Abstract
This study aims to examine if small for gestation age (SGA) status is correlated with alterations in body composition at prepuberty, independently of other factors, comparing SGA-born children with appropriate for gestational age (AGA)-born children. We examined anthropometrics, waist circumference, body mass index (BMI), six skinfold thickness, and body composition using the method of bioelectrical impedance in 636 children aged 7 to 10 years. We also considered age, gender, birth mode, mother’s age, prepregnancy weight, weight gain during pregnancy, social status, parental BMI, type of feeding, and daily exercise. We examined 636 children at a mean age of 9 years: 106 SGA-born and 530 AGA-born children. SGA as compared to AGA-born children had a lower BMI z-score (0.26 ± 0.89 kg/cm2 vs 0.46 ± 0.84 kg/cm2, p < 0.050) and a lower lean mass, although that was not statistically significant (24.0 ± 6.6 kg vs 25.6 ± 6.4 kg, p < 0.100). SGA-born children presented no difference in waist circumference or fat mass in comparison to children born AGA. Logistic regression analysis revealed a strong independent negative association between SGA status and BMI (beta = − 2.33, OR = 0.70 p = 0.019) and SGA status and lean mass (beta = − 2.43, OR = 0.95 p = 0.010).
Conclusion: Our findings suggest that SGA-born children had a lower BMI as compared to AGA-born subjects, whereas SGA status was negatively associated with BMI and lean mass.
What is Known: • Deviant birth weight for gestation has been associated with an increased risk of childhood adiposity. • Evidence remains scarce on whether small for gestational age status affects body composition and obesity later in childhood. | |
What is New: • Among school-aged children, small for gestational age subjects had a lower body mass index as compared to appropriate for gestational age counterparts, whereas small for gestational age status was negatively associated with body mass index and lean mass. • A meticulous observation is needed during childhood in children born with deviant birth weight. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Childhood obesity has emerged as a global public health problem, associated with certain morbidities including type two diabetes mellitus, hypertension, coronary artery disease, stroke, nonalcoholic liver disease, obstructive sleep apnea, dyslipidemia, and several types of cancer, as well as severe psychological consequences [1]. Several genetic, environmental, and biological factors have been implicated in the pathophysiology of excess weight gain during childhood [2,3,4]. Among them, birth weight has been identified as a significant indicator of the in utero experiences of the fetus that may have an impact on later life [3].
The term “small for gestational age (SGA)” is used to define an infant whose birth weight is less than the 10th percentile for gestational age or two standard deviations below the population norms on the growth charts, not considering the intrauterine growth trajectory [5]. The term “intrauterine growth restriction (IUGR)” refers to the condition characterized by a fetal growth deceleration and/or clinical features of intrauterine malnourishment, irrespective of the birth weight percentile in relation to gestational age [5]. IUGR fetuses represent those fetuses that have failed to reach their growth potential, due to genetic, infectious, maternal, or placental disorders [6]. Current evidence suggests that SGA and IUGR neonates are at an increased risk of develo** metabolic complications in later life [5].
Previous studies have identified that SGA status is a significant factor associated with decreased body mass index (BMI) or fat mass during childhood [7,8,9], whereas, on the other hand, several others have suggested that SGA-born subjects present an increased risk of later fat accumulation and childhood obesity [10,11,12,13]. As previously suggested, fetal growth restriction and adiposity accumulation in later life share environmental and genetic antecedents [14]. It has been assumed that growth-retarded fetuses develop a remodeling of their body composition in utero, whereas they show faster postnatal growth [14], and they experience a significant adiposity accumulation when exposed to a nutrient-rich environment [15]. On the other hand, recent evidence has suggested that numerous other factors, irrespective of birth weight, are important in determining later fat mass and body composition [16]. Given the conflicting results arising from different studies, a debate is still ongoing [14, 16, 17].
This study aimed to examine if SGA status is correlated with alterations in body composition at prepuberty independent of other prenatal, perinatal, and postnatal factors by comparing children born SGA with those born appropriate for gestational age (AGA).
Material and methods
Study design and population
This prospective study was conducted from 2019 to 2020 in the city of Ioannina, in Northwestern Greece, including children between 7 to 10 years of age. The local education bureau gave us permission to conduct the study in school, whereas the study was approved by the Science and Research Ethics Committee of the University of Ioannina (No. 934).
A representative sample of schools in the urban area was selected for the initial recruitment of the children. Children and their caregivers were approached in their school settings or were contacted by telephone and asked to participate in the study. After the purpose of the study was explained, written informed consent was obtained from each parent, in addition to oral consent that was obtained from the early school-aged participants.
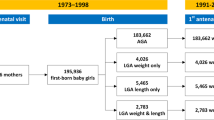
A total of 710 children were enrolled to participate, and they were further classified based on their birth weight for gestation. Anthropometrics were obtained from the children’s medical records. The AGA group consisted of children with birth weight for gestational age between the 10th and 90th centile, according to the new INTERGROWTH charts [18]. SGA group consisted of children with a birth weight below the tenth centile, whereas the large for gestational age (LGA) consisted of children with a birth weight above the 90th centile, according to the same growth charts.
To avoid a potential bias due to constitutional factors leading to SGA, we excluded SGA-born children whose mothers’ height was below two standard deviations of the mean or whose mothers had a height between one to two standard deviations of the mean and had also given birth to an SGA infant previously [19, 20]. We excluded 24 children due to missing information on the predictor or outcome variables or suspicion of inaccurate measurements and 50 children as they were LGA. Thus, 636 children in total comprised the study population, 106 SGA-born and 530 AGA-born subjects. Based on the annual number of births in the urban area of Ioannina, it was estimated that the study sample represented 22–24% of the children aged 7 to 10 years living in the area.
Predictor variables
Data on the birth history of every child participating in the study, including gestational age, gender, birth weight, delivery order, mode of delivery, and birth length, was obtained from every individual’s medical record that was provided by his/her caregiver. Children born below 35 weeks were not included in this study, and also, none of the children included in the study had any evidence of congenital infections, genetic syndromes, or congenital malformations. The ponderal index (PI) was calculated according to the formula PI = weight × 100/length3 (g/cm3). The children’s caregivers were also asked to fulfill a questionnaire including information regarding pregnancy (i.e., maternal age, weight gain during pregnancy, pregnancy pathology including gestational diabetes, or hypertension), nutritional data (i.e., exclusively breastfeeding, formula feeding, or mixed feeding at birth, breastfeeding at 6 months, duration of breastfeeding, time of solid food introduction), duration of weekly exercise, parental BMI, and family socioeconomic status. The duration of weekly exercise was calculated by the sum of hours that children were dedicated to out-of-school sports activities. The socioeconomic status of the family was classified as high, medium, or low, based on the level of parental education and the annual income.
Outcome variables
Children were assessed at their school or at the outpatient clinic of the Neonatal Unit of the University Hospital, where we measured weight, height, waist circumference, six skinfold thickness, blood pressure, and body composition. All measurements were performed at least 3 h after the morning awakening and after a minimum of three-hour fasting. Children’s caregivers had been also advised to prevent children from intense exercise within the preceding 12 h before the measurement. Finally, children had their measurements performed after voiding. Weight was measured with an electronic scale (Tanita-DC 430-U, Tanita Corporation, Tokyo, Japan) to the nearest 0.1 kg and height by a Harpenden stadiometer to the nearest 0.5 cm (Seca, Vogel & Halke GmbH & CO. KG, Hamburg, Germany). Waist circumference was measured twice to the nearest 1 mm at the minimum circumference between the iliac crest and the rib cage, expressed as a z-score. The BMI was calculated based on the formula BMI = weight/height2 (kg/m2) and was expressed as a z-score. To calculate the z-score for any BMI value, we used centile charts of the World Health Organization [21]. All anthropometric measurements were performed twice, and the mean value was used for analysis. The sum of the thickness of the skinfolds was assessed (Calipers, UK), measuring the double fold of skin and subcutaneous fat at the triceps (the midpoint of the back of the upper arm), the biceps (at the same level, to the front of the upper left arm), the subscapular (below and laterally to the shoulder blade), the suprailiac obliquely, the midthigh, and the midcalf. The predicted mass density (PMD) was calculated according to the formula PMD = 1.1533 − (0.0598 × L) for males and PMD = 1.1369 − (0.0643 × L) for females (L represents the logarithm of the cumulative sum of the four-skinfold thickness in mm), whereas the percentage (%) of body fat was calculated from the Siri equation [% Body Fat = (495/PMD) − 450].
Blood pressure was measured non-invasively in the right arm twice in every child while sitting (HS 20S sphygmomanometer, Shenzhen, China), and the values were assessed according to the updated criterion of the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents of the American Academy of Paediatrics [22].
Body composition was evaluated with Bioelectrical Impedance Analysis (BIA) (Tanita-DC 430-U, Tanita Corporation, Tokyo, Japan), measuring absolute fat mass, fat mass percentage, absolute lean mass, lean mass percentage, absolute total body water, total body water percentage, and total body weight. Lean mass was calculated using the cross-validated equation by Houtkooper [23]. BIA measures the impedance of body tissues to the flow of an alternating electric current, based on the differing electrolyte and water content of tissues and the composition of cell membranes [24, 25].
Statistical analysis
Descriptive statistics were calculated for collected characteristics. Continuous variables were expressed as mean ± standard deviation, while categorical variables were expressed as n (percentage %). The normality of the distributions of continuous variables was assessed by the Kolmogorov–Smirnov test. Comparisons between continuous variables were performed utilizing the Student’s t test and between categorical variables with the chi-square (x2) test or Fisher’s exact test. Logistic regression analysis was used to estimate the association between SGA status (dependent variable) and body composition at prepuberty (either lean mass or fat mass) or BMI after taking into consideration factors that may affect body composition. Namely, we included gestational age, gender, delivery order, mode of delivery, maternal age, weight gain during pregnancy, and pregnancy pathology including gestational diabetes or hypertension, nutritional data, i.e., exclusively breastfeeding, formula feeding, or mixed feeding at birth, breastfeeding at 6 months, duration of breastfeeding, and time of solid food introduction, duration of weekly exercise, parental BMI, and family socioeconomic status.
In sample size analysis, a cohort of more than 500 subjects was powered to detect differences of 10% in fat mass, lean mass, or BMI between the SGA and AGA subjects, with a power of 0.8 and a type-I error of 0.05. All performed tests were two-sided, and a p value less than 0.05 was considered statistically significant (alpha 0.05). The data were analyzed using StatView (SAS, CA USA).
Results
Among 636 children who were examined at a mean age of 9 years, 106 (17%) were SGA and 530 (83%) were AGA. The gestational age (37.0 ± 1.9 weeks), birth weight (2262 ± 355 g), and length at birth (47.1 ± 2.0 cm) of SGA-born subjects were significantly lower in comparison to AGA-born subjects (38.6 ± 1.2 weeks, 50.5 ± 1.0 cm, and 3200 ± 282 g, respectively, p < 0.001). SGA as compared to AGA subjects were born predominantly with a cesarean section (72% in comparison to 34%, p < 0.001), whereas SGA had an SGA-born sibling in 24%, as compared to 3% of the AGA-born children (p < 0.001). No significant differences were noted between the two groups regarding gender, delivery order, pregnancy weight gain, gestational diabetes, or hypertension. Regarding the parental characteristics, we recorded a significantly lower maternal weight and height among mothers of the SGA as compared to AGA children, but no differences in maternal age, maternal BMI, paternal weight, height, or BMI, as depicted in Table 1.
SGA in comparison to AGA-born children had a lower ratio of exclusively breastfeeding at birth (30% as compared to 50%, p < 0.001), a shorter duration of breastfeeding (5.2 ± 4.5 months in comparison to 7.6 ± 4.3 months, p < 0.050), and a lower ratio of breastfeeding at 6 months of age (19% in comparison to 34%, p < 0.001). We recorded no differences between the two groups regarding the time of solid food initiation, the duration of weekly exercise, or socioeconomic status (Table 2).
The mean age of assessment was 9.1 ± 1.6 years for SGA-born children and 9.2 ± 1.6 years for AGA-born children. SGA had a significantly lower BMI z-score (0.26 ± 0.9 kg/cm3 in comparison to 0.46 ± 0.8 kg/cm3, p < 0.050). We recorded no significant differences between SGA and AGA-born children in the waist circumference z-score, the sum of six skinfold thickness, the percentage of fat mass estimated with the Siri equation, the PMD, the absolute fat mass, or the percentage of fat mass. We found a lower lean mass of 24.0 ± 6.6 kg in SGA-born children in comparison to 25.6 ± 6.4 kg in AGA-born children, although that was not statistically significant (p < 0.100). Finally, we recorded significantly lower systolic and diastolic blood pressure in the SGA, as compared to AGA-born subjects (Table 3).
In logistic regression analysis, we found a strong independent negative association between SGA status and lean mass (beta = − 2.43, OR = 0.95, p = 0.010) and SGA status and BMI (beta = − 2.33, OR = 0.70, p = 0.019) (Table 4).
Discussion
The findings of the current study suggest that SGA-born children that were evaluated at a mean age of 9 years had a significantly lower BMI, as compared to AGA-born counterparts. SGA status was negatively associated with BMI and lean mass at school age.
A previous study that was performed by Rogers et al. included more than 6000 children and examined their body composition according to their birth anthropometrics with dual-energy x-ray absorptiometry (DeXA) [9]. The authors concluded that birth weight and birth length were associated with body composition and fat distribution during childhood [9]. In particular, birth weight and the ponderal index were both positively associated with the accumulation of fat and lean mass [9]. Our findings are in part in agreement with the above study, suggesting that SGA-born children had a significantly lower BMI and lower lean mass, although that was not statistically significant, as compared to AGA-born subjects. Of note, Rogers et al. examined the association of birth weight with fat mass accumulation during childhood in a linear model rather than categorically with a subgroup analysis between SGA and AGA-born children [9]. Although birth weight is a continuous variable, SGA-born children are traditionally considered a pathophysiological district subgroup.
Hediger et al. examined the association between birth weight and body composition among SGA, AGA, and LGA-born children [7]. The study included 3192 children that were evaluated with skinfold thickness at 3 to 6 years of age [7]. The authors suggested that SGA-born subjects had a trend of reduced lean mass development and that various environmental factors might have influenced fat mass development; however, they found no significant association between birth weight and fat mass accumulation, apart from a short period of the study [7]. The differences in the findings regarding the fat mass accumulation between the previous and our study might be explained by the fact that, in the previous study, children were examined at a much younger age in comparison to our cohort and that the fat mass was solely assessed by skinfold thickness, which, although useful in large epidemiological studies, is less accurate in comparison to BIA of DeXA [7].
Besides, Kramer et al. performed a large pertinent study evaluating 17,046 children at 11.5 years of age with anthropometrics, skinfold thickness, and BIA [8]. The authors reported that children born SGA had indeed a lower BMI and a lower percentage of body fat as compared to AGA-born children [8]. Although the findings of our study were in agreement with the above regarding BMI, we could not find any significant association between body weight deviation and fat mass. Interestingly, the differences in the fat mass that were recorded in the previous study were only 0.5%. Among others, those differences might be explained by the fact that the study was performed in Belarus, a country with a low prevalence of obesity. Furthermore, the authors in the design of their study excluded newborns with a birth weight below 2500 g, which could represent those with the greatest degree of fetal growth restriction [8].
Our findings suggested that SGA subjects had no differences as compared to AGA children in the waist circumference and body fat mass, although we could not evaluate the central fat distribution. SGA as compared to AGA-born subjects presented a lower BMI, whereas lean mass was lower but did not reach statistical significance. A lower BMI in SGA children along with a similar waist circumference may indirectly imply that SGA-born children presented a concentric distribution of the fat. Previous studies have suggested that SGA-born children present an increased fat mass accumulation which is predominantly centrally distributed [10,11,12,13]. Crume et al. demonstrated that fetal growth-restricted children tended to present higher subcutaneous adipose tissue at a mean age of 10 years in MRI [10]. Nevertheless, Ibanez et al. in a longitudinal study of 51 children evaluated with DeXA from 2 to 4 years of age reported that SGA-born children had more body fat and abdominal fat mass compared to AGA-born children [11]. Furthermore, the same group evaluated the fat composition with DeXA and MRI in 64 children at 6 years of age and similarly found that SGA children had a viscerotropic redistribution of abdominal fat in comparison to AGA children [13].
Interestingly, in our study, SGA-born children had lower systolic and diastolic blood pressure in comparison to AGA-born subjects. Although we recorded a minimum level of difference (approximately 3%) between the two groups, that was statistically significant. Our findings contradict previous evidence that suggests that SGA-born children are at increased risk of hypertension [26,27,28]. Previous studies and meta-analyses support that birth weight is inversely associated with the risk of high blood pressure (the “small baby syndrome hypothesis”) [26,27,28], which can be in part explained by the small size, reduced number, and abnormal development of the nephrons in children with low birth weight [28]. In our study, SGA-born subjects had a significantly lower BMI in comparison to AGA-born counterparts; given that BMI in school age has a significant impact on arterial blood pressure, that might explain the differences in blood pressure noted in our cohort.
Finally, our findings revealed that the rate and duration of breastfeeding were lower in SGA-born in comparison to AGA-born children, suggesting that excessive formula feeding is a common practice in SGA-born infants. This may be partly attributable to the higher pressure that encounter parents of SGA infants seen them to grow rapidly. Breastfeeding has been suggested to have a protective effect against later overweight; however, evidence remains inconsistent since many studies could not find any association between breastfeeding and childhood overweight [29]. In our study, multiple regression analysis could not reveal any significant association between breastfeeding and BMI or lean mass.
The limitations of our study should be acknowledged. Firstly, the study was performed in a single center, including a relatively limited sample population. Although the study population was representative of 22–24% of the children aged 7 to 10 years living in the area, we should be cautious before generalizing our findings. Moreover, we performed the body fat analysis with BIA and the sum of six skinfold thickness, which are indirect methods to evaluate body composition. Air displacement plethysmography, DeXA, and magnetic resonance imaging (MRI) due to their accuracy in body composition evaluation are considered reference methods [30]. Plethysmography uses the principles of body density and is considered a good reference method in serial evaluation to estimate total adiposity, lean mass, and fat mass and can be used in children from 2 years of age. However, body composition can also be estimated by anthropometry and BIA, when population size is extensive, or when economic resources are not available [24, 31].
In conclusion, among school-aged children, SGA had a lower BMI as compared to AGA-born subjects. SGA status was negatively associated with BMI and lean mass. Given that childhood is a crucial period with a major impact on the later development of certain diseases such as obesity or metabolic syndrome, a longitudinal follow-up of SGA-born subjects is warranted beyond infancy and childhood.
Availability of data and material
Non-available.
Code availability
Not applicable.
Abbreviations
- AGA:
-
Appropriate for gestational age
- BMI:
-
Body mass index
- DeXA:
-
Dual-energy x-ray absorptiometry
- LGA:
-
Large for gestational age
- PI:
-
Ponderal index
- PMD:
-
Predicted mass density
- SGA:
-
Small for gestational age
References
Ong KK (2006) Size at birth, postnatal growth and risk of obesity. Horm Res 65(Suppl 3):65–69
Nam HK, Lee KH (2018) Small for gestational age and obesity: epidemiology and general risks. Ann Pediatr Endocrinol Metab 23:9–13
Saenger P, Czernichow P, Hughes I, Reiter EO (2007) Small for gestational age: short stature and beyond. Endocr Rev 28:219–251
Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG, Guo XR (2011) Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev 12:525–542
Manapurath R, Gadapani B, Pereira-da-Silva L (2022) Body composition of infants born with intrauterine growth restriction: a systematic review and meta-analysis. Nutrients 14
Bamberg C, Kalache KD (2004) Prenatal diagnosis of fetal growth restriction. Semin Fetal Neonatal Med 9:387–394
Hediger ML, Overpeck MD, McGlynn A, Kuczmarski RJ, Maurer KR, Davis WW (1999) Growth and fatness at three to six years of age of children born small- or large-for-gestational age. Pediatrics 104:e33
Kramer MS, Martin RM, Bogdanovich N, Vilchuk K, Dahhou M, Oken E (2014) Is restricted fetal growth associated with later adiposity? Observational analysis of a randomized trial. Am J Clin Nutr 100:176–181
Rogers IS, Ness AR, Steer CD, Wells JC, Emmett PM, Reilly JR, Tobias J, Smith GD (2006) Associations of size at birth and dual-energy X-ray absorptiometry measures of lean and fat mass at 9 to 10 y of age. Am J Clin Nutr 84:739–747
Crume TL, Scherzinger A, Stamm E, McDuffie R, Bischoff KJ, Hamman RF, Dabelea D (2014) The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: the EPOCH study. Obesity (Silver Spring) 22:608–615
Ibanez L, Ong K, Dunger DB, de Zegher F (2006) Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J Clin Endocrinol Metab 91:2153–2158
Meas T, Deghmoun S, Armoogum P, Alberti C, Levy-Marchal C (2008) Consequences of being born small for gestational age on body composition: an 8-year follow-up study. J Clin Endocrinol Metab 93:3804–3809
Ibanez L, Lopez-Bermejo A, Suarez L, Marcos MV, Diaz M, de Zegher F (2008) Visceral adiposity without overweight in children born small for gestational age. J Clin Endocrinol Metab 93:2079–2083
Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS (1993) Fetal nutrition and cardiovascular disease in adult life. Lancet 341:938–941
Hales CN, Barker DJ (2001) The thrifty phenotype hypothesis. Br Med Bull 60:5–20
Lucas A, Fewtrell MS, Cole TJ (1999) Fetal origins of adult disease-the hypothesis revisited. BMJ 319:245–249
Rallis D, Balomenou F, Tzoufi M, Giapros V (2021) A systematic review indicates an association between birth weight and body fat in childhood. Acta Paediatr
Villar J, Giuliani F, Bhutta ZA, Bertino E, Ohuma EO, Ismail LC, Barros FC, Altman DG, Victora C, Noble JA, Gravett MG, Purwar M, Pang R, Lambert A, Papageorghiou AT, Ochieng R, Jaffer YA, Kennedy SH (2015) International F, Newborn Growth Consortium for the C. Postnatal growth standards for preterm infants: the preterm postnatal follow-up study of the INTERGROWTH-21(st) Project. Lancet Glob Health 3:e681–691
McCowan LM, Figueras F, Anderson NH (2018) Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am J Obstet Gynecol 218:S855–S868
Figueras F, Gratacos E (2014) Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther 36:86–98
Scherdel P, Botton J, Rolland-Cachera MF, Leger J, Pele F, Ancel PY, Simon C, Castetbon K, Salanave B, Thibault H, Lioret S, Peneau S, Gusto G, Charles MA, Heude B (2015) Should the WHO growth charts be used in France? PLoS ONE 10:e0120806
Baker-Smith CM, Flinn SK, Flynn JT, Kaelber DC, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM (2018) Subcommittee On S, Management Of High Bp In C. Diagnosis, evaluation, and management of high blood pressure in children and adolescents. Pediatrics 142
Houtkooper LB, Going SB, Lohman TG, Roche AF, Van Loan M (1985) Bioelectrical impedance estimation of fat-free body mass in children and youth: a cross-validation study. J Appl Physiol 1992(72):366–373
Andrews ET, Beattie RM, Johnson MJ (2019) Measuring body composition in the preterm infant: evidence base and practicalities. Clin Nutr 38:2521–2530
Demerath EW, Fields DA (2014) Body composition assessment in the infant. Am J Hum Biol 26:291–304
Mu M, Wang SF, Sheng J, Zhao Y, Li HZ, Hu CL, Tao FB (2012) Birth weight and subsequent blood pressure: a meta-analysis. Arch Cardiovasc Dis 105:99–113
Basioti M, Giapros V, Kostoula A, Cholevas V, Andronikou S (2009) Growth restriction at birth and kidney function during childhood. Am J Kidney Dis 54:850–858
Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, Vikse BE (2013) Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 382:273–283
Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM (2016) Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med 50:761–779
Griffin IJ, Cooke RJ (2012) Development of whole body adiposity in preterm infants. Early Hum Dev 88(Suppl 1):S19-24
Gallo P, Cioffi L, Limauro R, Farris E, Bianco V, Sassi R, De Giovanni M, Gallo V, D'Onofrio A, Di Maio S (2016) SGA children in pediatric primary care: what is the best choice, large or small? A 10-year prospective longitudinal study. Glob Pediatr Health 3:2333794X16659993
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
FB, DR, and VG conceptualized and developed the study design. Data collection was performed by FB. VG performed the statistical analysis. DR wrote the draft and reviewed and revised the manuscript. FB, FE, AZ, KB, MT, ES, NT, and VG reviewed and revised the manuscript. All authors approved the final manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Science and Research Ethics Committee of the University of Ioannina (No 934).
Consent to participate
Written informed consent was provided by the parents for the participation of their children in this study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balomenou, F., Rallis, D., Evangelou, F. et al. Is small for gestational age status independently correlated with body composition during childhood?. Eur J Pediatr 182, 661–668 (2023). https://doi.org/10.1007/s00431-022-04723-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04723-1