 ppm in 24 h. Compounds 37 exhibited superior molluscicidal activity than those of 36a–h. The substitution of F and Cl groups on pyrazol rings of substrates should enhance the molluscicidal activity.
3-Hydroxy-arylpropanenitriles
Vasconcellos et al. evaluated the molluscicidal activity of 3-hydroxy-arylpropanenitriles (38–40, Table 1) against B. glabrata snails (Vasconcellos et al. 2006). Compound 38 showed the highest activity among the tested compounds in 24 h (38, LC50 = 6.64 ppm, LC90 = 9.23 ppm; 39, LC50 = 7.30 ppm, LC90 = 10.64 ppm; 40, LC50 = 17.71 ppm, LC90 = 22.7 ppm). Compound 40 displayed the lowest toxicity to snails, which could be attributed to the different hydrophilic-lipophilic ability of the naphthalene nucleus. The detailed structural-activity relationships of the 3-hydroxy-arylpropanenitriles should be further explored in future experiments.
Five-membered heterocylces
Kanawade et al. explored novel thiophenedicarboxamide and dicyanothiopheneacetamide derivatives for the control of Indoplanorbis exustus snails (Kanawade et al. 2011). The LC50 values of compounds 41a and 42a (Table 1) were 0.6043 and 0.6511 ppm in immersion experiments, respectively. Fadda et al. evaluated the molluscicidal activity of thiophene, thiadiazole, and pyrazole derivatives (43–45, Table 1) against B. alexandrina snails (Fadda et al. 2009). Thiadiazole derivatives (44a–c) possessed higher activity than that of thiophene derivatives (43a–d) apparently because of the strong electron-donating effect of the thiadiazole ring. For example, compounds 44b and 44c demonstrated the best activity with the LC50 value of 5.5 ppm and 6 ppm, respectively. The surface of snails demonstrated the effusion of the gelatinous materials, which could cause the toxic effect on the cell membranes and resulted in hemolysis and subsequent death (Fadda et al. 2009).
El Shehry et al. carried out the molluscicidal activity of 3-((2,4-dichlorophenoxy)methyl)-1,2,4-triazolo(thiadiazoles and thiadiazines) (46–50, Table 1) against B. alexandrina snails (El Shehry et al. 2010a, b). Compounds 46 and 47 displayed 100% mortality of snails at 10 ppm in 24 h. However, the other compounds (48–50) showed moderate to low activity against snails. Abdelrazek et al. developed a serial of pyrazole derivatives for the control of B. alexandrina snails. Products 51a–d (Table 1) exhibited good molluscicidal activity in immersion experiments (LC50 = 11–13 ppm; LC90 ≥ 14 ppm) (Abdelrazek et al. 2006a, b).
Six-membered N-heterocyles
Some novel functionally substituted pyridines and pyridazines (52–54, Table 1) were prepared and evaluated against B. alexandrina snails in 24 h (LC100 = 6–15 ppm) (Abdelrazek and Fathy 2005). The electron-withdrawing groups on the aromatic ring (Ar group) of the tested compounds (52b, 53b, 54b) showed higher molluscicidal activity in 24 h.
El-Bayouki et al. discovered the molluscicidal activity of some thiazolo[5,4-d]pyrimidines (55, Table 1) against B. alexandrina snails (El-Bayouki and Basyouni 1988). Compounds 55a and 55i showed more effective activity, and the mortalities of snails were up to 100% at 25 ppm in 24 h. Bakhotmah et al. evaluated the molluscicidal activity of the phosphorus compounds bearing an amino pyrimidine-substituted pyrazolo[3,4-d]pyrimidine moiety against B. alexandrina snails (Bakhotmah 2019). For compounds 56 and 57 (Table 1), the mortalities of snails were all only 30% at 50 ppm in immersion experiments in 24 h.
Abdelrazek et al. developed new cinnoline derivatives and evaluated the molluscicidal activity to B. alexandrina snails (Abdelrazek et al. 2006a). Compounds 58–59 (Table 1) possessed good effects on the snails (58a, LC50 = 8 ppm, LC90 = 11 ppm; 58b, LC50 = 7 ppm, LC90 = 15 ppm; 58c, LC50 = 7 ppm, LC90 = 9 ppm; 59a, LC50 = 8 ppm, LC90 = 13 ppm; 59b, LC50 = 9 ppm, LC90 > 15 ppm; 59c, LC50 = 7 ppm, LC90 = 9 ppm). Compounds 58c and 59c showed superior activities mainly attributed to the 4-chlorophenyl group. Ali et al. explored the molluscicidal activity of phosphorus-containing 3-hydrazino-1,2,4-triazines against B. alexandrina snails (Ali et al. 2008). However, the tested products (60–62, Table 1) showed low activity at 50 ppm in 24 h. Abdel-Rahman et al. investigated a serial of 6-methyl-5-styryl-1,2,4-triazin-3-thiol derivatives for the control of B. alexandrina snails (Abdel-Rahman et al. 2003). Compounds 63, 64, and 66 (Table 1) had the moderate molluscicidal activity at 50 ppm in immersion experiments in 24 h. The p-dimethylamino, 1, 2, 4-dichloro phenyl, and β-naphthol moieties should play an essential role against snails. The molluscicidal activity of phthalazin-one 67 (Table 1) was also investigated for the control of B. alexandrina snails, and the LC50 value was 9 ppm in 24 h (67, LC90 = 13 ppm) (Abdelrazek et al. 2006a, b).
Pyrane derivatives
Souza et al. evaluated the molluscicidal activity of the 5,6-dimethyl-dihydro-pyran-2,4-dione and 6-substituted analogous (de Souza et al. 2004). Compounds 68–70 (Table 1) showed moderate activity against B. glabrata egg masses. The substitution of the propenyl or phenyl group in C-6 position of dihydro-pyran-2,4-diones could improve the molluscicidal activity. Flavones 71 and 72 (Table 1) were developed for the control of Bulinus truncatus snails, and the LC50 values of them were 5.47 ppm and 8.91 ppm in 24 h, respectively (71, LC90 = 9 ppm; 72, LC90 = 17 ppm) (Lahlou 2004).
Abdelrazek et al. synthesized and evaluated the molluscicidal activity of new chromene and pyrano[2,3-c]pyrazole derivatives (73–77, Table 1) against B. alexandrina snails (Abdelrazek et al. 2007). Compounds 75 and 76b afforded the most effective activity. The decoration of dimethyl substituents in the cyclohexanone ring and the fused pyran ring might improve molluscicidal bioactivity. For 76b, the NH of the indolyl moiety afforded the chelation effect, resulted in the enhancement of molluscicidal activity. The pyrane derivatives 78–82 (Table 1) were prepared for the control of B. alexandrina snails. All synthesized products exhibited generally moderate molluscicidal activity in immersion experiments. The most effective of them are 79a, 80a, 82a, and 82b (LC50 < 10 ppm). It seemed that pyranopyrazole derivative 79a should be a promising leading compound after appropriate modifications in future (Abdelrazek et al. 2006a, b).
Abdelrazek et al. discovered the molluscicidal activity of 5-oxo-5,6,7,8-tetrahydro-4H-chromene derivatives against B. alexandrina snails (Abdelrazek et al. 2004). The LC50 values of compounds 83a and 84a (Table 1) were 5 ppm (17.61 nM) and 8 ppm (28.17 nM) in 24 h, respectively, which are still far inferior to niclosamide (LC100 = 1 ppm). New pyrano derivative 85a (Table 1) exhibited the highest activity against B. alexandrina snails within the pyrano[2,3-c]pyrazole series (Abdelrazek et al. 2006a), which should be modified and considered in future research.
Benzofuran derivatives
El Shehry et al. screened the molluscicidal activity of some pyrazole, isoxazole, pyridine, pyrimidine, 1,4-thiazine, and 1,3,4-thiadiazine derivatives incorporating benzofuran moiety (86–89, Table 1) against B. alexandrina snails (El Shehry et al. 2010a, b). Compound 86 showed the highest molluscicidal activity and the mortality of snails was up to 100% at 10 ppm in immersion experiments in 24 h. Compounds 87–89 displayed good to moderate activity among the tested products. Giri et al. found that 1-aryl-1-(substituted benzofur-2-yl)-2-benzylcarbinols (90, Table 1) and 1-aryl-1-(substituted benzofur-2-yl)-2-phenylethylenes (91, Table 1) exhibited moderate activity against Lymnea acuminata snails (Giri and Mishra 1984a, b). Hassan et al. developed furo-salicylanilides for the control of B. alexandrina snails (Hassan et al. 2006). Compounds 92b and 92d showed lower molar concentration than that of Bayluscicide. However, cyclization of compound 92b to afford compound 93b (Table 1) resulted in the decrease of molluscicidal activity.
Benzimidazole derivatives
Nofal et al. developed the molluscicidal activity of benzimidazole derivatives for the control of B. alexandrine snails (Nofal et al. 2002). Compounds 94 and 95 (Table 1) exhibited moderate molluscicidal activity at 24 h in immersion experiments. However, the polycyclic benzimidazole derivatives 96–97 showed worse molluscicidal activity.
Coumarin derivatives
Giri et al. developed the molluscicidal activity of 3-substituted 4-hydroxycoumarin derivatives for the control of Lymnaea acuminata snails (Giri and Mishra 1984a, b). The compounds 98 and 99 (Table 1) showed the good molluscicidal activity at 5 ppm, and the mortalities of snails were 58.33% and 68.33% in 24 h, respectively. Schönberg et al. explored the molluscicidal activity of furocoumarins against Biomphalaria alexandrina snails (Schönberg and Latif 1954). Compounds 100 and 101 (Table 1) killed 100% and 69% of the snails at 5 ppm in immersion experiments in 24 h, respectively.
Lapachol and its derivatives
Lapachol (102, Table 1) is a notable natural product from Tecoma heptaphylla (Vell Mart.) (Bignoniaceae). Lapachol showed poor molluscicidal activity against snails in earlier reports (Marston et al. 1984). Santos et al. re-investigated the activity of lapachol and its derivatives against the B. glabrata snails (dos Santos et al. 2000). In immersion experiments, the tested compounds 102–105 (Table 1) showed a significant molluscicidal activity against adult snails in 24 h (compound 102, LC50 = 2.57 ppm, LC90 = 6.18 ppm; compound 103, LC50 = 1.53 ppm, LC90 = 4.30 ppm; compound 104, LC50 = 4.02 ppm, LC90 = 7.15 ppm; and compound 105, LC50 = 5.19 ppm, LC90 = 18.46 ppm). Additionally, compound 104 exhibited the highest molluscicidal activity against snail eggs (LC50 = 0.014 ppm, LC90 = 0.070 ppm) in immersion experiments in 24 h. Furthermore, the activity of 2-hydroxy-3-substituted-aminomethyl derivatives was also investigated against snail eggs. However, the activity of them was lower than compounds 102–105. Santos et al. developed a soluble potassium salt of lapachol 106 (Table 1) from the reaction of lapachol with KOH (dos Santos et al. 2001). The LC50 values of 106 against B. glabrata adult snails and eggs reached 2.70 and 1.43 ppm after 24 h in the immersion experiments, respectively. Silva et al. developed a series of amino and hydrogenated lapachol derivatives (107–108, Table 1) for the control of B. glabrata snails (Silva et al. 2005). Compounds 107a, 107b, 108a, 108c, 108d, 108e, and 108 h showed medium toxicity against snails (23.8 < LC50 < 89.0 mmol/L) in immersion experiments. However, 107c, 108b, and 108f were less active (LC50 > 200 mmol/L). It is notable that compounds 108 g and 109 (Table 1) exhibited significant molluscicidal activity and the LC50 values were 13.8 and 7.6 mmol/L in 24 h, respectively.
The molluscicidal activity of the potassium salt of isolapachol (110, Table 1) was explored for the control of B. glabrata (Lima et al. 2002a, b). Compound 110 exhibited good activity against adult snails (24 h LC50 3.05 ppm, LC90 4.71 ppm) and snail eggs (24 h LC50 0.33 ppm, LC90 0.48 ppm) in immersion experiments. However, 110 showed high toxicity against fishes (T. nilotica) (24 h LC50 2.05 ppm, LC90 2.43 ppm) and planktonic crustaceae (A. salina) (24 h LC50 2.05 ppm, LC90 2.43 ppm). Lima et al. evaluated the molluscicidal activity of lapachol and its derivatives and analogues for the control of B. glabrata snails (Lima et al. 2002a). The LC50 values of compounds 111a and 111c (Table 1) against adult snails were 0.98 and 1.66 ppm in 24 h, respectively. Compounds 111b, 111d, 111e, and 112 (Table 1) also displayed the moderate molluscicidal activity, with LC50 values in the range of 3.82–7.72 ppm in 24 h.
Ribeiro et al. prepared some naphthoquinones and evaluated the molluscicidal activity of compounds 113–114 (Table 1) for the control of B. glabrata snails (Ribeiro et al. 2009). The LC50 values of the tested products ranged from 0.475 to 3.049 ppm in 24 h. Camara et al. evaluated the molluscicidal activity of naphthoquinone derivatives for the control of B. glabrata snails (Camara et al. 2008). The presence of a bromine group in compound 115c (Table 1) resulted in almost 15-fold molluscicidal activity compared to compound 115a. However, these synthesized products exhibited obvious toxicity to the brine shrimp A. salina (Camara et al. 2008). Barbosa et al. developed new 2-aminoalkyl substituted anthraquinones from norlapachol for the control of B. glabrata snails (Barbosa et al. 2005). Compounds 116a–h (Table 1) displayed moderate activity. The increase of polarity of molecules can lead to the decrease of bioactivity (for 116b and 116c), which exhibits a similar trend to that of amino lapachol derivatives reported by Silva’s group (Silva et al. 2005). Therefore, compound 116 g displays the best molluscicidal activity among the tested products. However, the molluscicidal activity was sharply diminished when the aminoalkyl substituted compounds were converted into the cyclic molecules 117 and 118 (Table 1).
The above findings confirm the importance of lapachol and its derivatives as promising molluscicides.
Quinoline derivatives
Serials of novel enaminones derived from 4-hydroxyquinolinones (119–122, Table 1) were prepared by Abass’ group (Abass and Mostafa 2005). Part of these products exhibited good molluscicidal activity of against B. alexandrina and Lymnaea natalensis snails (LC50 < 20 ppm). For compounds 122a–c, the structure–activity relationship showed that the molluscicidal activity increased with the improvement of the lipophilicity of the molecules. In addition, the acute toxicity of the tested molluscicides against D. magna was also explored, and the experimental results revealed that the candidate compounds showed 0% mortality at LC50 concentrations after 48 h. Kujime et al. evaluated the molluscicidal activity of acridone alkaloids against B. glabrata snails (Kujime et al. 1992). Compounds 123 and 124 (Table 1) showed moderate bioactivity in 24 h. El Bardicy et al. evaluated the molluscicidal activity of aminoalkylamino substituted neo- and norneocryptolepine derivatives (125–131, Table 1) against B. alexandrina snails (El Bardicy et al. 2012). The LC50 values of the tested products were 0.63–3.9 ppm in 24 h.
Our group discovered the molluscicidal activity of 3-substituted quinazolinone derivatives 132 (Table 1) through a scaffold hop** approach using a pseudo-ring based on the intramolecular hydrogen bond formation (Guo et al. 2016). Most of the tested compounds showed good molluscicidal activity against O. hupensis with the LC50 values ranged from 2.69 to 10 ppm. The preliminary structure–activity relationship exhibited that the aromatic rings are essential for C section and the electron-donating groups on the C ring can promote the molluscicidal activity.
Thiaxanthene derivatives
El-Sakka et al. evaluated thiaxanthene derivatives (133–137, Table 1) against B. alexandrina snails (El-Sakka et al. 1994). Compounds 136–137 showed considerable activity, and 135 possessed the highest mortality in 24 h at 25 ppm.
Development of plant molluscicides
Chemical molluscicides are still the most convenient option for the control of snails and have been used successfully in practical applications (Perrett and Whitfield 1996). However, the registered chemical molluscicides (such as niclosamide, copper sulfate, and sodium pentachlorophenate) are still rare due to high cost, toxicity to non-target organisms, high residual risk, and relatively complicated synthesis process (Brackenbury and Appleton 1998). Some plant molluscicides are considered inexpensive and environmental friendly to the health of aquatic organisms and mammals, and some pure compounds have also been isolated from plant and proven to have molluscicidal activity against snails. Development of natural plant molluscicides may be a suitable alternative for snail control (Zhu et al. 2010).
Sesquiterpene lactones
Borkosky et al. evaluated the molluscicidal activity of sesquiterpene lactones from the tribe Vernonieae, family Asteraceae (Borkosky et al. 2009). The tested products 138–147 (Table 1) demonstrated moderate molluscicidal activity against B. peregrina snails. The LC50 values ranged from 27.99 to 88.25 ppm in 24 h.
Monoterpenoids
Radwan et al. evaluated the molluscicidal activity of monoterpenoid derivatives (148–155, Table 1) for the control of B. alexandrina snails (Radwan et al. 2008). Most of the compounds showed molluscicidal activity; especially compound 155b was the most effective among the tested compounds (LC50 = 5.43 ppm). Furthermore, mixing compounds 154a and 155a with piperonyl butoxide (PBO) afforded the LC50 values of 2.69 and 2.72 ppm in 24 h, respectively.
Diterpenoids
Dos Santos et al. discovered the molluscicidal activity of diterpenoids (156–157, Table 1) isolated from Jatropha elliptica (Pohl) Muell. Arg (dos Santos and Sant’Ana 1999). Compared with product 157, products 156 showed molluscicidal with the LC50 values of 1.16 ppm (adult snails) and 1.14 ppm (snail eggs) in 24 h. However, product 157 was not active against B. glabrata snails at a concentration of 100 ppm. Abdelgaleil et al. extracted some diterpenes (158–161, Table 1) from Euphorbia paralias L and evaluated the molluscicidal activity against B. alexandrina snails (Abdelgaleil et al. 2002). Most of the obtained products showed moderate molluscicidal activity except product 160c. Notably, among the isolated products, product 158b showed the best activity with the 100% mortality at 15 ppm in 24 h. The preliminary structure–activity relationship of the tested products exhibited that paraliane diterpenes (158a–b) showed higher activity than segetane diterpenes (159, 160a–c) to snails. For paraliane diterpenes, the acetoxyl group at C1 in compound 158a was unfavorable for the improvement of activity. In the case of 160a–c, the present of acetoxy group (R2) facilitated the molluscicidal activities, which were similar to 161a–c.
Triterpenoid saponins
Molluscicidal activity of triterpenoid saponins from Maesa lanceolata was assessed by Apers et al. (2001). Among the tested products, compound 162c (Table 1) showed the best activity against B. glabrata snails and the LC50 value was 0.5 ppm in 24 h. Novel triterpene saponins (163a–d, Table 1) were isolated from African medicinal plant, Pachyelasma tessmannii for the control of B. glabrata snails (Nihei et al. 2005). The LC50 values of 163a–d were 2, 2, 2, and 8 ppm within 24 h, respectively. Triterpenoid saponins 164 and 165 (Table 1) were extracted from the roots of Pueraria peduncularis (Chen et al. 2020). The two products 164 and 165 displayed moderate molluscicidal activity with the mortalities of 15% and 21.25% at 30 ppm in 24 h, respectively. A pentacyclic triterpenoid saponin 166 (Table 1) was exacted from the seed pomace of Camellia oleifere, which was widely cultivated in South China (Jia et al. 2019). Product 166 has been named as tea-seed distilled saponin (TDS) and been registered as Luo-Wei by the Ministry of Agriculture (MoA) of China. Compound 166 showed significant molluscicidal activity against O. hupensis, B. alexandrina, and Bulinus truncates, and the LC50 values were 0.701 ppm, 1.975 ppm, and 1.396 ppm in 24 h, respectively. In addition, TDS showed moderate toxicity to Japanese quail and shrimp, and high toxicity to zebrafish (96 h LC50 = 0.15 ppm). For all that, TDS may be a promising candidate molluscicide of plant origin to eliminate snails in high-risk areas. Ekabo et al. tested the novel saponins (167–168, Table 1) extracted from Serjania salzmanniana for the control of B. alexandrina snails (Ekabo and Farnsworth 1996). For products (167a–b, 168), the mortalities of snails were 70–100% at 10 ppm in 24 h. However, product 167c exhibited no activity at 10 ppm.
Cardiac glycosides
The two molluscicidal active cardiac glycosides, cerberin 169 and neriifolin 170 (Table 1), were isolated from the stems of Adenium obesum (Alzabib et al. 2019). Products 169 and 170 had significant molluscicidal activity against Monacha obstructa snails with LC50 values of 5.39 and 4.30 ppm in 24 h, respectively.
Ginkgolic acids
Yang et al. evaluated the molluscicidal activity of ginkgolic acids (171a–c, Table 1), isolated from Ginko sarcotesta against O. hupensis (Yang et al. 2005). However, the stability of these new formulations should be screened, and simultaneously require more field experiments in future works.
Plant molluscicides attract more and more attention. However, some unfavorable factors should not be overlooked (Li et al. 2002): (1) low content of the active ingredients resulted in low molluscicidal activity; (2) regional limitations for some specific molluscicidal plants; (3) relative complicated extraction process resulting in high cost; (4) environmental pollution because of the application of fresh molluscicidal plants. In addition, for plant molluscicides, the characterization of the structures of active ingredients is also very important, which could provide the reference for the development of novel chemical molluscicides and later modifications (Whitfield 1996). However, develo** novel, low-toxicity, and highly effective plant molluscicides and elucidating the possible mechanism in detail are still great challenges.
For laboratory and field testing of molluscicides for schistosomiasis (WHO (World Health Organization) 2019), we have: (1) for the new candidate molluscicide compound and for new end-use formulations of existing molluscicide compounds, an optimum effective dose should be determined based on the dose–response curve. The manufacturer’s label claim may also be considered to decide the optimum dose to be tested; (2) the efficacy of exposure in immersion bioassay and residual activity at this effective dose is then determined according to the immersion bioassay. The efficacy cut-off is the 80% mortality and the effective residual action is the duration until when the mortality in treatments remains ≥ 80%; (3) a dose–response curve is needed to determine LC50 and LC95 values. The results are recorded in a standard data recording form. The relationship between dose and mortality is analyzed using log-probit (Finney 1971) or logit regression; (4) from the dosages tested against a target species in the small-scale or simulated field trials, the minimum efficacious dosage (mortality and residual effect) should be selected as the optimum field application dosage for each type of habitat; (5) the WHO cut-off for the efficacy of a molluscicide formulation against adult snails is ≥ 80% mortality in 24 h post-exposure. The residual efficacy of the molluscicide is calculated as the duration (days) when mortality in snails remains ≥ 80%.
The present mechanism investigations for chemical and natural molluscicides focus on the simple interactions between molluscicides and some enzymes, and lack visual evidence in structural biology. Therefore, investigating the structure biology of target enzymes/proteins and studying the corresponding the action sites could help in designing novel molluscicides to combat snails (Wu et al. 2006). We believe that the original and innovative molluscicides will soon emerge after the above issues are resolved by more scientists, followed by a prompt growth in advancements.
References
Abass M, Mostafa BB (2005) Synthesis and evaluation of molluscicidal and larvicidal activities of some novel enaminones derived from 4-hydroxyquinolinones: Part IX. Bioorg Med Chem 13:6133–6144
Abdelgaleil SAM, El-Aswad AF, Nakatani M (2002) Molluscicidal and anti-feedant activities of diterpenes from Euphorbia paralias L. Pest Manag Sci 58:479–482
Abdel-Gawad MM, El-Amin SM, Ohigashi H, Watanabe Y, Takeda N, Sugiyama H, Kawanaka M (2000) Molluscicidal saponins from Anagallis arvensis against schistosome intermediate hosts. Jpn J Infect Dis 53:17–19
Abdel-Rahman TM, Shalaby AA, Nassar IF (2003) Synthesis of heterobicyclic nitrogen compounds as mollusicicde agents derived from 6-methyl-5-styryl-1,2,4-triazin-3-thiol: Part I. Phosphorus Sulfur 178:279–292
Abdelrazek FM, Fathy AEDM (2005) A novel synthesis and molluscicidal activity of some functionally substituted pyridine, pyrido-[3,2-c]pyridazine, and pyrido[3,2-c]pyridazino-[2’,3’-a]quinazoline derivatives. Arch Pharm Chem Life Sci 338:329–334
Abdelrazek FM, Metz P, Farrag EK (2004) Synthesis and molluscicidal activity of 5-oxo-5,6,7,8-tetrahydro-4H-chromene derivatives. Arch Pharm Pharm Med Chem 337:482–485
Abdelrazek FM, Metz P, Metwally NH, El-Mahrouky SF (2006a) Synthesis and molluscicidal activity of new cinnoline and pyrano [2,3-c]pyrazole derivatives. Arch Pharm Chem Life Sci 339:456–460
Abdelrazek FM, Michael FA, Mohamed AE (2006b) Synthesis and molluscicidal activity of some 1,3,4-Triaryl-5-chloropyrazole, pyrano[2,3-c]pyrazole, pyrazolylphthalazine and pyrano[2,3-d]thiazole derivatives. Arch Pharm Chem Life Sci 339:305–312
Abdelrazek FM, Metz P, Kataeva O, Jäger A, El-Mahrouky SF (2007) Synthesis and molluscicidal activity of new chromene and pyrano[2,3-c]pyrazole derivatives. Arch Pharm Chem Life Sci 340:543–548
Adewunmi CO, Ogungbamila FO, Oluwadiya JO (1987) Molluscicidal activities of some synthetic chalcones. Planta Med 53:110–112
Ali TE, Abdel-Rahman RM, Hanafy FI, El-Edfawy SM (2008) Synthesis and molluscicidal activity of phosphorus-containing heterocyclic compounds derived from 5,6-bis (4-bromophenyl)-3-hydrazino-1,2,4-triazine. Phosphorus Sulfur 183:2565–2577
Al-Sarar A, Hussein H, Abobakr Y, Bayoumi A (2012) Molluscicidal activity of methomyl and cardenolide extracts from Calotropis procera and Adenium arabicum against the land snail Monacha cantiana. Molecules 17:5310–5318
Alzabib AA, Abobakr Y, Al-Sarar AS, Hussein HI, Basudan OA, El-Gamal AA, Abdel-Kaderd MS, El-Komy MH (2019) Molluscicidal activity of cardiac glycosides isolated from Adenium obesum. Pest Manag Sci 75:2770–2775
Apers S, Baronikova S, Sindambiwe J-B, Witvrouw M, Clercq ED, Berghe D, Marck EV, Vlietinck A, Pieters L (2001) Antiviral, haemolytic and molluscicidal activities of triterpenoid saponins from Maesa lanceolata: establishment of structure-activity relationships. Planta Med 67:528–532
Bakhotmah DA (2019) Synthesis of fluorine and phosphorus compounds bearing an amino pyrimidine-substituted pyrazolo[3,4-d]pyrimidine moiety as molluscicidal agents against some snails. Polycycl Aromat Comp. https://doi.org/10.1080/10406638.2019.1625066
Barbosa TP, Camara CA, Silva TMS, Martins RM, Pinto AC, Vargasc MD (2005) New 1,2,3,4-tetrahydro-1-aza-anthraquinones and 2-aminoalkyl compounds from norlapachol with molluscicidal activity. Bioorg Med Chem 13:6464–6469
Barsoum FF, Hosnib HM, Girgisb AS (2006) Novel bis(1-acyl-2-pyrazolines) of potential anti-inflammatory and molluscicidal properties. Bioorg Med Chem 14:3929–3937
Bond HW, O’Grodnick JS, Pringle BH (1969) Tests of compounds for molluscicidal activity. VI. 4-(substituted phenoxy)-3, β-dinitrostyrenes. J Med Chem 12:1107–1108
Borkosky S, de Leóna SP, Juárez G, Sierra MG, Bardón A (2009) Molluscicidal sesquiterpene lactones from species of the Tribe vernonieae (Compositae). Chem Biodivers 6:513–519
Brackenbury TD, Appleton CC (1998) Plant molluscicides in South Africa: a registration dilemma. Parasitol Today 14:83–84
Brimer L, Elsheik S, Furu P (2007) Preliminary investigation of the disposition of the molluscicidal saponin deltonin from Balanites aegyptiaca in a snail species (Biomphalaria glabrata) and in mice. J Pestic Sci 32:213–221
Bringmann G, Holenz J, Assi LA, Zhao C, Hostettmann K (1996) Molluscicidal activity of naphthylisoquinoline alkaloids from triphyophyllum and ancistrocladus species. Planta Med 62:556–557
Bringmann G, Holenz J, Assi LA, Hostettmann K (1998) Molluscicidal activity (Biomphalariaglabrata) of Dioncophylline A: structure-activity investigations. Planta Med 64:485–486
Camara CA, Silva TMS, Da-Silva TG, Martins RM, Barbosa TP, Pinto AC, Vargas MD (2008) Molluscicidal activity of 2-hydroxy-[1,4]naphthoquinone and derivatives. An Acad Bras Ciênc 80:329–334
Cao ZG, Wang TP (2008) Research progress on snail control with drugs in China. Int J Med Parasit Dis 35:276–280 (in Chinese)
Changbunjong T, Wongwit W, Leemingsawat S, Tongtokit Y, Deesin V (2010) Effect of crude extract of Solanum xanthocarpum against snails and mosquito larvae. Southeast Asian J Trop Med Public Health 41:320–325
Chen Y, Fang P, Yi YR, Chen BP, WangY NYH, Ke WS (2001) Observation of influence of fluid extracted from Nerium Indicum leaves of soft tissues of Oncomelania hupensis by scanning electron microscope. Chin J Parasitol Dis Control 14:217–218 (in Chinese)
Chen SX, Wu L, Yang XM, Jiang XG, Li LG, Zhang RX, **a L, Shao SH (2007) Comparative molluscicidal action of extract of Ginko biloba sarcotesta, arecoline and niclosamide on snail hosts of Schistosoma japonicum. Pestic Biochem Physiol 89:237–241
Chen Q, Wang WX, Ke WS, Liu X, **ang XL (2008) Influences of stemona alkaloids on esterase isozymes activities and glycogen content in Oncomelania hupensis. Chin J Schisto Control 20:130–132 (in Chinese)
Chen Z, Wang W, Yao J, Li S, Zhang X, Hu H, Liu X, Luo B, Liu Y, Xu H, Duan L (2019) Toxicity of a molluscicide candidate PPU07 against Oncomelania hupensis (Gredler, 1881) and local fish in field evaluation. Chemosphere 222:56–61
Chen HB, Lv TX, Zhang M, Liao JX, Chang XL, Yue GZ, Li PL, Zhao XM, Qiu XY, Qian Y, Yang CP (2020) Two new compounds from the roots of Pueraria peduncularis and their molluscicidal effects on Pomacea canaliculata. J Asian Nat Prod Res 22:144–152
Colley DG, Bustinduy AL, Secor E, King CH (2014) Human schistosomiasis. Lancet 383:2253–2264
Dai J, Wu F, Gao Z, Zhang Y, Jiang Y, ** W, Huang Y, Zhou X (2001) Study on the molluscicidal effect of shachongding against oncomelania in laboralory and field. Chin J Zoonoses 17:68–70 (in Chinese)
Dai JR, Liang YS, Li HJ, Tao YH, Tang JX (2007) Comparison of the molluscicidal effect of three formulations of niclosamide against Oncomelania snails. Chin J Schisto Control 19:179–182 (in Chinese)
Dai JR, Wang W, Liang YS, Li HJ, Guan XH, Zhu YC (2008) A novel molluscicidal formulation of niclosamide. Parasitol Res 103:405–412
de Abreu FC, de Paula FS, dos Santos AF, Sant’Ana AEG, de Almeida MV, Cesar ET, Trindade MN, Goulart MOF (2001) Synthesis, electrochemistry, and molluscicidal activity of nitroaromatic compounds: effects of substituents and the role of redox potential. Bioorg Med Chem 9:659–664
de Souza CP (1995) Molluscicide control of snail vectors of Schistosomiasis. Mem Inst Oswaldo Cruz 90:165–168
de Paula-Andrade C, Coelho PRS, Nascimento RAP, Mota PMPC, Romano-Silva MA, de Alvarenga KAF, Schall VT, Pimenta DN, Coelho PMZ, Oliveira E (2019) Development of a natural molluscicide prototype kit (MoluSchall) for the control of schistosomiasis mansoni transmission. Rev Soc Bras Med Trop 52:e20190252
de Souza LC, dos Santos AF, SantAna AEG, de Oliveira ID (2004) Synthesis and evaluation of the molluscicidal activity of the 5,6-dimethyl-dihydro-pyran-2,4-dione and 6-substituted analogous. Bioorg Med Chem 12:865–869
Dong Y, Du CH, Zhang Y, Wang LF, Song J, Wu MS, Yang WC, Lv S, Zhou XN (2018) Role of ecological approaches to eliminating schistosomiasis in Eryuan County evaluated by system modeling. Infect Dis Poverty 7:129
dos Santos AF, Ferraz PAL, Pinto AV, Pinto M do CFR, Goulart MOF, Sant’Ana AEG (2000) Molluscicidal activity of 2-hydroxy-3-alkyl-1,4-naphthoquinones and Derivatives. Int J Parasitol 30: 1199–1202
dos Santos AF, Ferraz PAL, de Abreu FC, Chiari É, Goulart MOF, Sant’Ana AEG (2001) Molluscicidal and trypanocidal activities of lapachol derivatives. Planta Med 67:92–93
dos Santos AF, Sant’Ana AEG (1999) Molluscicidal activity of the diterpenoids jatrophone and jatropholones A and B isolated from Jatropha elliptica (Pohl) Muell. Arg Phytother Res 13:660–664
Ekabo OA, Farnsworth NR (1996) Antifungal and molluscicidal saponins from Serjania salzmanniana. J Nat Prod 59:431–435
El Bardicy S, El Sayed I, Yousif F, Van der Veken P, Haemers A, Augustyns K, Pieters L (2012) Schistosomicidal and molluscicidal activities of aminoalkylamino substituted neo- and norneocryptolepine derivatives. Pharm Biol 50:134–140
El Shehry MF, Abu-Hashem AA, El-Telbani EM (2010a) Synthesis of 3-((2,4-dichlorophenoxy)methyl)-1,2,4-triazolo(thiadiazoles and thiadiazines) as anti-inflammatory and molluscicidal agents. Eur J Med Chem 45:1906–1911
El Shehry MF, Swellem RH, Abu-Bakr SM, El-Telbani EM (2010b) Synthesis and molluscicidal evaluation of some new pyrazole, isoxazole, pyridine, pyrimidine, 1,4-thiazine and 1,3,4-thiadiazine derivatives incorporating benzofuran moiety. Eur J Med Chem 45:4783–4787
El-Bayouki KAM, Basyouni WM (1988) New thiazolo[5,4-d]pyrimidines with molluscicidal properties. Bull Chem Soc Jpn 61:3794–3796
El-Sakka IA, Zeid IF, Abdel-Bary HM, Abdel-Mageed AES (1994) Reactions with thiaxanthen-9-01: new thiaxanthene derivatives with molluscicidal and nematocidal activity. Arch Pharm (weinheim) 327:133–135
Fadda AA, Abdel-Latif E, El-Mekawy RE (2009) Synthesis and molluscicidal activity of some new thiophene, thiadiazole and pyrazole derivatives. Eur J Med Chem 44:1250–1256
Feng JC, Luo SL, Yang JM, Yang YY (2008) EST Isoenzymes of Oncomelania hupensis affected by Alternanthera philoxeroides. Environ Sci Technol 31:5–7 (in Chinese)
Fenwick A (1987) The role of molluscicides in schistosomiasis control. Parasitol Today 3:70–73
Finney DJ (1971) Probit analysis. Cambridge University Press, London
Giri S, Mishra AK (1984a) Fungicidal and molluscicidal activity of some 3-substituted 4-hydroxycoumarin derivatives. J Agric Food Chem 32:759–762
Giri S, Mishra AK (1984b) Fungicidal and molluscicidal activity of some heteroarylcarbinols and ethylenes. J Agric Food Chem 32:762–765
Gonnert R (1961) Results of laboratory and field trials with molluscicide Bayer 73. Bull Wld Hlth Org 25:483–501
Gryseels B, Polman K, Clerinx J, Kestens L (2006) Human schistosomiasis. Lancet 368:1106–1118
Guo W, Zheng LY, Wu YQ, Fan XL (2008) Synthesis and cercaricidal activities of a serial of novel self-diffused cercaricides derived from niphensamide. Chin Chem Lett 19:406–408
Guo D, Chen J, Liu Y, Yao H, Han FA, Pan J (2011) A high-performance molluscicidal ingredient against Oncomelania hupensis produced by a rhizospheric strain from Phytolacca acinosa Roxb. Pharmacogn Mag 7:277–283
Guo W, Zheng LY, Wu RM, Fan XL (2015) Design, synthesis, and cercaricidal activity of novel high-efficient, low-toxic self-spreading PEG –N-salicylanilide derivatives against cercariae larvae of Schistosome Japonicum floating on the water surface. Chem Biol Drug Des 85:527–533
Guo W, Zheng LY, Li YD, Wu RM, Chen Q, Yang DQ, Fan XL (2016) Discovery of molluscicidal and cercaricidal activities of 3-substituted quinazolinone derivatives by a scaffold hop** approach using a pseudo-ring based on the intramolecular hydrogen bond formation. Eur J Med Chem 115:291–294
Han B, Chen J (2014) Acacetin-7-rutinoside from Buddleja lindleyana, a new molluscicidal agent against Oncomelania hupensis. Z Naturforsch C 69:186–190
Han BX, Guo DZ, Chen J, Mao J (2012) Effects of AIBL on Oncomelania hupensis, the intermediate snail host of Schistosoma japonicum: an enzyme histochemical study. Asian Pac J Trop Med 5:966–969
Hang PY, Dai JR, Liang YS, Wang R (2001) Study on influence of B002 and niclosamide to nitric oxide synthase in Oncomelania snail. Chin J Schito Control 13:278–279 (in Chinese)
Hassan GS, Hegazy GH, Safwat HM (2006) Synthesis of furo-salicylanilides and their heterocyclic derivatives with anticipated molluscicidal activity. Arch Pharm Chem Life Sci 339:448–455
Hassan SE, Rahman EHA, El Monem ARA (2010) Molluscicidal activity of butanol fraction of Meryta denhamii flowers against Lymnaea natalensis and Biomophalaria alexandrina. Global Vet 4:15–21
He J, Wang H, Wu M, Chen X, Shu Q, Tao W, Xu Y, Zhang S (2007) Observation on the molluscicidal effect of niclosamide ethanolamine salt dustable powder by dusting in fields. J Trop Dis Parasitol 5(153–154):172 (in Chinese)
He P, Wang W, Sanogo B, Zeng X, Sun X, Lv Z, Yuan D, Duan L, Wu Z (2017) Molluscicidal activity and mechanism of toxicity of a novel salicylanilide ester derivative against Biomphalaria species. Parasite Vector 10:383
Hoffman DO, Zakhary R (1953) The relationship of exposure time to the molluscicidal activity of copper sulfate. Am J Trop Med Hyg 2:332–336
Huang YX (2019a) Strengthening the research on novel molluscicides to accelerate the progress towards elimination of schistosomiasis. Chin J Schisto Control 31:107–108 (in Chinese)
Huang YX (2019b) Research and field application of molluscicides in China. Chin J Schisto Control 31:679–684 (in Chinese)
Jia TW, Wang W, Sun LP, Lv S, Yang K, Zhang NM, Huang XB, Liu JB, Liu HC, Liu RH, Gawish FA, Habib MR, El-Emam MA, King CH, Zhou XN (2019) Molluscicidal effectiveness of Luo-Wei, a novel plant-derived molluscicide, against Oncomelania hupensis, Biomphalaria alexandrina and Bulinus truncates. Infect Dis Poverty 8:27
Kady MM, Brimer L, Furu P, Lemmich E, Nielsen HM, Thiilborg ST, Thastrup O, Christensen SB (1992) The molluscicidal activity of coumarins from Ethulia conyzoides and of Dicumarol. Planta Med 58:334–337
Kajihara N, Horimi T, Minai M, Hosaka Y (1979) Laboratory assessment of the molluscicidal activity of B-2, a new chemical against Oncomelania nosophora. Jpn J Med Sci Biol 32:185–188
Kanawade S, Toche RB, Patil SP, Desai AE, Bhamare SS (2011) Aminothiophenedicarboxamides and dicyanothiopheneacetamides as effective synthetic molluscicides against Indoplanorbis exustus snail. Eur J Med Chem 46:4682–4686
Karunamoorthi K, Bishaw D, Mulat T (2008) Laboratory evaluation of Ethiopian local plant Phytolacca dodecandra extract for its toxicity effectiveness against aquatic macroinvertebrates. Eur Rev Med Pharmacol Sci 12:381–386
Ke W, Wang W, Yang Y, Ma A, Liang H, Chen Y (1999) The electron microscopy scanning on injuring of head, foot and liver of Oncomelania hupensis by Pterocarya stenoptera. J Hubei Univ Nat Sci 21:383–385 (in Chinese)
Ke W, Yang Y, Chen Q, Wang W, Ma A (2000) Effect of water extract of Pterocarya stenoptera and Rumex japonicas on liver functions of Oncomelania hupensis. J Hubei Univ Nat Sci 22:77–79 (in Chinese)
Ke WS, Yang JL, **ong ZT (2006) The toxicity effect of Arisaema erubescens schott on Oncomlania hupensis. Asian J Ecotoxicol 1:283–288 (in Chinese)
Kenawy ER, Rizk ES (2004) Polymeric controlled release formulations of niclosamide for control of Biomphalaria alexandrina, the vector snail of Schistosomiasis. Macromol Biosci 4:119–128
Kenawy ER, Harras SF, Rizk EST, Mona MH, ElMehlawy MH (2020) New polymeric molluscicide-attractant (niclosamide-L-glutamate) for control of Biomphalaria alexandrina. Egyptian J Aquatic Res 46:13–18
King CH, Sutherland LJ, Bertsch D (2015) Systematic review and meta-analysis of the impact of chemical-based mollusciciding for control of Schistosoma mansoni and S haematobium transmission. PLoS Negl Trop Dis 9:e0004290
Kubo I, Matsumoto T, Klocke JA, Kamikawa T (1984a) Molluscicidal and insecticidal activities of isobutylamides isolated from Fagara macrophylla. Experientia 40:340–341
Kubo I, Matsumoto T, Kozuka M, Chapya A, Naoki H (1984b) Quinolizine alkaloids from the African medicinal plant Calpurnia aurea: molluscicidal activity and structural study by 2D-NMR. Agric Biol Chem 48:2839–2841
Kujime H, Yamagiwa Y, Kamikawa T (1992) Molluscicidal acridone alkaloids from Angostura paniculata: isolation, structures, and synthesis. J Nat Prod 55:1112–1117
Lahlou M (2004) Study of the molluscicidal activity of some phenolic compounds: structure-activity relationship. Pharm Biol 42:258–261
Lardans V, Dissous C (1998) Snail control strategies for reduction of schistosomiasis transmission. Parasitol Today 14:413–417
Li WG, Huang SX, Xu MX, Jiang YM, Hu LL, Gao ZQ, Cheng ZY, Wang Y, Zhou HL, Zhan ZW (1997) Ultrastructural changes of cerebral ganglion of Oncomelania hupensis after immersion in niclosamide. Chin J Parasite Dis Control 10:42–45 (in Chinese)
Li J, Qian WH, Huang YX, Jiang ZK, Zhang KY (2002) Advance of plant molluscicide studies. Chin J Schisto Control 14:67–69 (in Chinese)
Li H, Liang Y, Dai J, Xu M, Ru W, Xu Y (2006) Enzyme-histochemical observation on influence of suspension concentrate of niclosamide in Oncomelania hupensis snails. Chin J Schisto Control 18:427–430 (in Chinese)
Li ZJ, Ge J, Dai JR, Wen LY, Lin DD, Madsen H, Zhou XN, Lv S (2016) Biology and control of snail intermediate host of Schistosoma japonicum in the People’s Republic of China. Adv Parasit 92:197–236
Liang S, Abe EM, Zhou XN (2018) Integrating ecological approaches to interrupt schistosomiasis transmission: opportunities and challenges. Infect Dis Poverty 7:124
Lima NMF, dos Santos AF, Porfírio Z, Goulart MOF, Sant’Ana AEG (2002b) Toxicity of lapachol and isolapachol and their potassium salts against Biomphalaria glabrata, Schistosoma mansoni cercariae. Artemia salina and Tilapia nilotica. Acta Trop 83:43–47
Lima NMF, Correia CS, Ferraz PAL, Pinto AV, Pint, M do CRF, Santana AEG, Goulart MOF (2002a) Molluscicidal hydroxynaphthoquinones and derivatives: correlation between their redox potentials and activity against Biomphalaria glabrata. J Braz Chem Soc 13:822–829
Limpanont Y, Phuphisut O, Reamtong O, Adisakwattana P (2020) Recent advances in Schistosoma mekongi ecology, transcriptomics and proteomics of relevance to snail control. Acta Trop 202:105244
Liu HC, Chen W, Mi LX, Yang XX, Tu ZB (2001) Studies on the molluscicidal effect of Dioscorea zingiberensis against Oncomelania hupensis. Chin J Parasitol Parasit Dis 19:126–127 (in Chinese)
Lo NC, Gurarie D, Yoon N, Coulibaly JT, Bendavid E, Andrews JR, King CH (2018) Impact and cost-effectiveness of snail control to achieve disease control targets for schistosomiasis. P Natl Acad Sci USA 115:E584–E591
Madsen H (1990) Biological methods for the control of freshwater snails. Parasitol Today 6:237–241
Mahmoud MB, Ibrahim WL, Abou-El-Nour BM, El-Emam MA, Youssef AA (2011) Biological and biochemical parameters of Biomphalaria alexandrina snails exposed to the plants Datura stramonium and Sesbania sesban as water suspensions of their dry powder. Pestic Biochem Physiol 99:96–104
Marston A, Msonthi JD, Hostettmann K (1984) Naphthoquinones of diospyros usambarensis; their molluscicidal and fungicidal activities. Planta Med 50:279–280
McCullough FS, Gayral P, Duncan J, Christie JD (1980) Molluscicides in schistosomiasis control. B World Health Organ 58:681–689
Mello-Silva CC, Vilar MM, de Vasconcellos MC, Pinheiro J, de A Rodrigues ML (2010) Carbohydrate metabolism alterations in Biomphalaria glabrata infected with Schistosoma mansoni and exposed to Euphorbia splendens var. hislopii latex. Mem Inst Oswaldo Cruz 105:492–495
Molla E, Giday M, Erko B (2013) Laboratory assessment of the molluscicidal and cercariacidal activities of Balanites aegyptiaca. Asian Pac J Trop Biomed 3:657–662
Moon AP, Frick LP, Asakura S (1958) Laboratory screening of compounds for molluscicidal activity against Oncomelania nosophora with an immersion test and a modified plate test. Am J Trop Med Hyg 7:295–297
Nabih I, Metri J (1973) Structure and activity in molluscicides IV: new substituted tetralins with potential molluscicidal effect. J Pharm Sci 62:323–325
Nawwar GAM (1994) Salicylamides containing amino acid or pyran moieties with molluscicidal activity. Arch Pharm 327:201–205
Nawwar GAM, Haggag BM, Swellam RH (1993) Synthesis and molluscicidal activity of new derivatives of l-(hydroxy/substituted phenyl)-3-arylpropenones. Arch Pharm 326:831–836
Nawwar GAM, Swellem RH, Ibrahim AM (1994) Oxazole, pyrazole and piperidine derivatives having an o-hydroxy-aryl moiety with anticipated molluscicidal activity. Arch Pharm Res 17:66–70
Nie WY, Tan P, Zhang XJ, Yi TJ (2009) Progresses in researches on plant-derived molluscacides. Nat Prod Res Dev 21:273–278 (in Chinese)
Nihei KI, Ying BP, Murakami T, Matsuda H, Hashimoto M, Kubo I (2005) Pachyelasides A-D, novel molluscicidal triterpene saponins from Pachyelasma tessmannii. J Agric Food Chem 53:608–613
Njoroge M, Njuguna NM, Mutai P, Ongarora DSB, Smith PW, Chibale K (2014) Recent approaches to chemical discovery and development against malaria and the neglected tropical diseases human African trypanosomiasis and schistosomiasis. Chem Rev 114:11138–11163
Nofal ZM, Fahmy HH, Mohamed HS (2002) Synthesis, antimicrobial and molluscicidal activities of new benzimidazole derivatives. Arch Pharm Res 25:28–38
Ohmae H, Iwanaga Y, Nara T, Matsuda H, Yasuraaok K (2003) Biological characteristics and control of intermediate snail host of Schistosoma japonicum. Parasitol Int 52:409–417
Peng F, Liu L, Huang Q, Liu N, Yang H, Sun H, Hu Q, Feng F, Jiang C (2011) Molluscicidal effect of Eomecon chionantha alkaloids against Oncomelania hupensis snails. Southeast Asian J Trop Med Public Health 42:289–296
Pereira AR, Etzbach L, Engene N, M€ulle R, Gerwick WH (2011) Molluscicidal metabolites from an assemblage of Palmyra Atoll cyanobacteria. J Nat Prod 74:1175–1181
Perrett S, Whitfield PJ (1996) Currently available molluscicides. Parasitol Today 12:156–159
Pointier JP, Jourdane J (2000) Biological control of the snail hosts of schistosomiasis in areas of low transmission: the example of the Caribbean area. Acta Trop 77:53–60
Pu ZQ, Huang JS, Liu J, Zhang YP (2008) Research progress in the mechanism of the plant snail control. J Anhui Agri Sci 36:5724–5726
Radwan MA, El-Zemity SR, Mohamed SA, Sherby SM (2008) Potential of some monoterpenoids and their new N-methyl carbamate derivatives against Schistosomiasis snail vector, Biomphalaria alexandrina. Ecotoxicol Environ Saf 71:889–894
Rawi SM, El-Gindy H, Abd-El-Kader A (1996) New possible molluscicides from Calendula micrantha officinalis and Ammi majus. Ecotoxicol Environ Saf 35:261–267
Ribeiro KAL, de Carvalho CM, Molina MT, Lima EP, López-Montero E, Reys JRM, de Oliveira MBF, Pinto AV, Santana AEG, Goulart MOF (2009) Activities of naphthoquinones against Aedes aegypti (Linnaeus, 1762) (Diptera: Culicidae), vector of dengue and Biomphalaria glabrata (Say, 1818), intermediate host of Schistosoma mansoni. Acta Trop 111:44–50
Schönberg A, Latif N (1954) Furochromones and coumarins. XI. The molluscicidal activity of bergapten, isopimpinillin and xanthotoxin. J Am Chem Soc 76:6208
Silva TMS, Camara CA, Barbosa TP, Soares AZ, da Cunha LC, Pinto AC, Vargas MD (2005) Molluscicidal activity of synthetic lapachol amino and hydrogenated derivatives. Bioorg Med Chem 13:193–196
Singh A, Singh VK (2009) Molluscicidal activity of Saraca asoca and Thuja orientalis against the fresh water snail Lymnaea acuminate. Vet Parasitol 164:206–210
Sturrock RF (1995) Current concepts of snail control. Mem Inst Oswaldo Cruz 90:241–248
Sukumaran D, Parashar BD, Gupta AK, Jeevaratnam K, Prakash S (2004) Molluscicidal effect of nicotinanilide and its intermediate compounds against a freshwater snail lymnaea luteola, the Vector of Animal Schistosomiasis. Mem Inst Oswaldo Cruz 99:205–210
Sun H, Huang Q, Peng F, Liu N, Xu X, Hu Q, Feng F (2005) Effect of Eomecon chionantha alkaloids on ultrastructure of genital system of Oncomelania hupensis. Chin J Schisto Control 17:377–378 (in Chinese)
Sun WX, Yuan SS, Huang QY, Peng F, Liu NM, Yang SQ (2011) Study on liver injury of Oncomelania hupensis caused by Eomecon chinanthe sanguinarine. Chin J Schisto Control 23:82–84 (in Chinese)
Tan P, He C, Liu B, Chang H, Deng W (2001) SEM Observation of the changes of the head and foot’s muscle of Oncomelania hupensis after exposed to mixture of arecoline with molluscicides. Chin J Schisto Control 13:21–23 (in Chinese)
Thétiot-Laurent SAL, Boissier J, Robert A, Meunier B (2013) Schistosomiasis Chemotherapy. Angew Chem Int Ed 52:7936–7956
Tiwari F (2012) Bait formulation toxicity of plant derived molluscicides in attractant food pellets against vector snail. Lymnaea Acuminate World J Zool 7:54–59
Vasconcellos ML, Silva TM, Camara CA, Martins RM, Lacerda KM, Lopes HM, Pereira VL, de Souza RO, Crespo LT (2006) Baylis-Hillman adducts with molluscicidal activity against Biomphalaria glabrata. Pest Manag Sci 62:288–292
Vinaud MC, Lino RS Jr, Bezerra JCB (2008) Activity of Stryphnodendron polyphyllum, a plant from the Brazilian savannah, against hemocytes of Biomphalaria glabrata, an intermediate host of Schistosoma mansoni. Rev Patol Trop 37:237–246
Wang G, Shen B, Wang J, Song G (1997) Influence of the seed of Camellia sinensis (L) O. Kuntze on the tegument of soft tissues of Oncomelania hupensis. Chin J Parasitol Parasit Dis 15:243–245 (in Chinese)
Wang H, Cai WM, Wang WX, Yang JM (2006) Molluscicidal activity of Nerium Indicum Mill, Pterocarya stenoptera DC, and Rumex japonicum Houtt on Oncomelania hupensis. Biomed Environ Sci 19:245–248 (in Chinese)
Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, **ong JJ, Wu XH, Wang XH, Wang LY, **a G, Hao Y, Chin DP, Zhou XN (2009) A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med 360:121–128
Wang W, Qin Z, Zhu D, Wei Y, Li S, Duan L (2016) Synthesis, bioactivity evaluation, and toxicity assessment of novel salicylanilide ester derivatives as cercaricides against Schistosoma japonicum and molluscicides against Oncomelania hupensis. Antimicrob Agents Chemother 60:323–331
Wang W, Zhang X, Zhang H, Hu H, Li S, Liu X, Duan L (2017) Field evaluation of a novel molluscicide (niclosamidate) against Oncomelania hupensis, intermediate host of Schistosoma japonicum. Parasitol Res 116:3423–3427
Wang W, Mao Q, Yao J, Yang W, Zhang Q, Lu W, Deng Z, Duan L (2018) Discovery of the pyridylphenylureas as novel molluscicides against the invasive snail Biomphalaria straminea, intermediate host of Schistosoma mansoni. Parasite Vector 11:291
Wei WY, Bao ZP, Li GP, Lü GL, **a M, Guo JG (2008) Study on molluscicidal effect of calcium cyanamide. Chin J Schisto Control 20:54–57 (in Chinese)
Whitfield PJ (1996) Novel anthelmintic compounds and molluscicides from medicinal plants. Trans R Soc Trop Med Hyg 90:596–600
WHO (World Health Organization) (1993) The control of Schistosomiasis: second report of the WHO expert committee. WHO Tech Rep Series 830:1–86
WHO (World Health Organization) (2019) Guidelines for laboratory and field testing of molluscicides for control of schistosomiasis. Licence: CC BY-NC-SA 3.0 IGO
WHO (World Health Organization) (2021) https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
Wu F (2007) Progress of research on molluscicidal effect of nereistoxin pesticide. Chin J Schisto Control 19(482–484):487 (in Chinese)
Wu F, Chen Y, Dai J, Zhou X, Gao Z (1998) Molluscicidal effect of evisect against snail and its ova. J Practical Parasitic Dis 6:31–33 (in Chinese)
Wu XY, Yang LQ, Zhang LH, Ge QJ (2006) Progress of research on molluscicides. Chin J Schisto Control 18:474–476 (in Chinese)
** WP, Wu F, Jiang YJ, Huang YX (2000) Observation on the effect of four molluscicides eliminating snails. Chin J Schisto Control 12:11 (in Chinese)
** Y, Dong XL, Meng Z, Zhu ZL, Zhang SX (2016) A study on the development toxicity and genetoxicity induced by sodium pentachlorophenate. Prev Med 28:1081–1087 (in Chinese)
**a M, Ding L, Wei WY, Li GP, Guo FY, Chen XL, Guo JG (2010) Histological and morphological changes of Oncomelania hupensis snails by calcium cyanamide. Chin J Schisto Control 22:174–175 (in Chinese)
**a J, Yuan Y, Xu X, Wei F, Li G, Liu M, Li J, Chen R, Zhou Z, Nie S (2014) Evaluating the effect of a novel molluscicide in the endemic Schistosomiasis japonica area of China. Int J Environ Res Public Health 11:10406–10418
**ong T, Zhao QP, Xu XJ, Liu R, Jiang MS, Dong HF (2016) Morphological and enzymatical observations in Oncomelania hupensis after molluscicide treatment: implication for future molluscicide development. Parasitol Res 115:4139–4152
**ong T, Zhao QP, Liu R, Jiang MS, Dong HF (2018) Enzymology of snails under treatment of molluscicides. Chin J Schisto Control 30(237–240):243 (in Chinese)
Xu FS, ** JY, Gu XG, Yin HZ, Mao Y (2003) Effect of dipterex for killing schistosome egg and snail. Parasitoses Infect Dis 1:28–30 (in Chinese)
Xu SF, Wang H, Jiang LX (2005) Progress of research on various preparations of niclosamide and snail control. Chin J Schisto Control 17:478–480 (in Chinese)
Xu XJ, Yuan Y, Li CL, Wei FH, Zhao YB, Tu ZW, Liu M, Cao MM, He H, Fan HP (2007) Molluscicidal effect of a novel molluscicide, LDS. Chin J Schisto Control 19:328–333 (in Chinese)
Yang X, Chen S, **a L, Chen J (2008) Molluscicidal activity against Oncomelania hupensis of Ginkgo biloba. Fitoterapia 79:250–254
Yang GJ, Li W, Sun LP, Wu F, Yang K, Huang YX, Zhou XN (2010) Molluscicidal efficacies of different formulations of niclosamide: result of meta-analysis of Chinese literature. Parasite Vector 3:84
Yang GJ, Sun LP, Hong QB, Zhu HR, Yang K, Gao Q, Zhou XN (2012) Optimizing molluscicide treatment strategies in different control stages of schistosomiasis in the People’s Republic of China. Parasite Vector 5:260
You BR, Huang YX, Hu HG, Hang DR, **g SB, Mei QF (2016) Molluscicidal effect of niclosamide ethanolamine salt powder-granula against Oncomelania hupensis. Chin J Schisto Control 28:237–240 (in Chinese)
Younes A, El-Sherief H, Gawish F, Mahmoud M (2017) Biological control of snail hosts transmitting schistosomiasis by the water bug, Sphaerodema urinator. Parasitol Res 116:1257–1264
Yuan Y, Xu XJ, Dong HF, Jiang MS, Zhu HG (2005) Transmission control of schistosomiasis japonica: implementation and evaluation of different snail control interventions. Acta Trop 96:191–197
Yuan Y, Dong H, Xu X, Li G, Wei F, Zhao Y, Tu Z, Liu M, Cao M, He H, Tang L, Zhu H, Fan H (2011) Evaluation of a new molluscicide for counteracting the intermediate snail host of schistosoma japonicum. Malacologia 53:217–227
Zhang HM, Tan YG, Li XM, Jiang H, Yuan TQ, Lin R, Huang FM, Wei ZW (2006) Laboratory and field observation on the molluscicidal effect of a new formula of sodium pentachlorophenate on Oncomelania hepensis from mountainous area in Guangxi. Chin Trop Med 6:966–967 (in Chinese)
Zhang Y, Zhu D, Li H-J, Liu H-X, Liang Y-S, Xu X-N, Li W-X (2007) Mechanism of action of a plant molluscicide HL on Oncomelania hupensis. Chin J Zoonoses 23:978–981 (in Chinese)
Zhang P, Pan J, Duan W, Li X, Zhang Y, Zhou Y, Jiang Q, Mao Z, Yu P (2011) Synthesis of ginkgolic acid analogues and evaluation of their molluscicidal activity. Molecules 16:4059–4069
Zhang X, Zhang H-M, Liu X, Rong X-B, Yang R (2013) Molluscicidal effect of 10% LDS in fields. Chin J Schisto Control 25(182–183):186 (in Chinese)
Zhang LJ, Xu ZM, Guo JY, Dai SM, Dang H, Lü S, Xu J, Li SZ, Zhou XN (2019) Endemic status of schistosomiasis in People’s Republic of China in 2018. Chin J Schisto Control 31:576–582 (in Chinese)
Zhu DP, Gu JR, Yin SY, Hong GB, Chen PL, Guo YH (1984) Study on a new mollusccide, bromoacetamide. Chin J Parasitol Parasit Dis 2:17–20 (in Chinese)
Zhu D, Zhou XN, Zhang SQ, Zhang G, Liu HX, Lu DB, Cai GY, Ni QZ, Cao ZG, Wu WD (2006) Study on the molluscicidal effect of META-Li against Oncomelania hupensis. Chin J Parasitol Parasit Dis 24:200–203 (in Chinese)
Zhu D, Li HJ, Liu HX, Zhang Y, Guo J, Liang YS, Zhou XN (2007) Enzyme histochemistry: the effect of META-Li on Oncomelania hupensis. Chin J Parasitol Parasit Dis 25:198–201 (in Chinese)
Zhu GY, Zu DL, Guan YG, Yang WD, Lu YH (2010) Experimental study and application of molluscicidal constituents and mechanisms in plants. Chin J Vector Biol Control 21:509–511 (in Chinese)
Zou FC, Duan G, **e YJ, Zhou Y, Dong GD, Lin RQ, Zhu XQ (2009) Molluscicidal activity of the plant Eupatorium adenophorum against Oncomelania hupensis, the intermediate host snail of Schistosoma japonicum. Ann Trop Med Parasitol 103:549–553
Acknowledgements
The work is supported by the National Natural Science Foundation of China (21867001, 22167002, 21662002 and 21602031), the Academic &Technical Leadership Development Program of Jiangxi Province (20194BCJ22014), the Double-Thousand Talents Plan of Jiangxi Province (2019) and the Jiangxi Natural Science Foundation (20202ACBL206022, 20171BAB203010), the Science Foundation of Jiangxi Provincial Department of Education (GJJ201429), and the Graduate Innovation Research Project of Jiangxi Province (YCX20A009).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Christoph G. Grevelding
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, L., Deng, L., Zhong, Y. et al. Molluscicides against the snail-intermediate host of Schistosoma: a review. Parasitol Res 120, 3355–3393 (2021). https://doi.org/10.1007/s00436-021-07288-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07288-4