Abstract
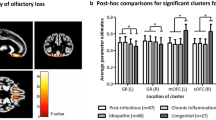
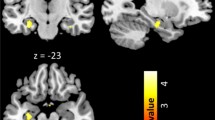
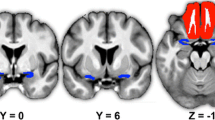
Brain structural features of healthy individuals are associated with olfactory functions. However, due to the pathophysiological differences, congenital and acquired anosmia may exhibit different structural characteristics. A systematic review was undertaken to compare brain structural features between patients with congenital and acquired anosmia. A systematic search was conducted using PubMed/MEDLINE and Scopus electronic databases to identify eligible reports on anosmia and structural changes and reported according to PRISMA guidelines. Reports were extracted for information on demographics, psychophysical evaluation, and structural changes. Then, the report was systematically reviewed based on various aetiologies of anosmia in relation to (1) olfactory bulb, (2) olfactory sulcus, (3) grey matter (GM), and white matter (WM) changes. Twenty-eight published studies were identified. All studies reported consistent findings with strong associations between olfactory bulb volume and olfactory function across etiologies. However, the association of olfactory function with olfactory sulcus depth was inconsistent. The present study observed morphological variations in GM and WM volume in congenital and acquired anosmia. In acquired anosmia, reduced olfactory function is associated with reduced volumes and thickness involving the gyrus rectus, medial orbitofrontal cortex, anterior cingulate cortex, and cerebellum. These findings contrast to those observed in congenital anosmia, where a reduced olfactory function is associated with a larger volume and higher thickness in parts of the olfactory network, including the piriform cortex, orbitofrontal cortex, and insula. The present review proposes that the structural characteristics in congenital and acquired anosmia are altered differently. The mechanisms behind these changes are likely to be multifactorial and involve the interaction with the environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The etiologies of olfactory dysfunction include congenital causes, ageing, idiopathic changes, infections of the upper respiratory tract (URTI), sinonasal disease (SND), traumatic brain injury (TBI), and neurologic illnesses including Alzheimer’s disease, multiple sclerosis and Parkinson’s disease (Wiesmann et al. 2001; Barresi et al. 2012). Olfactory dysfunction can affect all areas of life—from preparation and enjoyment of foods to the appreciation of the fragrances of flowers, detection and avoidance of hazardous odours, maintenance of personal hygiene, and the social or intimate interactions with others (Stevenson 2009; Croy et al. 2014).
The sense of smell allows us to identify the chemical nature in the surroundings. Sensory neurons in the nose detect odour molecules and transmit signals to the olfactory bulb (OB), a structure in the forebrain where initial odour processing occurs (Han et al. 2019). The OB collects the sensory afferents of the olfactory receptor cells located in the olfactory neuroepithelium. The axonal projections are then conveyed with a relay in the OB to the piriform cortex through the olfactory tract (Gottfried 2010). The piriform cortex projects to multiple brain regions within the limbic system. This system is directly connected with the frontal cortex through pathways to the posterior orbitofrontal cortex (OFC) as well as indirectly via the mediodorsal thalamic nucleus (Carmichael et al. 1994; Illig 2005). The OB is closely related to the olfactory sulcus (OS), located in the frontal lobe (Rombaux et al. 2009b). Previous studies suggest the OB volume (Mazal et al. 2016; Shehata et al. 2018; Yousem et al. 1996b), and OS depth (Miao et al. 2015) change according to different types of olfactory disorders suggesting that peripheral olfactory input influences the OB volume, in which OB volume is smaller, and OS depth is shallow in anosmic patients. Little is known about cortical brain areas beyond the OB and OS. Only a few studies reported pattern and variability of grey matter (GM) and white matter (WM) in patients with olfactory loss (Bitter et al. 2010a, b; Peng et al. b, 2021; Yap et al. 2021). The search was performed to identify studies reporting congenital anosmia and acquired anosmia using magnetic resonance imaging (MRI). Article search was conducted between the earliest record and 26 July 2021. Search terms were as follow: ((((((((((anosmia)) OR (congenital anosmia)) OR (acquired anosmia)) OR (smell impairment)) OR (olfactory loss)) OR (olfactory dysfunction)) OR (olfactory deficit)) OR (smell blindness)) OR (smell dysfunction)) AND ((( (Magnetic resonance imaging)) OR (MRI)) OR (MR Imaging)). We also manually checked for related articles in references and citations through the Google Scholar database. There was no limitation on the publication date. All records were grouped into a final database after removing duplicates, followed by screening the titles and abstracts and finally full-text article screening and eligibility by HAM and NY, independently, the details of the selected studies are tabulated in Fig. 1. Consensus for eligibility was reached through discussion. We used an assessment tool from the National Heart, Lung and Blood Institute, Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, to assess the quality of included studies https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
Inclusion criteria and exclusion criteria
Original studies reported in peer-reviewed journals describing research on structural brain changes in anosmia using MRI were included. We included studies describing research on patients with anosmia (congenital and acquired) that include studies that used standardised measures of olfactory function, e.g., the measurement of odour threshold, discrimination and identification (TDI score) (Hummel et al. 2017), and assessing any olfactory component; OB volume, OS depth, WM, and GM. We excluded all review articles as well as case reports and case series studies. We also exclude functional MRI (fMRI), diffusor tensor imaging (DTI), electro-encephalography (EEG), and magneto-encephalography (MEG) studies. We also exclude olfactory dysfunction in relation to neurodegenerative diseases and neuropsychiatric disorders such as; Alzheimer's and syndromes such as Kallmann and Bardet Biedl syndromes. Finally, we exclude anosmia in patients with SARS-CoV-2 or COVID-19 infection because findings on COVID-19 are currently develo** and unclear. We estimate that olfactory loss due to COVID-19 requires an independent review due to its emerging nature. Following the removal of duplicates and citations from non-English language journals, those evidently outside the review’s scope were rejected. From the eligible studies, the following variables were recorded: year of publication, country, study author(s), analysis mode, participants’ demographics, including age, handedness, duration of olfactory loss, psychophysical and physiological tests, and principal findings.
Results
Study demographics and details
Table 1 provides a summary of demographic information of anosmic patients with various etiologies, including congenital anosmia, idiopathic olfactory loss (IOL), infections of the upper respiratory tract (URTI), and traumatic brain injury (TBI). Across 28 studies, 2024 participants have investigated; 1639 patients and 385 healthy controls. Generally, studies have reasonable quality, as shown in Supplementary A. Sample size calculations were rarely mentioned, and the number of anosmic patients reported was between 3 and 378 patients per study. None of the studies was blinded due to the nature of the studies, which require the direct involvement of personnel-in-charge. Gender of participants were reported in some of the studies and indicated slightly more females (anosmia: males = 581, females = 625 and not mentioned = 433, healthy controls: male = 173, female = 196 and not mentioned = 16). The studies were conducted in multiple countries, including South Korea, The Netherlands, Germany, Belgium, PR China, the USA, and Taiwan. The age of the participants ranged from 9 to 73 years old. Seventeen studies compared anosmic patients to age-and sex-matched healthy controls. However, no study conducted separate analyses based on age and gender. Seven studies exclusively accrued right-handed participants (Bitter et al. 2010a, b; Peng et al. 2011).
Limitations in the literature
The heterogeneity of the findings was high due to the differences in many factors. The results show morphological variations, particularly in GM and WM atrophy. For example, both congenital and acquired anosmia studies reported inconsistency in brain areas and laterality. Even though some of the studies reported similar aetiology, their findings were at times contradictory. Six studies reported congenital anosmia; however, only three studies reported larger volumes of GM and WM compared to healthy controls in a few areas related to olfactory processing. Most importantly, the areas involved and the laterality of the involved areas were varied from one study to another. Furthermore, most of the existing studies also have a very small sample size, and future research requires further investigation in a larger sample.
Future directions for research in this area
We would like to suggest more comprehensive research in the future for both congenital and acquired olfactory loss. This includes more regions being considered, more comprehensive measures of anosmia, and the usage of MRI methods like DTI. In the current study, we proposed that the neural mechanism of brain alterations is different between aetiology, but the underlying details of these differences are unclear. More importantly, congenital anosmia shows larger GM and WM volume than HC, which is not found in acquired anosmia. This finding is fascinating, but the current data show discrepancies in terms of areas involved and brain laterality.
Conclusion
The present review suggests that MRI evaluations of OB volume could objectively diagnose olfactory dysfunction in patients with subjective olfactory loss. However, because the correlation between OS depth and olfactory dysfunction is not apparent and contradictory, a combined OB volume and OS depth evaluation is suggested. We also observed the right dominance of OB volume and OS depth, which is in line with the idea that the right hemisphere is relatively more important for olfactory processing than the left hemisphere. The present review also observed both primary and secondary olfactory areas show GM and WM alterations and conclude that brain alteration is more pronounced with longer disease duration. Finally, congenital anosmia shows larger GM and WM volumes in a few regions in primary and secondary olfactory areas. This is opposite to the presence of smaller GM and WM volumes in patients with acquired olfactory loss. The present study suggests that the difference in the volume and thickness of GM and WM between congenital and acquired anosmia is due to different mechanisms responsible for the olfactory dysfunction. We further suggest that the mechanisms underlying congenital anosmia differ from those involved in acquired loss. The mechanism behind these structural and neural changes are likely to be multifactorial. This result motivates further neuroimaging research into the pathophysiology of lifelong olfactory dysfunction.
Availability of data and material (data transparency)
Not applicable.
Code availability (software application or custom code)
Not applicable.
Abbreviations
- URTI:
-
Infections of the upper respiratory tract
- SND:
-
Sinonasal disease
- TBI:
-
Traumatic brain injury
- OB:
-
Olfactory bulb
- OS:
-
Olfactory sulcus
- GM:
-
Grey matter
- WM:
-
White matter
- MRI:
-
Magnetic resonance imaging
- fMRI:
-
Functional magnetic resonance imaging
- DTI:
-
Diffusor tensor imaging
- EEG:
-
Electroencephalography
- MEG:
-
Magnetoencephalography
- VBM:
-
Voxel-based morphometry
- TDI score:
-
Threshold-Discrimination-Identification score
- OFC:
-
Orbital frontal cortex
- SFS:
-
Superior frontal sulcus
- MFG:
-
Middle frontal gyrus
- MPC:
-
Medial prefrontal cortex
- DLPFC:
-
Dorsolateral prefrontal cortex
- ACC:
-
Anterior cingulate cortex
- MTG:
-
Middle temporal gyrus
- STG:
-
Superior temporal gyrus
- SMG:
-
Supramarginal gyrus
- SFG:
-
Superior frontal gyrus
- MOG:
-
Middle occipital gyrus
- MCC:
-
Middle cingulate cortex
- ACC:
-
Anterior cingulate cortex
- ITG:
-
Inferior temporal gyrus
- SFS:
-
Superior frontal sulcus
- MPC:
-
Medial prefrontal cortex
- SCG:
-
Subcallosal gyrus
- NAc:
-
Nucleus accumbens
- SOG:
-
Superior occipital gyrus
- IC:
-
Anterior insular cortex
- SMG:
-
Supramarginal gyrus
References
Abolmaali ND, Kühnau D, Knecht M, Köhler K, Hüttenbrink KB, Hummel T (2001) Imaging of the human vomeronasal duct. Chem Senses. https://doi.org/10.1093/chemse/26.1.35
Abolmaali ND, Volker H, Thomas JV, Karl BH, Thomas H (2002) MR evaluation in patients with isolated anosmia since birth or early childhood. Am J Neuroradiol 23:157–164
Albouy P, Mattout J, Bouet R, Maby E, Sanchez G, Aguera PE, Daligault S et al (2013) Impaired pitch perception and memory in congenital amusia: the deficit starts in the auditory cortex. Brain. https://doi.org/10.1093/brain/awt082
Arnold TC, You Y, Ding M, Zuo X-N, de Araujo I, Li W (2020) Functional connectome analyses reveal the human olfactory network organization. Eneuro. https://doi.org/10.1523/eneuro.0551-19.2020
Barresi M, Ciurleo R, Giacoppo S, Cuzzola VF, Celi D, Bramanti P, Marino S (2012) Evaluation of olfactory dysfunction in neurodegenerative diseases. J Neurol Sci. https://doi.org/10.1016/j.jns.2012.08.028
Bengtsson S, Berglund H, Gulyas B, Cohen E, Savic I (2001) Brain activation during odor perception in males and females. NeuroReport. https://doi.org/10.1097/00001756-200107030-00048
Bitter T, Brüderle J, Gudziol H, Burmeister HP, Gaser C, Guntinas-Lichius O (2010a) Gray and white matter reduction in hyposmic subjects—a voxel-based morphometry study. Brain Res 1347(August):42–47. https://doi.org/10.1016/j.brainres.2010.06.003
Bitter T, Gudziol H, Burmeister HP, Mentzel HJ, Guntinas-Lichius O, Gaser C (2010b) Anosmia leads to a loss of gray matter in cortical brain areas. Chem Senses. https://doi.org/10.1093/chemse/bjq028
Boesveldt S, Postma EM, Boak D, Welge-Luessen A, Schöpf V, Mainland JD, Martens J, Ngai J, Duffy VB (2017) Anosmia-a clinical review. Chem Senses. https://doi.org/10.1093/chemse/bjx025
Bridge H, Cowey A, Ragge N, Watkins K (2009) Imaging studies in congenital anophthalmia reveal preservation of brain architecture in “visual” cortex. Brain. https://doi.org/10.1093/brain/awp279
Buschhüter D, Smitka M, Puschmann S, Gerber JC, Witt M, Abolmaali ND, Hummel T (2008) Correlation between olfactory bulb volume and olfactory function. Neuroimage. https://doi.org/10.1016/j.neuroimage.2008.05.004
Carmichael ST, Clugnet M-C, Price JL (1994) Central olfactory connections in the macaque monkey. J Comp Neurol 346(3):403–434. https://doi.org/10.1002/cne.903460306
Chung MS, Choi WR, Jeong HY, Lee JH, Kim JH (2018) MR imaging-based evaluations of olfactory bulb atrophy in patients with olfactory dysfunction. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A5491
Coppola DM (2012) Studies of olfactory system neural plasticity: the contribution of the unilateral naris occlusion technique. Neural Plast. https://doi.org/10.1155/2012/351752
Croy I, Nordin S, Hummel T (2014) Olfactory disorders and quality of life-an updated review. Chem Senses. https://doi.org/10.1093/chemse/bjt072
Çullu N, Yeniçeri İÖ, Güney B, Özdemir MY, Koşar İ (2020) Evaluation of olfactory bulbus volume and olfactory sulcus depth by 3 T MR. Surg Radiol Anat. https://doi.org/10.1007/s00276-020-02484-w
Delon-Martin C, Plailly J, Fonlupt P, Veyrac A, Royet JP (2013) Perfumers’ expertise induces structural reorganisation in olfactory brain regions. Neuroimage 68(March):55–62. https://doi.org/10.1016/j.neuroimage.2012.11.044
Doty RL, Shaman P, Kimmelman CP, Dann MS (1984) University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. https://doi.org/10.1288/00005537-198402000-00004
Doty RL, Yousem DM, Pham LT, Kreshah AA, Gechle R, William Lee V (1997) Olfactory dysfunction in patients with head trauma. Arch Neurol. https://doi.org/10.1001/archneur.1997.00550210061014
Folstein MF, Folstein SE, McHugh PR (1975) ‘Mini-Mental State’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. https://doi.org/10.1016/0022-3956(75)90026-6
Fonteyn S et al (2014) Non-sinonasal-related olfactory dysfunction: a cohort of 496 patients. Eur Ann Otorhinolaryngol Head Neck Dis. https://doi.org/10.1016/j.anorl.2013.03.006
Frasnelli J, Hummel T (2005) Olfactory dysfunction and daily life. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-004-0796-y
Frasnelli J, Fark T, Lehmann J, Gerber J, Hummel T (2013) Brain structure is changed in congenital anosmia. Neuroimage. https://doi.org/10.1016/j.neuroimage.2013.07.070
Fujii M, Fukazawa K, Takayasu S, Sakagami M (2002) Olfactory dysfunction in patients with head trauma. Auris Nasus Larynx. https://doi.org/10.1016/S0385-8146(01)00118-3
Gardner AJ, Shih SL, Adamov EV, Zafonte RD (2017) Research frontiers in traumatic brain injury: defining the injury. Phys Med Rehabil Clin N Am. https://doi.org/10.1016/j.pmr.2016.12.014
Goektas O, Fleiner F, Sedlmaier B, Bauknecht C (2009) Correlation of olfactory dysfunction of different etiologies in mri and comparison with subjective and objective olfactometry. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2008.10.039
Gottfried JA (2010) Central mechanisms of odour object perception. Nat Rev Neurosci. https://doi.org/10.1038/nrn2883
Gottfried JA, Zelano C (2011) The value of identity: olfactory notes on orbitofrontal cortex function. Ann N Y Acad Sci. https://doi.org/10.1111/j.1749-6632.2011.06268.x
Gudziol V, Paech I, Hummel T (2010) Unilateral reduced sense of smell is an early indicator for global olfactory loss. J Neurol. https://doi.org/10.1007/s00415-009-5445-3
Han P, Winkler N, Hummel C, Hähner A, Gerber J, Hummel T (2018) Alterations of brain gray matter density and olfactory bulb volume in patients with olfactory loss after traumatic brain injury. J Neurotrauma. https://doi.org/10.1089/neu.2017.5393
Han P, Zang Y, Akshita J, Hummel T (2019) Magnetic resonance imaging of human olfactory dysfunction. Brain Topogr 32(6):987–997. https://doi.org/10.1007/s10548-019-00729-5
Hasson U, Andric M, Atilgan H, Collignon O (2016) Congenital blindness is associated with large-scale reorganisation of anatomical networks. Neuroimage. https://doi.org/10.1016/j.neuroimage.2015.12.048
Huart C, Meusel T, Gerber J, Duprez T, Rombaux P, Hummel T (2011) The depth of the olfactory sulcus is an indicator of congenital anosmia. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A2632
Hummel T, Damm M, Vent J, Schmidt M, Theissen P, Larsson M, Klussmann JP (2003) Depth of olfactory sulcus and olfactory function. Brain Res. https://doi.org/10.1016/S0006-8993(03)02589-7
Hummel T, Doty RL, Yousem DM (2005) Functional MRI of intranasal chemosensory trigeminal activation. Chem Senses. https://doi.org/10.1093/chemse/bjh186
Hummel T, Urbig A, Huart C, Duprez T, Rombaux P (2015) Volume of olfactory bulb and depth of olfactory sulcus in 378 consecutive patients with olfactory loss. J Neurol. https://doi.org/10.1007/s00415-015-7691-x
Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, Damm M et al (2017) Position paper on olfactory dysfunction. Rhinology. https://doi.org/10.4193/Rhino16.248
Huttenlocher PR, Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. https://doi.org/10.1002/(SICI)1096-9861(19971020)387:2%3c167::AID-CNE1%3e3.0.CO;2-Z
Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H (1982) Synaptogenesis in human visual cortex - evidence for synapse elimination during normal development. Neurosci Lett. https://doi.org/10.1016/0304-3940(82)90379-2
Hyde KL, Lerch JP, Zatorre RJ, Griffiths TD, Evans AC, Peretz I (2007) Cortical thickness in congenital amusia: when less is better than more. J Neurosci. https://doi.org/10.1523/JNEUROSCI.3039-07.2007
Illig KR (2005) Projections from orbitofrontal cortex to anterior piriform cortex in the rat suggest a role in olfactory information processing. J Compar Neurol 488(2):224–231. https://doi.org/10.1002/cne.20595
Innocenti GM, Price DJ (2005) Exuberance in the development of cortical networks. Nat Rev Neurosci. https://doi.org/10.1038/nrn1790
Jiang J, Zhu W, Shi F, Liu Y, Li J, Qin W, Li K, Chunshui Yu, Jiang T (2009a) Thick visual cortex in the early blind. J Neurosci. https://doi.org/10.1523/JNEUROSCI.5451-08.2009
Jiang RS, Chai JW, Chen WH, Fuh WB, Chiang CM, Chen CCC (2009b) Olfactory bulb volume in taiwanese patients with posttraumatic anosmia. Am J Rhinol Allergy. https://doi.org/10.2500/ajra.2009.23.3370
Jones-Gotman M, Zatorre RJ (1993) Odor recognition memory in humans: role of right temporal and orbitofrontal regions. Brain Cogn. https://doi.org/10.1006/brcg.1993.1033
Karstensen HG, Vestergaard M, Baaré WFC, Skimminge A, Djurhuus B, Ellefsen B, Brüggemann N et al (2018) Congenital olfactory impairment is linked to cortical changes in prefrontal and limbic brain regions. Brain Imaging Behav. https://doi.org/10.1007/s11682-017-9817-5
Kolb B, Gibb R (2011) Brain plasticity and behaviour in the develo** brain. J Canad Acad Child Adolesce Psychiatry 20:265
Kondo H, Matsuda T, Hashiba M, Baba S (1998) A study of the relationship between the T&T olfactometer and the University of Pennsylvania Smell Identification Test in a Japanese population. Am J Rhinol. https://doi.org/10.2500/105065898780182390
Kondo K, Kikuta S, Ueha R, Suzukawa K, Yamasoba T (2020) Age-related olfactory dysfunction: epidemiology, pathophysiology, and clinical management. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2020.00208
Lee S, Changsu H, Meungho R, Jae EO, Jie S, Yan C, Guoxing L, Perdana, Fallis AG (2012) 済無No Title No Title. J Chem Inf Model 53(9):1689–1699. https://doi.org/10.1017/CBO9781107415324.004
Liu J et al (2018) Evaluation of idiopathic olfactory loss with chemosensory event-related potentials and magnetic resonance imaging. Int Forum Allergy Rhinol. https://doi.org/10.1002/alr.22144
Manan HA, Franz EA, Yahya N (2020a) Functional connectivity changes in patients with brain tumours—a systematic review on resting state-FMRI. Neurol Psychiatry Brain Res. https://doi.org/10.1016/j.npbr.2020.03.003
Manan HA, Franz EA, Yahya N (2020b) Utilisation of functional MRI language paradigms for pre-operative map**: a systematic review. Neuroradiology. https://doi.org/10.1007/s00234-019-02322-w
Manan HA, Elizabeth AF, Noorazrul Y (2021) The Utilisation of resting-state FMRI as a pre-operative map** tool in patients with brain tumours in comparison to task-based FMRI and intraoperative map**: a systematic review. Eur J Cancer Care. https://doi.org/10.1111/ecc.13428
Mazal PP, Haehner A, Hummel T (2016) Relation of the volume of the olfactory bulb to psychophysical measures of olfactory function. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-014-3325-7
Miao X, Yang L, Hua Gu, Ren Y, Chen G, Liu J, Wei Y (2015) Evaluation of post-traumatic anosmia with MRI and chemosensory ERPs. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-014-3278-x
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, Atkins D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. https://doi.org/10.1371/journal.pmed.1000097
Mueller A, Rodewald A, Reden J, Gerber J, Von Kummer R, Hummel T (2005) Reduced olfactory bulb volume in post-traumatic and post-infectious olfactory dysfunction. NeuroReport. https://doi.org/10.1097/00001756-200504040-00011
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Nogueira R, Abolafia JM, Drugowitsch J, Balaguer-Ballester E, Sanchez-Vives MV, Moreno-Bote R (2017) Lateral orbitofrontal cortex anticipates choices and integrates prior with current information. Nat Commun. https://doi.org/10.1038/ncomms14823
Park HJ, Lee JD, Kim EY, Park B, Maeng Keun Oh, Lee SC, Kim JJ (2009) Morphological alterations in the congenital blind based on the analysis of cortical thickness and surface area. Neuroimage. https://doi.org/10.1016/j.neuroimage.2009.03.076
Peng P, Gu H, **ao W, Si LF, Wang JF, Wang SK, Zhai RY, Wei YX (2013) A voxel-based morphometry study of anosmic patients. Br J Radiol. https://doi.org/10.1259/bjr.20130207
Peter MG, Mårtensson G, Postma EM, Nordin LE, Westman E, Boesveldt S, Lundström JN (2020) Morphological changes in secondary, but not primary, sensory cortex in individuals with life-long olfactory sensory deprivation. Neuroimage. https://doi.org/10.1016/j.neuroimage.2020.117005
Potter H, Butters N (1980) An assessment of olfactory deficits in patients with damage to prefrontal cortex. Neuropsychologia. https://doi.org/10.1016/0028-3932(80)90101-3
Qin W, Liu Y, Jiang T, Chunshui Yu (2013) The development of visual areas depends differently on visual experience. PLoS ONE. https://doi.org/10.1371/journal.pone.0053784
Rombaux P, Mouraux A, Bertrand B, Nicolas G, Duprez T, Hummel T (2006a) Olfactory function and olfactory bulb volume in patients with postinfectious olfactory loss. Laryngoscope. https://doi.org/10.1097/01.MLG.0000195291.36641.1E
Rombaux P, Weitz H, Mouraux A, Nicolas G, Bertrand B, Duprez T, Hummel T (2006b) Olfactory function assessed with orthonasal and retronasal testing, olfactory bulb volume, and chemosensory event-related potentials. Arch Otolaryngol Head Neck Surg. https://doi.org/10.1001/archotol.132.12.1346
Rombaux P, Mouraux A et al (2006c) Retronasal and orthonasal olfactory function in relation to olfactory bulb volume in patients with post-traumatic loss of smell. Laryngoscope. https://doi.org/10.1097/01.mlg.0000217533.60311.e7
Rombaux P, Martinage S, Huart C, Collet S (2009a) Post-infectious olfactory loss: a cohort study and update. B-ENT. https://doi.org/10.3389/conf.neuro.09.2009.12.012
Rombaux P, Duprez T, Hummel T (2009b) Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology 47:3
Rombaux Ph, Potier H, Markessis E, Duprez T, Hummel T (2010a) Olfactory bulb volume and depth of olfactory sulcus in patients with idiopathic olfactory loss. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-010-1230-2
Rombaux P et al (2010b) Increased olfactory bulb volume and olfactory function in early blind subjects. NeuroReport. https://doi.org/10.1097/WNR.0b013e32833fcb8a
Rombaux P et al (2012) Prognostic value of olfactory bulb volume measurement for recovery in postinfectious and post-traumatic olfactory loss. Otolaryngol Head Neck Surg. https://doi.org/10.1177/0194599812459704
Schofield PW, Moore TM, Gardner A (2014) Traumatic brain injury and olfaction: a systematic review. Front Neurol. https://doi.org/10.3389/fneur.2014.00005
Shehata EM, Tomoum MO, Amer MA, Alarabawy RA, Eltomey MA (2018) Olfactory bulb neuroplasticity: a prospective cohort study in patients with chronic rhinosinusitis with nasal polyps. Clin Otolaryngol 43(6):1528–1534. https://doi.org/10.1111/coa.13202
Shepherd GM (2006) Smell images and the flavour system in the human brain. Nature. https://doi.org/10.1038/nature05405
Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Gabrieli JDE, Sullivan EV (1998) Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. https://doi.org/10.1523/jneurosci.18-21-08990.1998
Sobel N, Khan RM, Saltman A, Sullivan EV, Gabrieli JDE (1999) The world smells different to each nostril. Nature. https://doi.org/10.1038/46944
Stevenson RJ (2009) An initial evaluation of the functions of human olfaction. Chem Senses. https://doi.org/10.1093/chemse/bjp083
Tierney AL, Nelson CA (2009) Brain development and the role of experience in the early years. Zero Three 30:9
Veldhuizen MG, Nachtigal D, Teulings L, Gitelman DR, Small DM (2010) The insular taste cortex contributes to odor quality coding. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2010.00058
Voss P, Zatorre RJ (2012) Occipital cortical thickness predicts performance on pitch and musical tasks in blind individuals. Cereb Cortex. https://doi.org/10.1093/cercor/bhr311
Wandell BA, Wade AR (2003) Functional imaging of the visual pathways. Neurol Clin. https://doi.org/10.1016/S0733-8619(03)00003-3
Wang J, Eslinger PJ, Smith MB, Yang QX (2005) Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol Ser A Biol Sci Med Sci. https://doi.org/10.1093/gerona/60.4.510
Weiss T, Soroka T, Gorodisky L, Furman-Haran E, Dhollander T, Shushan S, Snitz K, Weissgross R, Sobel N (2019) Human olfaction without apparent olfactory bulbs case study human olfaction without apparent olfactory bulbs. Neuron. https://doi.org/10.1016/j.neuron.2019.10.006
Weiss T, Soroka T, Gorodisky L, Shushan S, Snitz K, Weissgross R, Furman-Haran E, Dhollander T, Sobel N (2020) Human olfaction without apparent olfactory bulbs. Neuron. https://doi.org/10.1016/j.neuron.2019.10.006
Wiesmann M, Yousry I, Heuberger E, Nolte A, Ilmberger J, Kobal G, Yousry TA, Kettenmann B, Naidich TP (2001) Functional magnetic resonance imaging of human olfaction. Neuroimaging Clin N Am. https://doi.org/10.1002/9780470995716.ch7
Yahya N, Manan HA (2019) Utilisation of diffusion tensor imaging in intracranial radiotherapy and radiosurgery planning for white matter dose optimization: a systematic review. World Neurosurg. https://doi.org/10.1016/j.wneu.2019.06.027
Yahya N, Manan HA (2020a) Diffusion tensor imaging indices to predict cognitive changes following adult radiotherapy. Eur J Cancer Care. https://doi.org/10.1111/ecc.13329
Yahya N, Manan HA (2020b) Neurocognitive impairment following proton therapy for paediatric brain tumour: a systematic review of post-therapy assessments. Support Care Cancer. https://doi.org/10.1007/s00520-020-05808-z
Yahya N, Chua X-J, Manan HA, Ismail F (2018) Inclusion of dosimetric data as covariates in toxicity-related radiogenomic studies: a systematic review. Strahlenther Onkol. https://doi.org/10.1007/s00066-018-1303-5
Yao L, Pinto JM, Yi X, Li Li, Peng P, Wei Y (2014) Gray matter volume reduction of olfactory cortices in patients with idiopathic olfactory loss. Chem Senses. https://doi.org/10.1093/chemse/bju047
Yao L, Yi X, Pinto JM, Yuan X, Guo Y, Liu Y, Wei Y (2018) Olfactory cortex and olfactory bulb volume alterations in patients with post-infectious olfactory loss. Brain Imaging Behav. https://doi.org/10.1007/s11682-017-9807-7
Yap KH, Manan HA, Sharip S (2021) Heterogeneity in brain functional changes of cognitive processing in ADHD across age: a systematic review of task-based FMRI studies. Behav Brain Res. https://doi.org/10.1016/j.bbr.2020.112888
Yousem DM, Geckle RJ, Bilker WB, McKeown DA, Doty RL (1996a) Post-traumatic olfactory dysfunction: MR and clinical evaluation. Am J Neuroradiol 17:1171–1179
Yousem DM, Geckle RJ, Bilker W, McKeown DA, Doty RL (1996b) MR evaluation of patients with congenital hyposmia or anosmia. Am J Roentgenol. https://doi.org/10.2214/ajr.166.2.8553963
Yousem DM, Maldjian JA, Siddiqi F, Hummel T, Alsop DC, Geckle RJ, Bilker WB, Doty RL (1999) Gender effects on odor-stimulated functional magnetic resonance imaging. Brain Res. https://doi.org/10.1016/S0006-8993(98)01276-1
Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E (1992) Functional localisation and lateralisation of human olfactory cortex. Nature. https://doi.org/10.1038/360339a0
Zhou W, Chen D (2008) Encoding human sexual chemosensory cues in the orbitofrontal and fusiform cortices. J Neurosci. https://doi.org/10.1523/JNEUROSCI.3148-08.2008
Funding
This work was supported by the Geran Galakan Penyelidik Muda (Incentive Grant for Young Researchers) Universiti Kebangsaan Malaysia (UKM) GGPM-2017-016, Dana Fundamental Pusat Perubatan Universiti Kebangsaan Malaysia (PPUKM) (PPUKM Fundamental Fund) FF-2020-013 and Publication Incentive Fund GP-2020-K021856.
Author information
Authors and Affiliations
Contributions
Original idea: TH. Articles search and selection: HAM and NY. Conceptualisation, writing the original draft: HAM. Review and editing: HAM, NY, PH and TH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
This article does not need informed consent by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manan, H.A., Yahya, N., Han, P. et al. A systematic review of olfactory-related brain structural changes in patients with congenital or acquired anosmia. Brain Struct Funct 227, 177–202 (2022). https://doi.org/10.1007/s00429-021-02397-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02397-3