Abstract
Background
To assess the frequency, clinical features, and outcome of peri-ictal delirium in adult patients experiencing seizures during intensive care.
Methods
This observational study was conducted at a Swiss intensive care unit from 2015 to 2020. Patients aged ≥ 18 years with seizures were categorized as peri-ictal delirious (Intensive Care Delirium Screening Checklist [i.e., ICDSC] ≥ 4) or not (i.e., ICDSC < 4) within 24 h of seizures. The frequency of peri-ictal delirium and in-hospital death were defined as the primary endpoints. Illness severity and treatment characteristics between delirious and non-delirious patients were secondary endpoints. Logistic regression was used to compare in-hospital death and differences regarding clinical characteristics between delirious and non-delirious patients.
Results
48% of 200 patients had peri-ictal delirium for a median of 3 days. Delirious patients were older (median age 69 vs. 62 years, p = 0.002), had lower Simplified Acute Physiology Scores II (SAPS II; median 43 vs. 54, p = 0.013), received neuroleptics more frequently (31 vs. 5%, p < 0.001), were mechanically ventilated less often (56% vs. 73%, p = 0.013) and shorter (median 3 vs. 5 days, p = 0.011), and had decreased odds for in-hospital death with delirium (OR = 0.41, 95% CI 0.20–0.84) in multivariable analyses.
Conclusions
Delirium emerged in every second patient experiencing seizures and was associated with lower SAPS II, shorter mechanical ventilation, and better outcomes, contradicting assumptions that altered cerebral function, from seizures and delirium, are linked to unfavorable outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several studies have investigated the emergence of transient cerebral dysfunction in critically ill patients admitted to intensive care units (ICUs) with frequent clinical manifestations, such as delirium [1, 7,8,9, 13, 22], and less frequently reported complications, such as seizures [4, 8, 31]. The term delirium is defined as a complex syndrome with a varying duration and combining at least four well-defined clinical symptoms including symptom fluctuation, disturbed sleep/wake-cycles, inappropriate speech or mood, psychomotor agitation or retardation, hallucinations/delusions/psychosis, disorientation, inattention, and altered consciousness [5]. In contrast, seizures are a sudden and temporary disturbance in the electrical activity of the brain, which can cause changes in behavior, sensation, motor activity or consciousness, lasting less than 5 min. They are often accompanied by focal or bilateral motor symptoms typically involving body parts according to their representation on the hyperactive motor cortex as outlined in the diagnostic manual of the International League Against Epilepsy (ILAE).
Despite their different clinical manifestation and frequency, both appear to be associated with increased morbidity, prolonged and intensified treatment, and adverse outcomes. However, the concurrent presentation of these neurological complications in critically ill patients remains inadequately explored, and there is a lack of understanding of the associated clinical characteristics and specific outcomes.
We, therefore, aimed to assess the frequency, clinical features and associated short-term outcome of peri-ictal delirium in critically ill patients experiencing seizures during intensive care.
Methods
Setting, study design and ethics
In the current study, we use data from a previously registered cohort study conducted at the ICU of the University Hospital of Basel, a Swiss academic tertiary medical center (NCT03860467; https://classic.clinicaltrials.gov/ct2/show/NCT03860467). The study was granted approval by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz), in accordance with the ethical principles outlined in the 1964 Declaration of Helsinki, along with its subsequent revisions. As per the committee's ethical evaluation (EKNZ No. 2018-02361), the requirement for patients’ informed consent was waived. To ensure the quality and uniformity in the reporting of observational studies, the STROBE guidelines were adhered to [29].
Data collection
All adult (≥ 18 years of age) ICU patients from January 1st 2015 to December 31st 2020 with reported isolated seizures were retrospectively assessed as previously reported (details regarding the definition of seizures outlined below) [30, 31]. Patients with persistent seizures fulfilling the criteria of status epilepticus (SE) were excluded. The prospectively recorded digital electroencephalographic (EEG) and ICU information system MetaVision (iMDsoft, Wakefield, MA) databases were screened to retrospectively collect and enter the following data into a predefined case report form: demographics, history of previous seizures and/or delirium, presumed etiology of seizures categorized as potentially non-fatal or fatal as previously defined [21, 30, 31], the Charlson Comorbidity Index [6], and the Simplified Acute Physiology Score II (SAPS II) [18]. In addition, several markers of systemic inflammation, including maximal core body temperature, serum concentrations of C-reactive protein (CRP) expressed in mg per liter, and white blood cell counts were assessed. Furthermore, the Glasgow Coma Score (GCS) [17] documented at seizure onset, seizure semiology (if reported, including single or repetitive seizures), and seizure evolution (defined as focal, focal to bilateral or primarily bilateral) were recorded. In addition, delirium-associated symptoms as assessed by the Intensive Care Delirium Screening Checklist (ICDSC) [5] with the highest ICDSC score on the day of seizure onset and 24 h prior and after seizure were assessed, as well as the emergence of delirium within this timeframe defined as an ICDSC ≥ 4 (details regarding ICDSC assessment outlined below).
Treatment parameters that were evaluated included the length of sedation and mechanical ventilation, the number of inserted drainages and catheters, administration of antipsychotic (neuroleptic) and antiseizure medications, as well as the duration of in-hospital treatment and ICU stay. Additionally, complications that emerged during intensive medical care were documented, such as organ failure and infections identified within a 7-day period preceding the onset of seizures. Infections were detected using the protocol outlined in earlier investigations [3, 24, 27], in accordance to the guidelines published by the Centers for Disease Control and Prevention (CDC) [11]. Outcome at hospital discharge, such as return to premorbid neurologic function and death, were assessed.
Definition of seizures
As in our previous study [31], patients were categorized as having seizures using predefined criteria. The diagnosis of seizures depended on the additional following three point: (1) patients needed to show improvement in consciousness and/or neurologic function after their seizure has been observed while in the intensive care unit; (2) the patients’ EEGs following seizures had to show evidence of repeated epileptiform discharges, such as spikes and/or sharp waves, following the first seizure; (3) or seizures had to be detected by EEG. In addition, patients with motor symptoms had to exhibit motor symptoms that align with typical seizures, as outlined by the ILAE. In accordance with the ILAE (diagnostic manual) motor symptoms had to be focal or bilateral and typically involve body parts linked to their representation on the motor cortex. These symptoms may include rhythmic myocloni, Jacksonian march, tonic muscle contractions, mutual contractions of agonist and antagonist muscles producing athetosis or twists, contraction clusters for milliseconds (jerks), sudden loss or diminution of muscle tone, flexion, extension or both for seconds (spasms) in series involving proximal and truncal muscles, hyperkinetic movements such as pedaling, jum**, pelvic thrusting, thrashing and/or rocking movements, automatisms defined as repetitive movements resembling voluntary action but undertaken without volition, dysarthria/anarthria while language functions remain intact, and forced conjugate ocular, cephalic, and/or truncal version, rotation, or lateral deviation (left or right).
All patients who exhibited clinical, electrographic, or electro-clinical seizures with incomplete recovery of neurologic function and consciousness within 30 min following the seizure received EEGs. Two trained and board-certified EEG specialists visually evaluated all EEGs, and in cases of disagreement, consensus was reached through additional joint review.
Definition and screening of delirium
As described in our previous study on postictal delirium following SE [4], in our institution, the ICDSC was routinely used to screen patients for the emergence of delirium. According to the studies and guidelines mentioned above, an ICDSC ≥ 4 was defined as delirium [2, 5]. An ICDSC ≥ 4 documented prior to seizure onset was defined as pre-ictal delirium, an ICDSC ≥ 4 in the aftermath of seizures as post-ictal delirium. Specialized nurses conducted systematic screenings using the ICDSC every 8 h throughout the entire study duration. First, the nurses assessed alterations in consciousness from baseline, inattention, disorganized thinking, and hallucinations or delusions. In the subsequent step, psychomotor activity (agitation or retardation), speech/mood, sleep–wake cycle, and symptom fluctuations were evaluated over the same duration of the 8-h shift. Patients’ Richmond Agitation-Sedation Scale (RASS) was also assessed every 8 h, ranging from − 5 (deep coma) to + 4 (combative) [10]. The ICDSC was evaluated once patients had recovered to a RASS level greater than − 4. In patients with delirium, the screening for ICDSC was expanded in both directions, the time prior and after the 24 h of seizure onset until ICDSC was < 4 to calculate the overall duration of delirium.
Outcomes
The frequency of peri-ictal delirium among critically ill patients experiencing seizures and in-hospital death were defined as the primary endpoints and differences regarding demographics, illness severity, and treatment characteristics between delirious and non-delirious patients were secondary endpoints.
Statistics
Patients were first categorized into patients with seizures during their ICU stay with and without peri-ictal delirium. Further, patients were categorized as patients with and without in-hospital death. Univariable comparisons of proportions were performed between the two groups of each categorization using Chi-square and Fisher exact test (where appropriate). Continuous variables were compared using the Student t test for normal distributions and the Mann–Whitney U test for non-normal distributions. Categorical variables were presented as counts (percentage), while continuous variables were expressed as medians and interquartile ranges (IQRs). Demographics, clinical, and treatment-related variables with significant differences among these groups were included in the uni- and multivariable logistic regression analyses to calculate the odds for each variable for in-hospital death. The final multivariable logistic regression model was assessed for goodness-of-fit using the Hosmer–Lemeshow χ2 test, which compares observed and estimated outcomes and provides measures of calibration [15]. Given that delirium occurring before (i.e., pre-ictal) and after (i.e., post-ictal) seizures could potentially signify distinct clinical entities and exhibit varying associations with specific outcomes, sensitivity analyses were conducted to evaluate the relationship between each of these two entities and outcome measures.
A two-tailed p value of ≤ 0.05 was considered statistically significant. All statistical analyses were performed using STATA®16.1 (Stata Corp., College Station, TX, USA).
Results
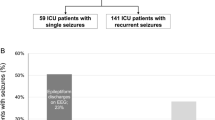
Among 26,370 critically ill patients treated in the intensive care unit from 2015 to 2020, 200 patients (0.76%) were identified as having seizures during intensive care. Of those, 96 (48%) were delirious 24 h around seizure onset according to the ICDSC (Fig. 1A), with a median duration of delirium of 3 days. Post-ictal delirium was more common than pre-ictal delirium (64.6% versus 35.4%). Differences regarding symptoms in patients with and without peri-ictal delirium as assessed by the ICDSC are outlined in Fig. 1B, with disturbed sleep/wake cycle, psychomotor agitation or retardation, inattention, and altered consciousness being the most frequent symptoms in patients with peri-ictal delirium.
Univariable comparisons between delirious and non-delirious patients
Univariable comparisons of clinical characteristics known on the day of seizure onset between patients with and without concomitant peri-ictal delirium are presented in Table 1. Delirious patients were older and had a lower median Simplified Acute Physiology Score II. History of seizures and previous delirium, as well as seizure type (i.e., focal or bilateral, and isolated or repetitive) did not differ between delirious and non-delirious patients. Table 2 presents the univariable comparison of treatment characteristics, complications, and short-term (in-hospital) outcomes between patients with and without concomitant peri-ictal delirium. As compared to non-delirious patients, delirious patients received neuroleptic drugs more often following the detection of delirium, were mechanically ventilated less frequently and for a shorter median duration, had a longer median in-hospital treatment, and died less often during their hospital stay. Analyses after excluding non-survivors, however, revealed no differences regarding median in-hospital stay. Other treatment characteristics, such as the number of inserted catheters and drainages, number of antiseizure drugs, length of ICU-stay, and complications reported during intensive care did not differ significantly.
Uni- and multivariable comparisons between patients with and without in-hospital death
Table 3 presents univariable comparisons of patient-related clinical characteristics known at seizure onset between survivors (158; 79%) and non-survivors (42; 21%). Non-survivors were older, less commonly delirious, reached a lower level of consciousness on the day of seizure, had a higher SAPS II, and potentially fatal etiologies of seizures were more frequent. However, seizure characteristics did not differ significantly. Late complications in the course of intensive care were more frequent in non-survivors (renal failure 43% vs. 22%, p = 0.005; liver failure 19% vs. 3%, p = 0.001; multiorgan failure 21% vs. 1%, p < 0.001).
Uni- and multivariable logistic regression analyses including all clinical variables known at seizure onset are presented in Table 4. Multivariable analyses revealed peri-ictal delirium to be associated with decreased odds for in-hospital death independent of other potential confounders, including increasing age, potentially fatal etiologies, increasing level of consciousness on the day of seizure, and decreasing SAPS II. The Hosmer Lemeshow goodness-of-fit test revealed insignificant p values representing adequate model fit (χ2 7.45, p = 0.489) indicating an adequate fit of the performed multivariable model.
Sensitivity analyses regarding pre- and postictal delirium
Given that delirium occurring before (i.e., pre-ictal) and after (i.e., post-ictal) seizures might signify distinct clinical entities and exhibit varying associations with specific outcomes, sensitivity analyses were conducted to evaluate the relationship between each of these two entities and in-hospital death. Such analyses revealed insignificant associations either for increased or decreased odds regarding in-hospital death for the multivariable models adjusting for the same potential confounders (for pre-ictal delirium: adjusted ORfor death 1.52; 95% CI 0.50–4.64; for post-ictal delirium: adjusted ORfor death 0.46, 95% CI 0.18–1.18).
Discussion
Our investigation reveals that peri-ictal delirium manifests in nearly half of the critically ill patients who suffer from seizures while in intensive care, with post-ictal delirium being more frequent than pre-ictal delirium. It is commonly assumed that manifestations of altered cerebral function, as mirrored by seizures and delirium, are closely linked to unfavorable prognoses and outcomes in critically ill patients [1, 4, 9, 13, 22, 31]. However, our study of a severely ill patient cohort with seizures demonstrates that delirium is associated with lower illness severity, as quantified by the SAPS II. Surprisingly, our study further suggests that patients with peri-ictal delirium experience shorter periods of mechanical ventilation and die less frequently during their hospital stay. As patients who died during their hospital stay may have died relatively early during the course of disease, subsequent analyses regarding duration of ICU and hospital stay were performed after excluding non-survivors. These analyses revealed no significant differences between delirious and non-delirious patients regarding ICU and hospital stay. However, mechanical ventilation remained significantly shorter in delirious patients. These findings and especially the shorter mechanical ventilation of delirious patients contradict other studies who suggest an association of ICU delirium and prolonged mechanical ventilation as well as increased mortality [19]. Multivariable analyses indicate that, independent of potential confounders identified in our univariable comparisons, patients with peri-ictal delirium have low odds of short-term in-hospital death. Although sensitivity analyses for both pre- and post-ictal delirium did not observe a consistent and significant reduction in the odds of in-hospital mortality, an increased risk of delirium with such adverse short-term outcomes that would have been in line with the current assumptions was not found in any of our analyses.
As the current evidence from the literature clearly indicates that delirium emerging as a complication during intensive care promotes increased mortality rather than survival [16], the findings of our study call for an explanation. As in our institution patients are not routinely sent to the ICU because of single seizures not transforming into status epilepticus, a selection towards less critically ill patients, for example, patients with uncontrolled epilepsy but no other critical illnesses can be excluded. However, the fact that non-delirious patients had a higher SAPS II indicating being more critically ill as compared to delirious patients suggests a potential underlying selection bias towards less critically ill patients. As in contrast to single seizures, delirium is a criterion to be admitted to the ICU, especially if the intermediate care unit has no capacity, the admission of delirious but otherwise not critically ill patients may be a possible explanation. However, as only 7 patients (3.5% of our cohort) were delirious on the day of admission to the ICU, it is unlikely that admission for treatment of delirium is the only explanation for such potential self-fulfilling prophecy. Another possible explanation that cannot be excluded from our study may be that our study population was not representative of all critically ill patients with delirium.
The high proportion of critically ill patients with delirium in close temporal relation to seizures seems surprising at first glance. However, it appears to be in line with several prior studies on delirium following SE [3, 4] and studies on delirium in critically ill populations without epileptic complications [8, 12, 14] but higher than in mixed cohorts of neurocritically ill patients with delirium in 12–43% [20] and 22% in post-surgery ICU patients [23]. The larger proportion of patients with delirium emerging in the post-ictal phase compared to delirium evolving hours before registered seizure onset suggests that seizures in critically ill patients may be important promotors for delirium, a hypothesis that calls for further studies.
When accounting for previous reports of “post-ictal encephalopathies”, the coincidence of delirium with seizures seems less surprising. However, it is crucial to note that the terms “post-ictal encephalopathy” and “delirium” should not be used interchangeably, even though distinguishing between the two may be challenging. Manifestation of altered consciousness, disorientation, and/or inattention in “post-ictal encephalopathies” cannot be equated with delirium, the latter requiring a more complex combination of at least four well-defined symptoms, as outlined in the ICDSC [5]. These insights prompted ten societies to issue an extensive statement regarding the nomenclature of delirium and encephalopathy, recommending that delirium be regarded as a subcategory of encephalopathy [25].
The fact that none of the investigated inflammatory parameters were associated with the occurrence of seizures and delirium, and on the contrary, the leukocyte count in delirious patients was lower than in non-delirious patients, may seem surprising, since in earlier studies, systemic inflammation or infections in SE patients were associated with a worse disease course and outcome [24, 26,27,28, 33]. However, the serum concentrations of CRP in our cohort increased with every day of observation. Possible explanations for not reaching significance are the limited sample size and the relatively short time of observation.
With a median of 3 days, the duration of delirium in our cohort was slightly longer as compared to delirium seen in patients following SE [3, 4]. However, a finding common to both patient with isolated seizures and SE is delirium emerging more frequently in patients with a higher level of consciousness at seizure onset. One possible albeit daring interpretation of this finding is that a higher level of consciousness at seizure onset and the occurrence of delirium may signify the preservation of complex neuronal functions in the central nervous system, a notion that warrants further exploration. While our results do not imply that the occurrence of delirium in critically ill patients with seizures is a reliable indicator of improved outcomes, they do challenge the prevailing notion that multiple symptoms of impaired brain function are associated with a faster disease progression or poor outcomes. In the absence of compelling evidence to the contrary, clinicians are urged to refrain from embracing the conventional assumption that an unfavorable prognosis is probable for this patient cohort.
Strengths and limitations
The strength of our study is the relatively large cohort, the observation period of 5 years at a tertiary academic medical care center, and the use of comprehensive prospectively monitored clinical data during the entire study period with the digital ICU information system MetaVision (iMDsoft, Wakefield, MA).
It is important to note that our study was designed as a single-center observational study, which may limit the generalizability of our findings. Nonetheless, the data we analyzed were systematically and prospectively recorded in a digital ICU information system, and was available for all patients, thereby reducing the possibility of selection bias. Additionally, the routine clinical practice in the ICU ensured the systematic collection of clinical data related to treatment, monitoring measures, and complications. Moreover, the standardized diagnostic procedures and management of seizures and delirium were performed by trained and specialized nurses and a consulting team of neurologists and neurocritical care specialists, which remained consistent throughout the entire study period. Another limitation is the fact that by the retrospective nature of the study, epileptic seizures that may have not been clinically overt may have been missed and are underrepresented by our cohort.
A similar limitation comes from the retrospective assessment of reported seizure semiology, which was lacking in some patients. Moreover, very short-lasting delirium might have been missed since the ICDSC was performed every eight hours only. This time span, however, reflects the daily clinical practice of many ICUs, as more frequent ICDSC assessments would be very labor intensive and interfere with patient care if performed by trained nurses. However, such screening every eight hours may be especially critical, as mental status of our patients may alter frequently in the post-ictal period. These limitations are likely to have affected the temporal allocation of delirium and seizures when applying our 24 h window. Moreover, the retrospective study design lacked the ability to discriminate between catatonia and delirium [32], which plausibly inflated the incidence of delirium. Additionally, the extent to which a “novel baseline neurologic function” manifested after seizures could not be evaluated, conceivably leading to an overestimation of the occurrence of post-ictal delirium. Finally and as discussed above, the retrospective nature of our study does not exclude the possibility of an underlying and undetected selection bias that may explain why delirious patients in our cohort were less severely ill. This, however, hardly detracts from the conclusion of our study that the co-occurrence of epileptic seizures and delirium can not necessarily be equated with a poor prognosis of ICU patients.
Conclusions
Peri-ictal delirium appears to be a common complication among patients experiencing seizures during intensive care, with delirium occurring in nearly every second patient and post-ictal delirium being more frequent than pre-ictal delirium. Interestingly, our observations suggest that peri-ictal delirium is associated with a decreased SAPS II, shorter mechanical ventilation, and better outcomes in our patient cohort. The observations made in this study challenge the prevalent assumption that altered cerebral function, as evidenced by seizures and delirium, is a harbinger of unfavorable outcomes. Further comprehensive investigations are required.
Data availability statement
The corresponding author has full access to all of the data in the study. He takes full responsibility for the integrity of the data, the accuracy of the data analysis and interpretation, and the conduct of the research. The authors have the right to publish any and all data, separate and apart from the guidance of any sponsor.
References
Abelha FJ, Luis C, Veiga D, Parente D, Fernandes V, Santos P, Botelho M, Santos A, Santos C (2013) Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Crit Care 17:R257
Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R, American College of Critical Care M (2013) Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 41:263–306
Baumann SM, Semmlack S, De Marchis GM, Rüegg S, Marsch S, Sutter R (2020) Frequency and implications of complications in the ICU after status epilepticus. No calm after the storm. Crit Care Med 48:1779–1789
Baumann SM, Semmlack S, Hunziker S, Kaplan PW, De Marchis GM, Ruegg S, Marsch S, Sutter R (2021) Prediction of postictal delirium following status epilepticus in the ICU: first insights of an observational cohort study. Crit Care Med 49:e1241–e1251
Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y (2001) Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Int Care Med 27:859–864
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Dittrich T, Marsch S, Ruegg S, De Marchis GM, Tschudin-Sutter S, Sutter R (2020) Delirium in meningitis and encephalitis: emergence and prediction in a 6-year cohort. J Intensive Care Med 36:566–575
Dittrich T, Tschudin-Sutter S, Widmer AF, Rüegg S, Marsch S, Sutter R (2016) Risk factors for new-onset delirium in patients with bloodstream infections: independent and quantitative effect of catheters and drainages—a four-year cohort study. Ann Intens Care Med 6:104
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, Inouye SK, Bernard GR, Dittus RS (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 291:1753–1762
Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, Sessler CN, Dittus RS, Bernard GR (2003) Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 289:2983–2991
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140
Girard TD, Exline MC, Carson SS, Hough CL, Rock P, Gong MN, Douglas IS, Malhotra A, Owens RL, Feinstein DJ, Khan B, Pisani MA, Hyzy RC, Schmidt GA, Schweickert WD, Hite RD, Bowton DL, Masica AL, Thompson JL, Chandrasekhar R, Pun BT, Strength C, Boehm LM, Jackson JC, Pandharipande PP, Brummel NE, Hughes CG, Patel MB, Stollings JL, Bernard GR, Dittus RS, Ely EW, Investigators M-U (2018) Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med 379:2506–2516
Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, Ely EW (2010) Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 38:1513–1520
Girard TD, Thompson JL, Pandharipande PP, Brummel NE, Jackson JC, Patel MB, Hughes CG, Chandrasekhar R, Pun BT, Boehm LM, Elstad MR, Goodman RB, Bernard GR, Dittus RS, Ely EW (2018) Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med 6:213–222
Hosmer DW, Lemeshow S (1980) A goodness of-fit test for the multiple logistic regression model. Commun Stat A9:1043–1069
Hughes CG, Hayhurst CJ, Pandharipande PP, Shotwell MS, Feng X, Wilson JE, Brummel NE, Girard TD, Jackson JC, Ely EW, Patel MB (2021) Association of delirium during critical illness with mortality: multicenter prospective cohort study. Anesth Analg 133:1152–1161
Jones C (1979) Glasgow coma scale. Am J Nurs 79:1551–1553
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Mart MF, Pun BT, Pandharipande P, Jackson JC, Ely EW (2021) ICU survivorship-the relationship of delirium, sedation, dementia, and acquired weakness. Crit Care Med 49:1227–1240
Patel MB, Bednarik J, Lee P, Shehabi Y, Salluh JI, Slooter AJ, Klein KE, Skrobik Y, Morandi A, Spronk PE, Naidech AM, Pun BT, Bozza FA, Marra A, John S, Pandharipande PP, Ely EW (2018) Delirium monitoring in neurocritically ill patients: a systematic review. Crit Care Med 46:1832–1841
Rossetti AO, Hurwitz S, Logroscino G, Bromfield EB (2006) Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry 77:611–615
Salluh JI, Soares M, Teles JM, Ceraso D, Raimondi N, Nava VS, Blasquez P, Ugarte S, Ibanez-Guzman C, Centeno JV, Laca M, Grecco G, Jimenez E, Arias-Rivera S, Duenas C, Rocha MG, Delirium Epidemiology in Critical Care Study G (2010) Delirium epidemiology in critical care (DECCA): an international study. Crit Care 14:R210
Scholz AF, Oldroyd C, McCarthy K, Quinn TJ, Hewitt J (2016) Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br J Surg 103:e21-28
Semmlack S, Tschudin-Sutter S, Widmer AF, Valenca M, Ruegg S, Marsch S, Sutter R (2016) Independent impact of infections on the course and outcome of status epilepticus: a 10-year cohort study. J Neurol 263:1303–1313
Slooter AJC, Otte WM, Devlin JW, Arora RC, Bleck TP, Claassen J, Duprey MS, Ely EW, Kaplan PW, Latronico N, Morandi A, Neufeld KJ, Sharshar T, MacLullich AMJ, Stevens RD (2020) Updated nomenclature of delirium and acute encephalopathy: statement of ten societies. Intensive Care Med 46:1020–1022
Sutter R, Grize L, Fuhr P, Rüegg S, Marsch S (2013) Acute phase proteins and mortality in status epilepticus: a five-year observational cohort study. Crit Care Med 41:1526–1533
Sutter R, Tschudin-Sutter S, Grize L, Fuhr P, Bonten MJ, Widmer AF, Marsch S, Ruegg S (2012) Associations between infections and clinical outcome parameters in status epilepticus: a retrospective 5-year cohort study. Epilepsia 53:1489–1497
Sutter R, Valenca M, Tschudin-Sutter S, Ruegg S, Marsch S (2015) Procalcitonin and mortality in status epilepticus: an observational cohort study. Crit Care 19:361
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
Wagner AS, Baumann SM, Semmlack S, Frei AI, Ruegg S, Hunziker S, Marsch S, Sutter R (2023) Comparing patients with isolated seizures and status epilepticus in intensive care units: an observational cohort study. Neurology 100:e1763–e1775
Wagner AS, Semmlack S, Frei A, Ruegg S, Marsch S, Sutter R (2022) Seizures and risks for recurrence in critically ill patients: an observational cohort study. J Neurol 269:4185–4194
Wilson JE, Carlson R, Duggan MC, Pandharipande P, Girard TD, Wang L, Thompson JL, Chandrasekhar R, Francis A, Nicolson SE, Dittus RS, Heckers S, Ely EW, Delirium CP, Cohort I (2017) Delirium and catatonia in critically ill patients: the delirium and catatonia prospective cohort investigation. Crit Care Med 45:1837–1844
Zelano J, Moller F, Dobesberger J, Trinka E, Kumlien E (2014) Infections in status epilepticus: a retrospective 5-year cohort study. Seizure 23:603–606
Funding
Open access funding provided by University of Basel. The funder (University Hospital Basel) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. This study was performed and designed without the input or support of any pharmaceutical company, or other commercial interest.
Author information
Authors and Affiliations
Contributions
AIF conceptualization, methodology, validation, investigation data curation, writing—review and editing. ASW conceptualization, methodology, validation investigation, data curation, investigation, writing—review and editing. SMB conceptualization, validation, investigation, data curation, writing—review and editing. PG validation, writing—review and editing. SB validation, writing—review and editing. SH validation, writing—review and editing. SR validation, writing—review and editing. SM validation, writing—review and editing. RS conceptualization, methodology, formal analysis, writing—original draft, visualization, supervision, funding acquisition. All authors approved the final submitted version.
Corresponding author
Ethics declarations
Conflict of interest
Anja I. Frei: none. Anna S. Wagner: none. Sira M. Baumann: none. Pascale Grzonka: none. Sebastian Berger: none. Sabina Hunziker reports to be supported by the Swiss National Foundation (SNF) (Ref 10001C_192850/1 and 10531C_182422), the Bangerter-Rhyner Foundation (8472/HEG-DSV), and the Swiss Society of General Internal Medicine (SSGIM). Stephan Rüegg received unconditional research grants from UCB-pharma. He received honoraria from serving on the scientific advisory boards of Arvelle, Eisai, and UCB-pharma, and from serving as a consultant for Arvelle, Eisai, Pfizer, Novartis, Sandoz, and UCB-pharma. He does not hold any stocks of any pharmaceutical industries or manufacturers of medical devices. He received funding from UCB-pharma, and Swiss National Science Foundation Grants: Grant Number 320030_169379/1 and coapplicant for grants numbers 33CM30_125115/1 and 33CM30_140338/1; he disclosed that he is the past-president of the Swiss League against Epilepsy (no payments), editor of EPILEPTOLOGIE (Journal of the Swiss League against Epilepsy) (no payments), and editor of the Swiss EEG Bulletin (payments from UCB). Stephan Marsch reports no disclosures. Raoul Sutter received research grants from the Swiss National Foundation (No 320030_169379), the Research Fund of the University Basel, the Scientific Society Basel, and the Bangerter-Rhyner Foundation. He received personal grants from UCB-pharma and holds stocks from Novartis, Roche, Alcon, and Johnson & Johnson.
Ethical standard statement
This study has been approved by the local ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frei, A.I., Wagner, A.S., Baumann, S.M. et al. Concurrence of seizures and peri-ictal delirium in the critically ill - its frequency, associated characteristics, and outcomes. J Neurol 271, 231–240 (2024). https://doi.org/10.1007/s00415-023-11944-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11944-3