Abstract
Lewy body diseases, such as Parkinson’s disease and dementia with Lewy bodies, vary in their clinical phenotype but exhibit the same defining pathological feature, α-synuclein aggregation. Microbiome–gut–brain dysfunction may play a role in the initiation or progression of disease processes, though there are multiple potential mechanisms. We discuss the need to evaluate gastrointestinal mechanisms of pathogenesis across Lewy body diseases, as disease mechanisms likely span across diagnostic categories and a ‘body first’ clinical syndrome may better account for the heterogeneity of clinical presentations across the disorders. We discuss two primary hypotheses that suggest that either α-synuclein aggregation occurs in the gut and spreads in a prion-like fashion to the brain or systemic inflammatory processes driven by gastrointestinal dysfunction contribute to the pathophysiology of Lewy body diseases. Both of these hypotheses posit that dysbiosis and intestinal permeability are key mechanisms and potential treatment targets. Ultimately, this work can identify early interventions targeting initial disease pathogenic processes before the development of overt motor and cognitive symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spectrum of Lewy body diseases ranges from incidental Lewy body disease, Parkinson’s disease (PD) with varying degrees of cognitive impairment, to dementia with Lewy bodies (DLB) at the most severe end [1]. PD is characterized by motor symptoms (bradykinesia, rigidity, and tremor [2]), with recent, increased appreciation that non-motor symptoms are common and significantly impact quality of life [3]. Estimated prevalence of PD ranges between 100 and 200 per 100,000 people and an annual incidence of 15 per 100,000 [4]. DLB is characterized by fluctuating cognition, recurrent visual hallucinations, REM sleep behavior disorder, and parkinsonism (including bradykinesia, rigidity, and tremor) [5]. Point and period prevalence estimates of DLB range from 0.02 to 63.5 per 1000 persons [6], with considerable variability likely related to underdiagnosis of the disorder [7, 8].
As PD and DLB are diagnosed after there has been significant degeneration (e.g. PD results in 50–70% loss of neurons in the substantia nigra before clinical diagnosis occurs [9]), it is crucial to develop diagnostic tools and interventions that can be used early in the disease course. Idiopathic REM sleep behavior disorder (iRBD) is one of the earliest and most specific prodromal indicators of Lewy body diseases [10,11,12] with one in three idiopathic iRBD patients converting to a Lewy body disease within 5 years, 82.4% at 10.5 years, and 96.6% at 14 years. Of the converters, 44% will convert to PD and 25% to dementia with Lewy bodies [13]. Therefore, iRBD cohorts provide a unique opportunity to identify early disease mechanisms and develop neuroprotective interventions for Lewy body diseases.
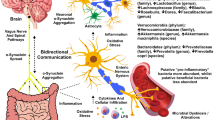
The phenotypic presentation of Lewy body diseases largely depends on the location of the pathological initiation and progression [14,15,16]. While the clinical phenotypes of Lewy body diseases differ, the aggregation of alpha-synuclein (α-syn) is a defining feature [17, 18]. Through careful assessment of the propagation pattern of PD pathology, Braak and colleagues proposed that PD may be caused by an intestinal pathogen [19]. Several reviews have discussed the evidence to suggest that the gut plays an important role in the initiation or progression of pathological processes [20,21,22,23,24], though many questions remain regarding the mechanisms by which gut dysfunction leads to the development of Lewy body diseases. In the current review, we discuss two main hypotheses by which gastrointestinal dysfunction contributes to pathogenesis or disease progression. The first builds on Braak’s initial theory and posits that Lewy body diseases are caused by α-syn aggregation in the gut which travels in a prion-like fashion to the central nervous system (CNS). The second, more recent hypothesis emphasizes chronic intestinal proinflammatory processes as key mechanisms [24]. These theories are not mutually exclusive as there are likely interactions between immune responses and α-syn at all stages of the disease process. How early in the disease course a proinflammatory response versus α-syn aggregation occurs is an area of controversy and active investigation. Notably, both of these hypotheses emphasize dysbiosis (an imbalance in the microbiota) and intestinal permeability (translocation of lumen products across the gut wall, also referred to as “leaky gut”) as key mechanisms in the initiation and progression of pathophysiology in Lewy body diseases.
Gastrointestinal dysfunction
Historically, PD research had focused on the motor symptoms of the disorder, though it is increasingly appreciated that gastrointestinal dysfunction, most commonly constipation, is one of the earliest prodromal symptoms of PD [25]. PD patients are three times more likely to experience constipation [26]. Constipation can precede motor deficits of PD by decades [27, 28] and is associated with worse outcomes, including earlier onset of dementia [29, 30]. In addition, patients may also experience sialorrhea and dysphagia, gastroparesis, and small intestinal bacterial overgrowth (SIBO), demonstrating pan-gut involvement [31, 32]. LBD patients experience similar, if not more severe gastrointestinal symptoms, with evidence that gastric emptying is slower in DLB patients relative to PD [33], though there is currently limited examination in DLB. Of note, objective colonic dysfunction is far more prevalent than subjective constipation in PD, highlighting the need to incorporate objective assessments to detect gastrointestinal dysfunction in Lewy body diseases [34].
SIBO was historically viewed as a cause of malabsorption and required invasive aspiration to obtain cultures of jejunal aspirate to diagnose [35]. Over time, it was recognized that intestinal bacteria were the sole source of certain gases, such as hydrogen and methane, that could be detected in exhaled breath. This was leveraged to develop glucose and lactulose breath tests for SIBO [36]. Using this approach, ~ ½ of PD patients test positive for SIBO [37, 38] and the occurrence of SIBO was associated with more severe motor fluctuations [38, 39]. SIBO eradication led to a significant improvement in patients OFF time and delayed ON episodes each day, though there is a high rate of SIBO relapse at 6 months (43%) [38]. SIBO was not directly related to small bowel transit delays [40] nor associated with worse gastrointestinal symptoms, but independently predicted worse motor function [41]. Gastrointestinal disorders that exhibit increased rates of SIBO, such as ulcerative colitis, Crohn’s disease, and irritable bowel syndrome are associated with an increased risk of PD [42, 43], suggesting SIBO may increase risk of PD or may play a role in the pathogenesis of Lewy body diseases. Evaluation of SIBO has been primarily conducted in more advanced patients. Given potential improvement in motor functioning, it is important to evaluate SIBO within prodromal, early stage PD, and DLB cohorts to understand the impact across phenotypes. For a detailed review of gastrointestinal symptoms in PD, refer to [26, 27, 32].
Pathological processes
Lewy bodies and Lewy neurites are the defining neuropathological characteristics of PD and DLB [44,45,46]. Point mutations in the gene encoding α-syn (SNCA) were found to be pathogenic for familial forms of PD [47], which led to the subsequent discovery that α-syn is the principal component of Lewy bodies [17]. α-syn in its normal form is found within the presynaptic regions of neurons, either unfolded or contained in alpha-helical membrane-bound forms. Aggregation refers to the process by which α-syn becomes partially folded and aggregates to form oligomers, protofibrils, fibrils, and mature Lewy bodies [17, 48, 49]. It is unclear whether these variants of protein structure reflect distinct pathologies or a continuum of conformations reflecting the different stages of Lewy body diseases. Further, it is likely that in addition to a “triggering” event that initiates α-syn aggregation, it is likely that additional mechanisms are necessary to facilitate (allowing the disease to spread to the CNS) and aggravate the disease process (promote neurodegeneration beyond the basal ganglia) [50].
As noted, Braak and colleagues proposed that PD may be caused by an intestinal pathogen which travels through enteric neurons before entering the CNS via the vagus nerve [19]. Increasing evidence supports this hypothesis, including evidence of α-syn pathology in the intestinal wall examined both antemortem [51, 52] and postmortem [53, 54]. The α-syn deposits have been observed up to 20 years prior to a PD diagnosis [55]. Examination of colonic biopsies in iRBD cohorts has also demonstrated the presence of α-syn in these prodromal cohorts [56]. Recent work has demonstrated that gut microbes are able to promote α-syn-mediated motor deficits, brain pathology, and neuroinflammation in a mouse model of PD [57]. This evidence has led to an interest in identifying how intestinal dysbiosis and intestinal permeability, may play a mechanistic role in the initiation of α-syn aggregation at the level of the gut.
However, neuropathological studies using large cohorts have failed to find evidence of cases in which α-syn pathology is present in the peripheral nervous system in the absence of CNS pathology [58, 59]. Alternatively, intestinal inflammation may be the driver of disease pathogenesis in the periphery [24, 60]. It is well established that neuroinflammation is present in PD, though it was initially considered a response to α-syn aggregation rather than a primary mechanism of disease initiation [61]. More recently, it is hypothesized that intestinal dysbiosis and inflammation are the earliest disease processes that initiate both innate and adaptive immune system activation [60]. Specifically, a toxic trigger or changes in microbiota may contribute to dysbiosis and facilitate a proinflammatory environment. These processes increase intestinal permeability, resulting in increased levels of circulating proinflammatory cytokines, innate and adaptive immune cell activation, increased blood–brain barrier permeability, peripheral cell infiltration of the central nervous system, and neuroinflammation. While this process upregulates native α-syn expression which could potentially trigger its aggregation in the peripheral nervous system, the mechanistic emphasis is on the inflammatory processes.
Clinical phenotypes
Clinically, the distinction between PD and DLB is made based on the temporal onset of motor versus cognitive symptoms (e.g., motor symptoms occur first = PD; cognitive symptoms occur first = DLB). However, both PD (including PDD) and DLB patients can exhibit similar clinical symptoms as the disease progresses. For instance, while hallucinations and RBD are diagnostic criteria for DLB, these same symptoms are present in 40–50% [62, 63] and 39–50% [64, 65] of PD patients, respectively. Additionally, cognitive impairment is common in PD, with up to 83% of patients exhibiting dementia after 20 years [66]. It is well established that the motor and cognitive symptoms of the disorders are closely linked to α-syn aggregation suggesting that disease mechanisms are the same across the disorders [1, 67], with differences in the clinical presentation related to the anatomical location of disease initiation and progression [16, 68].
Revisions of Braak’s original pathological staging have addressed some of the heterogeneity in pathological progression within the brain that leads to PDD versus DLB [69]. Given the evidence for a possible gut origin in some patients, a recent revision to Braak’s pathological staging has proposed two distinct paths of pathological initiation and progression. Either α-syn aggregation originates in the CNS (brain first) or the peripheral nervous system (body first) [15, 70], with the spread of pathology in a bidirectional manner. The neural connectome thus plays a crucial role in determining how α-syn propagates through the nervous system [16]. This theory is particularly useful for understanding the heterogeneity in phenotypes across Lewy body diseases. For example, in the body-first subtype, the α-syn pathology presumably originates in the enteric or autonomic nervous system and spreads to the CNS via the vagus and sympathetic connectome. These patients develop iRBD in the prodomal phase, have more autonomic and gastrointestinal symptoms, significant hyposmia, and faster motor and non-motor symptoms progression. This clinical presentation also largely overlaps with many symptoms observed in DLB, such as iRBD, autonomic dysfunction, and more severe cognitive dysfunction. Given the common neuropathology (α-syn aggregation) across these disorders and overlap in symptoms, this highlights the need to evaluate gastrointestinal mechanisms of pathogenesis across Lewy body diseases, as disease mechanisms likely span across diagnostic categories and may better account for the heterogeneity of clinical phenotypes.
Dysbiosis
Microorganisms that live inside and on humans, referred to as microbiota, have symbiotic relationships with the human host. However, dysbiosis can lead to many disease processes, such as SIBO, Crohn’s disease, and inflammatory bowel disease [71, 72], with more recent evidence supporting the role of microbiota in neurodegenerative conditions [73]. Initial estimates suggested a ratio of 10:1 between bacteria and human cells, with more recent evidence suggesting a 1:1 ratio with approximately 3.9 × 1013 bacteria in/on the human body [74]. The sheer number of microbiota leads to complex dynamics that raises considerable challenges in evaluating the patterns of microbiota variation and the impact of dysbiosis.
The most common methodological approach includes the use of 16S rRNA sequencing of either fecal or intestinal samples. The 16S rRNA gene is conserved in all bacteria allowing for taxonomic identification. Despite over 25 studies using this approach in fecal samples in PD, there is considerable variability among findings and over 100 differently abundant taxa between PD patients and controls [75, 76]. A meta-analysis and pooled re-analysis of ten available studies that used 16S rRNA-gene amplicon sequencing indicated that the gut microbiome significantly differs between PD patients and controls, though the interstudy variability was the main factor driving bacterial community structures and only 1% of the total variance was accounted for by group status [76]. When inconsistencies across studies (country of origin, sampling protocols, sample storage, DNA extractions, and sequencing strategies) were accounted for, PD patients exhibited a reduction of the genera Roseburia, Fusicategnibacter, Blautia, Anaerostipes (Lachnospiraceae family), and Faecalibacterium (Ruminococcaceae family) in addition to enrichment of the Lactobacillus, Akkermansia, and Bifidobacterium genera. No significant differences in enterotypes were observed. Similar reports were observed in a recent review [75]. Increased Akkermansia (14 out of 30 studies), followed by Lactobacillus (7 out of 30 studies), and Bifidobacterium (10 out of 30 studies) whereas decreased abundance of Roseburia (8 out of 30), Faecalibacterium (8 out of 30 studies), and Blautia (7 out of ten studies) were most consistently observed.
There are numerous reasons for variability across studies, including collection and assaying methods, with poor control of confounding factors, such as antibiotic use in early life or previous gastrointestinal infections [77]. There is an enormous amount of individual and geographic variability that raises challenges when attempting to quantify group differences in the microbiome [78, 79]. Finally, the majority of prior evaluations of the microbiome quantify microbial taxa and metabolic pathways as fractions of the sample sequence generated by each analysis, rather than disease-associated imbalances that may occur [77, 80].
Increases in rare species of microbiota
While the prior studies often detect changes in the more prevalent microbiota species, it can be challenging to detect rare species that, when they increase in number, can exert adverse biological effects. As part of the pooled re-analysis by Romano et al. [76] discussed above, microbial alpha-diversity and abundances of rare taxa were significantly increased in PD relative to control samples. This suggests a reduction in dominant species and an increase in rare/low abundant ones. When commensal bacteria increase in number to exert adverse biologic effects, they are known as pathobionts. For example, sulfate-reducing bacteria are rare members of the gut microbiome under normal conditions (a fraction of a percent) and help to support microbial fermentation by converting its metabolite, hydrogen, to hydrogen sulfide (H2S). However, when dysbiosis occurs, sulfate-reducing bacteria can increase in number (bloom in sulfate-reducing bacteria), becoming pathogenic as an increase can impair intestinal barrier and increase levels of potentially toxic H2S. The Desulfovibrionaceae family, the most prominent family of sulfate-reducing bacteria [81], is elevated in PD patients [82, 83] and the concentration of Desulfovibrio species correlates with the severity of PD [83]. As a consequence, PD patients may exhibit excess H2S. H2S can be beneficial as it acts as a gaseous neurotransmitter produced in small quantities by the host regulating a number of body functions including gastrointestinal, neuronal, cardiovascular, endocrine, respiratory, renal, and hepatic systems [84]. However, elevated levels of H2S produced by a bloom in sulfate-reducing bacteria can become harmful to the host and is associated with gastrointestinal disorders such as ulcerative colitis, Crohn’s disease, and irritable bowel syndrome [84,85,86]. As noted, these disorders are linked with an increased risk of PD [42, 43].
Consequences of dysbiosis
Reduction in short-chain fatty acids
Roseburia, Fusicategnibacter, Blautia, and Anaerostipes are butyrate producers, a short-chain fatty acid (SCFA). SCFAs are produced by the fermentation of dietary fiber by microbiota and are exclusively produced in the intestine. They exhibit anti-oxidant and anti-inflammatory processes and regulate the expression of tight junction proteins, which can impact intestinal barrier integrity [87]. Absolute concentrations of SCFAs are significantly reduced in human PD fecal samples, including butyrate, acetate, and propionate [88]. A decrease in fecal levels of butyrate has been associated with intestinal inflammation in PD patients [89]. Examination of plasma SCFAs suggested opposite effects, with increased SCFAs in PD relative to a matched cohort [90]. Taken together, the observed reductions in Roseburia, Fusicategnibacter, Blautia, and Anaerostipes may contribute to proinflammatory shifts in microbiota composition in PD.
Increase in self-peptides
Lactobacillus, Akkermansia, and Bifidobacterium genera are typically considered to be beneficial bacteria, suggesting either a role in PD or simply that these bacteria are well adapted to thrive in the context of dysbiosis. However, an enrichment of Akkermansia has been observed in multiple sclerosis patients and is associated with a proinflammatory response [91,92,93]. Recent evidence suggests that peptides produced by Akkermansia may interact with autoreactive T cells in multiple sclerosis [94]. Specifically, the human leukocyte antigen (HLA)-DR15 haplotype has been associated with the pathogenesis of multiple sclerosis [95] via the abundant production of HLA-DR-derived self-peptides. Akkermansia may mimic these peptides ultimately sensitizing activated CD4 T cells in the periphery which leads to pathogenic autoreactive T cells in the brain [94]. Given the increases in Akkermansia, it is possible that similar mechanisms may occur in Lewy body diseases.
Increase in lipopolysaccharide
The observed increase in the density of Gram-negative bacterial strains (including Akkermansia and Desulfovibrio) in PD fecal samples [96, 97] corresponds to an increase in endotoxin lipopolysaccharide (LPS) content, as LPS is a cell wall component of Gram-negative bacteria [98]. LPS is a known endotoxin that can lead to increased intestinal permeability [99,100,101]. Plasma and serum lipopolysaccharide-binding protein is reduced in PD [102,103,104]. Lipopolysaccharide-binding protein increases when LPS is elevated acutely [105, 106], but decreased when there has been chronic exposure, suggesting PD patients experience prolonged elevated LPS. LPS in this context may directly contribute to the initiation of α-synuclein at the level of the gut [107] as it has demonstrated the ability to promote α-synuclein aggregation via the formation of intermediate nucleating species [108,109,110]. Alternately, LPS may lead to systemic inflammatory processes in the context of increased intestinal permeability that contribute to disease pathogenesis, as it is a potent stimulator of microglial activation and has been associated with degeneration of the neurons in the SNc and motor deficits [111]. Specifically, LPS activates toll-like receptors initiating an innate immune response stimulating the production of inflammatory cytokines (such as IL-1, TNF-α, and IL-6) and reactive oxygen species [112, 113].
Increase in H2S
Several pathological processes may occur when Desulfovibrio colonizes the intestine, including increased generation of H2S in amounts that exceed detoxification capacity, increased inflammatory responses, and increased intestinal permeability (leaky gut) [114, 115]. Desulfovibrio also has the ability to produce magnetite (Fe3O4) [116], which may accelerate α-syn aggregation [117]. A recent model has proposed that the increase in H2S concentrations causes leaky membrane resulting in the release of cytochrome c from the mitochondria and an increase in cytosolic iron levels. This, in combination with magnetite nanoparticles originating from Desulfovibrio species may result in α-syn aggregation via production of reactive oxygen species [118].
Increase in curli protein
Preclinical studies have suggested that the amyloid protein, curli, produced by Escherichia coli (E. coli), may play an important role in the initiation of α-syn initiation. Specifically, in the context of increased proteobacteria (Gram-negative bacteria), there will be more E. coli, a bacterium that secretes curli. Rats exposed to curli-producing bacteria (E. coli) displayed increased neuronal α-syn deposition in both the gut and brain as well as enhanced microgliosis and astrogliosis. Together, this suggests that curli, a gut bacterial amyloid protein, may trigger the initiation of α-syn aggregation [119]. This role of curli was supported by the finding that curli expression was required for E. coli to exacerbate α-syn-induced behavioral deficits. In addition, oral treatment of mice with a gut-restricted inhibitor of amyloid prevented curli-mediated acceleration of PD-like pathology and behavioral abnormalities [120]. A separate line of work showed that LPS may contribute to the pathophysiology by accelerating the synthesis of curli fibrils [121].
Increased intestinal permeability
The intestinal barrier, which consists of physical (mucus, tight junction proteins), and chemical (anti-microbial peptides) components, shields the intestine from the contents of the lumen. Barrier integrity is reliant on the tight junctions, which include claudins, occludin, zonula occludens, adheren junctions, desmosomes, and gap junctions [122]. Damage to the barrier can allow α-syn, microbes, environmental toxins, or other luminal contents to gain access to the submucosal neuronal tissue or systemic circulation. Several studies have demonstrated that PD patients exhibit intestinal barrier dysfunction [89, 123,124,125], referred to as ‘leaky gut’ that is associated with microbial translocation across the intestinal mucosa. Factors resulting from dysbiosis, such as increases in LPS [99,100,101] and Desulfovibrio spp. [126] also lead to leaky gut. Recent work has demonstrated that Desulfovibrio spp. induced intestinal permeability via the snail pathway [126]. Snail is a transcription factor associated with increased intestinal permeability [127,128,129] via negatively regulating tight junctions [130, 131].
Leaky gut may contribute to the entry of known modulators of α-syn aggregation, such as curli, H2S, and LPS, into the systemic circulation and then beyond, to end organs such as the brain. For example, increased intestinal permeability and E. coli staining correlated with α-syn staining, supporting the contribution of leaky gut and this bacteria to α-syn aggregation [123]. Additional evidence has also demonstrated associations between intestinal permeability and pathological α-syn aggregation [132].
Inflammation
As we discussed above, many of the observations thus far suggest the observed patterns of altered microbiota composition facilitate a proinflammatory shift in PD, including a reduction in SCFAs and an increase in immune system activating peptides. LPS activates toll-like receptors that initiate an innate immune response and the production of inflammatory cytokines. The production of H2S functions as an endogenous regulator of the immune system [133] and thus an increase in H2S secondary to a bloom in sulfate-reducing bacteria could contribute to a proinflammatory response. Aspects of these processes can occur with intact intestinal barrier, though an increase in intestinal permeability facilitates the release of lumen products contributing to a systemic inflammatory response driven by the innate and adaptive immune systems [134, 135], which may play a key role in sustaining and exacerbating α-syn aggregation [24].
Pathways to the brain
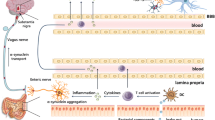
There are multiple paths for bidirectional gut–brain communications (Fig. 1), involving neural pathways as well as immune and endocrine mechanisms [136]. The vagal and spinal sensory neurons receive signals within the lamina propria and are directly connected to the brainstem and spinal cord, respectively. Additional pathways connect the enteric nervous system with CNS. For example, signaling from intrinsic primary afferent neurons are conveyed by intestinofugal nerves to the spinal cord via sympathetic ganglia.
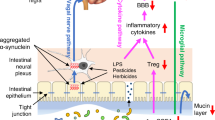
Proposed mechanisms of early disease processes in Lewy body diseases. Left panel: healthy gut. In a healthy gut, the commensal microbes, epithelium and immune cells maintain an equilibrium. Right panel: dysbiosis includes decrease in Roseburia, Fusicategnibacter, Blautia, and Anaerostipes, leading to a reduction in the production of short-chain fatty acids (SCFAs). An increase in Lactobacillus, Akkermansia, and Bifidobacterium is considered beneficial bacteria, though Akkermansia may produce peptides that mimic self-peptides that sensitize T cells. An increase in Gram-negative bacteria strains increase lipopolysaccharide (LPS), an endotoxin that can damage the intestinal barrier and initiate inflammatory processes. A bloom in sulfate-reducing bacteria (SRB) increases hydrogen sulfide (H2S) production, which may facilitate α-syn aggregation, increase intestinal permeability, and initiate an inflammatory response. E. coli increases levels of curli protein, which has been implicated as a modulator of α-syn aggregation. α-Syn may aggregate locally via these mechanisms within the enteroendocrine cells and travel via the vagus nerve to the brainstem. Additionally, the activation of both innate and adaptive inflammatory responses increases the circulating proinflammatory cytokines, reactive oxygen species (ROC), monocytes, macrophages, and T cells. The release of lumen products and triggering of inflammatory processes can damage the blood–brain barrier (BBB), facilitating infiltration of the pathogenic processes into the central nervous system (CNS)
Braak’s initial theory posited that α-syn pathology has the ability to spread from the gastrointestinal tract to the brain via the vagus nerve [19]. Epidemiological evidence has demonstrated that a full truncal vagotomy decreases the risk of PD [137, 138]. Animal models have also shown that recombinant α-syn injected into the intestinal wall could be transported via the vagal nerve to reach the dorsal motor nucleus in the brainstem [139]. Additionally, the injection of preformed α-syn fibrils into the muscle layers of the pylorus and duodenum, which is densely innervated by the vagus nerve, leads to their propagation to the CNS following a path similar to that characterized by Braak [140]. The potential cellular mechanisms of α-syn propagation have been reviewed elsewhere [141], with increasing evidence supporting the notion that it propagates in a prion-like fashion [142], which would provide a mechanism by which α-syn aggregation in the gut could propagate to the CNS.
There are multiple potential sites within these pathways in which α-syn aggregation could initiate, including the enteric nervous system [143]. However, recent efforts have proposed that gut enteroendocrine cells may serve as sites for the initial emergence of pathogenic α-syn [144]. Enteroendocrine cells are chemosensory cells dispersed throughout the mucosal lining of the intestine and their apical surface is open to the lumen of the intestine. Historically, they were viewed as hormone-producing cells, but subsequent observations have demonstrated that they are electrically excitable, possess many neuronal features, and can communicate directly with the nervous system [145, 146]. Additionally, α-syn is expressed by the enteroendocrine cells, both in the small and large intestine, highlighting that they may serve as loci for the initial pathological α-syn aggregation [144].
This work largely supports the notion that it is physiologically feasible for α-syn aggregation to begin in the gut and travel to the CNS. However, as noted, there has been limited evidence using immunohistochemical staining of cases in which α-syn pathology was observed in the gut in the absence of CNS pathology [58, 59]. Alternatively, dysbiosis and intestinal permeability may lead to an increase in the entry of lumen products (including LPS, H2S, and curli proteins) as well as cytokines and immune cells into systemic circulation [147, 148]. Rather than initiating α-syn aggregation in the periphery, these processes may contribute to disease mechanisms by impacting the blood–brain barrier (BBB) and facilitating a prolonged immune response. This would facilitate peripheral cell infiltration across the BBB which contributes to neuroinflammation.
Increased blood–brain barrier permeability
The BBB is a physiological barrier that protects the brain from unwanted molecules in the blood. Similar to the intestinal barrier, the BBB leakage is driven by damage to endothelial tight junctions, which include occludin, claudins, zonula occludens, and adheren junctions, though the BBB has added complexity given the sensitivity of the brain to toxins and pathogens [149]. In addition to changes in tight junctions, BBB permeability can also be altered by damage to endothelial cells or astrocytes as well as degradation of extracellular matrix components. Disruption of the BBB likely plays an important role across neurodegenerative conditions [150], with emerging evidence that permeability of the BBB is increased in PD [151, 152]. Specifically, an increased ratio of cerebrospinal fluid albumin to serum albumin was observed in PD [153]. Thinning and fragmentation of tight junction proteins was observed in postmortem immunofluorescence staining evaluations of PD cases [154]. PET imaging has also indicated reduced P-glycopreotein 1 activity, suggestive of BBB dysfunction, in the midbrain in PD patients [155].
The gut microbiota can regulate the BBB via several potential mechanisms [156], including the direct impact of intestinal microbial metabolites such as LPS [157, 158], immune and endocrine responses [159], or upregulation of α-syn [160]. In terms of the consequences of dysbiosis discussed above, there are several potential mechanisms by which dysbiosis and intestinal permeability may lead to increased BBB in PD. Specifically, LPS has been used to study the impact of systemic inflammation on BBB function, indicating potential BBB dysfunction in 60% of studies [161], with BBB change observed more consistently in mice versus rats. These studies, however, typically use septic doses of LPS, which limits the generalizability to Lewy body diseases. While α-syn in its non-pathologic form can travel bi-directionally across the BBB, transportation is enhanced in the presence of LPS [162, 163], suggesting potential upregulation of α-syn in the brain. Additionally, increased desulfovibrio spp. can induce leaky gut via the activation of the snail pathway. This same pathway has also been found to disrupt BBB by impacting integrity of tight junctions [164].
Systemic inflammation induced by dysbiosis and intestinal permeability may also increase BBB permeability. For example, systemic inflammation induces migration of microglia to the cerebral vasculature to maintain BBB integrity by expressing tight-junction proteins and connecting with the endothelial cells. However, during sustained inflammation, microglia phagocytose the astrocytic end-feet of the BBB, impairing the BBB function [165]. Given the consistent finding of activated microglia in the postmortem brains of PD patients [166, 167], this would suggest that systemic inflammation may be driving these processes. Herein we focus our review on the potential mechanisms driven by gastrointestinal factors we have identified, however, for detailed review of immune dysfunction and neuroinflammation, please refer to [24, 60].
REM sleep behavior disorder
As noted above, iRBD is a prodrome of Lewy body diseases, with up to 96% of iRBD patients converting to a synucleinopathy, the majority would be diagnosed with either PD or dementia with Lewy bodies. In the “body first” Lewy body disease phenotype mentioned above, the pathology would theoretically progress from the dorsal motor nerve of the vagus to first impact the locus coeruleus [16], which is inferior to the substantia nigra and associated with the development of iRBD [168, 169]. As the disease progresses to the substantia nigra or broader regions, individuals may begin to exhibit symptoms consistent with either PD or LBD.
Self-reported gastrointestinal symptoms are elevated in iRBD cohorts relative to healthy controls, with significantly greater endorsement of constipation and straining for defecation [170, 171]. Total gastrointestinal transit time, colonic volume, and 3D-Transit colonic transit time were significantly increased in an iRBD cohort relative to controls, though not to the extent observed in medicated PD patients [172]. iRBD patients exhibit a microbiome similar to that seen in patients with PD [173] and colonic biopsies in iRBD cohorts showed the presence of α-syn [56], though SCFA were not reduced in iRBD as in PD [174].
Additionally, interleukin-10 levels are upregulated in iRBD relative to controls [175] in addition to tumor necrosis factor-α levels, which were found to predict phenoconversion to an α-synucleinopathy [176]. Increased microglial activation was detected by PET in the substantia nigra in addition to reduced dopaminergic function in the putamen [177]. iRBD patients’ blood monocytic cells showed increased expression of CD11b and decreased expression of HLA-DR. iRBD patients had increased classical monocytes and mature natural killer cells. The levels of expression of toll-like receptor 4 on blood monocytes was correlated with the nigral immune activation measured with PET [178].
Taken together, early gastrointestinal symptoms, such as constipation and dysbiosis, as well as systemic inflammation, are present in the prodromal stages of LBDs, however, there is a great need to understand the earliest changes, identify potential mechanisms, and whether these predict phenoconversion.
Early detection of pathological changes and targets for intervention
There are currently no disease-modifying treatments for Lewy body diseases, largely due to the lack of mechanistic understanding of disease pathogenesis and the challenges associated with targeting α-syn aggregates [179]. While there are numerous research questions to answer regarding the mechanisms of gastrointestinal dysfunction and the pathogenesis of Lewy body disease, there are several potential treatments that are readily available spanning antibiotics, probiotics, and fecal microbiota transplantation [180]. For example, Rifaximin is a broad-range, gastrointestinal-specific antibiotic used to treat SIBO, which improves motor symptoms [38]. Relatedly, numerous studies have evaluated the impact of probiotics on constipation symptoms in PD [181], with evidence of a reduced MDS-UPDRS total score [182]. Based on the emerging research presented herein, targeting specific bacteria offer intriguing possibilities.
Additionally, a major limitation of current PD medications is that they lose efficacy over time, with recent evidence that gut microbiota has been found to moderate the metabolism of Parkinson’s medication [183,184,185]. While not disease modifying, targeting these bacteria may significantly improve efficacy of PD medications [186]. Evolving evidence support PD and Lewy body diseases more generally as both central and peripheral diseases. Targeting the pathophysiology taking place in the gut offers exciting opportunities for early intervention. Could targeting dysbiosis or intestinal permeability prior to the development of α-syn aggregation be an effective way of forestalling Lewy body diseases before the disease is clinically diagnosable?
References
Jellinger KA, Korczyn AD (2018) Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med 16:34
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601
Pfeiffer RF (2016) Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 22:S119–S122
Tysnes O-B, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transm 124:901–905
McKeith IG, Boeve BF, Dickson DW et al (2017) Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89:88–100
Hogan DB, Fiest KM, Roberts JI et al (2016) The prevalence and incidence of dementia with Lewy bodies: a systematic review. Can J Neurol Sci 43:S83–S95
Mok W, Chow TW, Zheng L et al (2004) Clinicopathological concordance of dementia diagnoses by community versus tertiary care clinicians. Am J Alzheimer’s Dis Other Dementias 19:161–165
Jones SAV, O’brien JT (2014) The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med 44:673–683
Brooks DJ (1998) The early diagnosis of Parkinson’s disease. Ann Neurol 44:S10–S18
Iranzo A, Tolosa E, Gelpi E et al (2013) Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 12:443–453
Postuma RB, Iranzo A, Hu M et al (2019) Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 142:744–759
Postuma RB, Berg D (2019) Prodromal Parkinson’s disease: the decade past, the decade to come. Mov Disord 34:665–675
Galbiati A, Verga L, Giora E et al (2019) The risk of neurodegeneration in REM sleep behavior disorder: a systematic review and meta-analysis of longitudinal studies. Sleep Med Rev 43:37–46
Just MK, Gram H, Theologidis V et al (2022) Alpha-synuclein strain variability in body-first and brain-first synucleinopathies. Front Aging Neurosci 14:907293
Horsager J, Andersen KB, Knudsen K et al (2020) Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain 143:3077–3088
Borghammer P (2021) The α-synuclein origin and connectome model (SOC Model) of Parkinson’s disease: explaining motor asymmetry, non-motor phenotypes, and cognitive decline. J Parkinsons Dis 11:455–474
Spillantini MG, Schmidt ML, Lee VM-Y et al (1997) α-synuclein in Lewy bodies. Nature 388:839–840
Baba M, Nakajo S, Tu P-H et al (1998) Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 152:879
Braak H, Rüb U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110:517–536
Lionnet A, Leclair-Visonneau L, Neunlist M et al (2018) Does Parkinson’s disease start in the gut? Acta Neuropathol 135:1–12
Klingelhoefer L, Reichmann H (2015) Pathogenesis of Parkinson disease—the gut–brain axis and environmental factors. Nat Rev Neurol 11:625–636
Breen DP, Halliday GM, Lang AE (2019) Gut–brain axis and the spread of α-synuclein pathology: vagal highway or dead end? Mov Disord 34:307–316
Baizabal-Carvallo JF, Alonso-Juarez M (2020) The link between gut dysbiosis and neuroinflammation in Parkinson’s disease. Neuroscience 432:160–173
Houser MC, Tansey MG (2017) The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Park Dis 3:1–9
Cersosimo MG, Raina GB, Pecci C et al (2013) Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol 260:1332–1338
Lubomski M, Davis RL, Sue CM (2020) Gastrointestinal dysfunction in Parkinson’s disease. J Neurol 267:1377–1388
Fasano A, Visanji NP, Liu LWC et al (2015) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 14:625–639
Stocchi F, Torti M (2017) Constipation in Parkinson’s disease. Int Rev Neurobiol 134:811–826
Camacho M, Macleod AD, Maple-Grødem J et al (2021) Early constipation predicts faster dementia onset in Parkinson’s disease. NPJ Park Dis 7:1–7
Leta V, Urso D, Batzu L et al (2021) Constipation is associated with development of cognitive Impairment in de novo Parkinson’s disease: a longitudinal analysis of two international cohorts. J Parkinsons Dis 11:1–11
Mukherjee A, Biswas A, Das SK (2016) Gut dysfunction in Parkinson’s disease. World J Gastroenterol 22:5742
Warnecke T, Schäfer KH, Claus I et al (2022) Gastrointestinal involvement in Parkinson’s disease: pathophysiology, diagnosis, and management. NPJ Park Dis 8:1–13
Sakakibara R, Masuda M, Tateno F et al (2019) Gastrointestinal function in dementia with Lewy bodies: a comparison with Parkinson disease. Clin Auton Res 29:633–638
Knudsen K, Fedorova TD, Bekker AC et al (2017) Objective colonic dysfunction is far more prevalent than subjective constipation in Parkinson’s disease: a colon transit and volume study. J Parkinsons Dis 7:359–367
Quigley EMM (2019) The spectrum of small intestinal bacterial overgrowth (SIBO). Curr Gastroenterol Rep 21:1–7
Rezaie A, Buresi M, Lembo A et al (2017) Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol 112:775
Li X, Feng X, Jiang Z, Jiang Z (2021) Association of small intestinal bacterial overgrowth with Parkinson’s disease: a systematic review and meta-analysis. Gut Pathog 13:1–10
Fasano A, Bove F, Gabrielli M et al (2013) The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord 28:1241–1249
Niu X-L, Liu L, Song Z-X et al (2016) Prevalence of small intestinal bacterial overgrowth in Chinese patients with Parkinson’s disease. J Neural Transm 123:1381–1386
Su A, Gandhy R, Barlow C, Triadafilopoulos G (2017) Utility of the wireless motility capsule and lactulose breath testing in the evaluation of patients with Parkinson’s disease who present with functional gastrointestinal symptoms. BMJ Open Gastroenterol 4:e000132
Tan AH, Mahadeva S, Thalha AM et al (2014) Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat Disord 20:535–540
Zhang X, Svn Z, Liv M et al (2021) Association between irritable bowel syndrome and risk of Parkinson’s disease: a systematic review and meta-analysis. Front Neurol 12:2
Zhu F, Li C, Gong J et al (2019) The risk of Parkinson’s disease in inflammatory bowel disease: a systematic review and meta-analysis. Dig Liver Dis 51:38–42
Okazaki H, Lipkin LE, Aronson SM (1961) Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion. J Neuropathol Exp Neurol 20:237–244
Kosaka K, Oyanagi S, Matsushita M, Hori A (1976) Presenile dementia with Alzheimer-, pick-and Lewy-body changes. Acta Neuropathol 36:221–233
Ince PG, Perry EK, Morris CM (1998) Dementia with lewy bodies. a distinct non-alzheimer dementia syndrome? Brain Pathol 8:299–324
Polymeropoulos MH, Lavedan C, Leroy E et al (1997) Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047
Iwatsubo T, Yamaguchi H, Fujimuro M et al (1996) Purification and characterization of Lewy bodies from the brains of patients with diffuse Lewy body disease. Am J Pathol 148:1517
Roberts RF, Wade-Martins R, Alegre-Abarrategui J (2015) Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain 138:1642–1657
Johnson ME, Stecher B, Labrie V et al (2019) Triggers, facilitators, and aggravators: redefining Parkinson’s disease pathogenesis. Trends Neurosci 42:4–13
Shannon KM, Keshavarzian A, Dodiya HB et al (2012) Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord 27:716–719
Hilton D, Stephens M, Kirk L et al (2014) Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol 127:235–241
Wakabayashi K, Takahashi H, Takeda S et al (1988) Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76:217–221
Braak H, de Vos RAI, Bohl J, Del Tredici K (2006) Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396:67–72
Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P (2016) Pathological α-synuclein in gastrointestinal tissues from prodromal P arkinson disease patients. Ann Neurol 79:940–949
Sprenger FS, Stefanova N, Gelpi E et al (2015) Enteric nervous system α-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology 85:1761–1768
Sampson TR, Debelius JW, Thron T et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167:1469–1480
Beach TG, Adler CH, Sue LI et al (2010) Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119:689–702
Beach TG, Adler CH, Sue LI et al (2021) Vagus nerve and stomach synucleinopathy in Parkinson’s disease, incidental Lewy body disease, and normal elderly subjects: evidence against the “body-first” hypothesis. J Parkinsons Dis 11:1–11
Tansey MG, Wallings RL, Houser MC et al (2022) Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol 2:1–17
Harms AS, Ferreira SA, Romero-Ramos M (2021) Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol 141:527–545
Williams DR, Lees AJ (2005) Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol 4:605–610
Fénelon G, Mahieux F, Huon R, Ziégler M (2000) Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 123:733–745
Zhang X, Sun X, Wang J et al (2017) Prevalence of rapid eye movement sleep behavior disorder (RBD) in Parkinson’s disease: a meta and meta-regression analysis. Neurol Sci 38:163–170
Chahine LM, Amara AW, Videnovic A (2017) A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev 35:33–50
Hely MA, Reid WGJ, Adena MA et al (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 23:837–844
Walker L, Stefanis L, Attems J (2019) Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies–current issues and future directions. J Neurochem 150:467–474
Aarsland D, Ballard CG, Halliday G (2004) Are Parkinson’s disease with dementia and dementia with Lewy bodies the same entity? J Geriatr Psychiatry Neurol 17:137–145
Beach TG, Adler CH, Lue L et al (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117:613–634
Borghammer P (2018) How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord 33:48–57
Baker PI, Love DR, Ferguson LR (2009) Role of gut microbiota in Crohn’s disease. Expert Rev Gastroenterol Hepatol 3:535–546
Nishida A, Inoue R, Inatomi O et al (2018) Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 11:1–10
Marizzoni M, Provasi S, Cattaneo A, Frisoni GB (2017) Microbiota and neurodegenerative diseases. Curr Opin Neurol 30:630–638
Sender R, Fuchs S, Milo R (2016) Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164:337–340
Tan AH, Lim SY, Lang AE (2022) The microbiome–gut–brain axis in Parkinson disease—from basic research to the clinic. Nat Rev Neurol 18:1–20
Romano S, Savva GM, Bedarf JR et al (2021) Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Park Dis 7:1–13
Haikal C, Chen Q-Q, Li J-Y (2019) Microbiome changes: an indicator of Parkinson’s disease? Transl Neurodegener 8:1–9
Nishiwaki H, Ito M, Ishida T et al (2020) Meta-analysis of gut dysbiosis in Parkinson’s disease. Mov Disord 35:1626–1635
Arumugam M, Raes J, Pelletier E et al (2011) Enterotypes of the human gut microbiome. Nature 473:174–180
Vandeputte D, Kathagen G, D’hoe K et al (2017) Quantitative microbiome profiling links gut community variation to microbial load. Nature 551:507–511
Loubinoux J, Bronowicki J-P, Pereira IAC et al (2002) Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol Ecol 40:107–112
Lin A, Zheng W, He Y et al (2018) Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord 53:82–88
Murros KE, Huynh VA, Takala TM, Saris PEJ (2021) Desulfovibrio bacteria are associated with Parkinson’s disease. Front Cell Infect Microbiol 11:652617
Singh SB, Lin HC (2015) Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms 3:866–889
Barton LL, Ritz NL, Fauque GD, Lin HC (2017) Sulfur cycling and the intestinal microbiome. Dig Dis Sci 62:2241–2257
Villumsen M, Aznar S, Pakkenberg B et al (2019) Inflammatory bowel disease increases the risk of Parkinson’s disease: a Danish nationwide cohort study 1977–2014. Gut 68:18–24
Dalile B, Van Oudenhove L, Vervliet B, Verbeke K (2019) The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol 16:461–478
Unger MM, Spiegel J, Dillmann K-U et al (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72
Schwiertz A, Spiegel J, Dillmann U et al (2018) Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord 50:104–107
Shin C, Lim Y, Lim H, Ahn T (2020) Plasma short-chain fatty acids in patients with Parkinson’s disease. Mov Disord 35:1021–1027
Jangi S, Gandhi R, Cox LM et al (2016) Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 7:1–11
Berer K, Gerdes LA, Cekanaviciute E et al (2017) Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci 114:10719–10724
Cekanaviciute E, Yoo BB, Runia TF et al (2017) Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci 114:10713–10718
Wang J, Jelcic I, Mühlenbruch L et al (2020) HLA-DR15 molecules jointly shape an autoreactive T cell repertoire in multiple sclerosis. Cell 183:1264–1281
Martin R, Sospedra M, Eiermann T, Olsson T (2021) Multiple sclerosis: doubling down on MHC. Trends Genet 37:784–797
Keshavarzian A, Green SJ, Engen PA et al (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30:1351–1360
Scheperjans F, Aho V, Pereira PAB et al (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30:350–358
Raetz CRH, Whitfield C (2002) Lipopolysaccharide endotoxins. Annu Rev Biochem 71:635
Nighot M, Al-Sadi R, Guo S et al (2017) Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by toll-like receptor 4/myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression. Am J Pathol 187:2698–2710
Guo S, Al-Sadi R, Said HM, Ma TY (2013) Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol 182:375–387
Guo S, Nighot M, Al-Sadi R et al (2015) Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. J Immunol 195:4999–5010
Hasegawa S, Goto S, Tsuji H et al (2015) Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS ONE 10:e0142164
Pal GD, Shaikh M, Forsyth CB et al (2015) Abnormal lipopolysaccharide binding protein as marker of gastrointestinal inflammation in Parkinson disease. Front Neurosci 9:306
Chen S-J, Chi Y-C, Ho C-H et al (2021) Plasma lipopolysaccharide-binding protein reflects risk and progression of Parkinson’s disease. J Parkinsons Dis 11:1129–1139
Schumann RR (2011) Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem Soc Trans 39:989–993
Ramadori G, Zum Buschenfelde K-HM, Tobias PS et al (1990) Biosynthesis of lipopolysaccharide-binding protein in rabbit hepatocytes. Pathobiology 58:89–94
Bhattacharyya D, Bhunia A (2021) Gut–brain axis in Parkinson’s disease etiology: the role of lipopolysaccharide. Chem Phys Lipids 235:105029
Bhattacharyya D, Mohite GM, Krishnamoorthy J et al (2019) Lipopolysaccharide from gut microbiota modulates α-synuclein aggregation and alters its biological function. ACS Chem Neurosci 10:2229–2236
Bhattacharyya D, Bhunia A (2020) Gut–Brain axis in Parkinson’s disease etiology: the role of lipopolysaccharide. Chem Phys Lipids 2:105029
Kim C, Lv G, Lee JS et al (2016) Exposure to bacterial endotoxin generates a distinct strain of α-synuclein fibril. Sci Rep 6:1–12
Deng I, Corrigan F, Zhai G et al (2020) Lipopolysaccharide animal models of Parkinson’s disease: recent progress and relevance to clinical disease. Brain Behav Immun Health 4:100060
Block ML, Zecca L, Hong J-S (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Rosadini CV, Kagan JC (2017) Early innate immune responses to bacterial LPS. Curr Opin Immunol 44:14–19
Singh SB, Coffman CN, Carroll-Portillo A et al (2021) Notch signaling pathway is activated by sulfate reducing bacteria. Front Cell Infect Microbiol 643:2
Weglarz L, Dzierzewicz Z, Skop B et al (2003) Desulfovibrio desulfuricans lipopolysaccharides induce endothelial cell IL-6 and IL-8 secretion and E-selectin and VCAM-1 expression. Cell Mol Biol Lett 8:991–1004
Pereira IAC, Ramos AR, Grein F et al (2011) A comparative genomic analysis of energy metabolism in sulfate reducing bacteria and archaea. Front Microbiol 2:69
Joshi N, Basak S, Kundu S et al (2015) Attenuation of the early events of α-synuclein aggregation: a fluorescence correlation spectroscopy and laser scanning microscopy study in the presence of surface-coated Fe3O4 nanoparticles. Langmuir 31:1469–1478
Murros KE (2022) Hydrogen sulfide produced by gut bacteria may induce Parkinson’s disease. Cells 11:978
Chen SG, Stribinskis V, Rane MJ et al (2016) Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci Rep 6:1–10
Sampson TR, Challis C, Jain N et al (2020) A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. Elife 9:e53111
Swasthi HM, Mukhopadhyay S (2017) Electrostatic lipid–protein interactions sequester the curli amyloid fold on the lipopolysaccharide membrane surface. J Biol Chem 292:19861–19872
Wells JM, Brummer RJ, Derrien M et al (2017) Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Liver Physiol 312:G171–G193
Forsyth CB, Shannon KM, Kordower JH et al (2011) Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 6:e28032
Clairembault T, Leclair-Visonneau L, Coron E et al (2015) Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun 3:1–9
Aho VTE, Houser MC, Pereira PAB et al (2021) Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol Neurodegener 16:1–14
Singh SB, Coffman CN, Varga MG et al (2022) Intestinal alkaline phosphatase prevents sulfate reducing bacteria-induced increased tight junction permeability by inhibiting snail pathway. Front Cell Infect Microbiol 627:2
Forsyth CB, Tang Y, Shaikh M et al (2011) Role of snail activation in alcohol-induced iNOS-mediated disruption of intestinal epithelial cell permeability. Alcohol Clin Exp Res 35:1635–1643
Elamin E, Masclee A, Troost F et al (2014) Activation of the epithelial-to-mesenchymal transition factor snail mediates acetaldehyde-induced intestinal epithelial barrier disruption. Alcohol Clin Exp Res 38:344–353
Liu W, Ruan T, Ji X et al (2020) The Gli1-snail axis contributes to salmonella typhimurium-induced disruption of intercellular junctions of intestinal epithelial cells. Cell Microbiol 22:e13211
Cano A, Pérez-Moreno MA, Rodrigo I et al (2000) The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83
Martínez-Estrada OM, Cullerés A, Soriano FX et al (2006) The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J 394:449–457
Kelly LP, Carvey PM, Keshavarzian A et al (2014) Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord 29:999–1009
Dilek N, Papapetropoulos A, Toliver-Kinsky T, Szabo C (2020) Hydrogen sulfide: an endogenous regulator of the immune system. Pharmacol Res 161:105119
Shannon K (2022) Gut-derived sterile inflammation and Parkinson’s disease. Front Neurol 521:2
Wang Q, Luo Y, Ray Chaudhuri K et al (2021) The role of gut dysbiosis in Parkinson’s disease: mechanistic insights and therapeutic options. Brain 144:2571–2593
Forsythe P, Kunze WA (2013) Voices from within: gut microbes and the CNS. Cell Mol life Sci 70:55–69
Svensson E, Horváth-Puhó E, Thomsen RW et al (2015) Vagotomy and subsequent risk of P arkinson’s disease. Ann Neurol 78:522–529
Liu B, Fang F, Pedersen NL et al (2017) Vagotomy and Parkinson disease: a Swedish register–based matched-cohort study. Neurology 88:1996–2002
Holmqvist S, Chutna O, Bousset L et al (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128:805–820
Kim S, Kwon S-H, Kam T-I et al (2019) Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103:627–641
Fares MB, Jagannath S, Lashuel HA (2021) Reverse engineering Lewy bodies: how far have we come and how far can we go? Nat Rev Neurosci 22:111–131
Uchihara T, Giasson BI (2016) Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 131:49–73
Lebouvier T, Chaumette T, Paillusson S et al (2009) The second brain and Parkinson’s disease. Eur J Neurosci 30:735–741
Chandra R, Hiniker A, Kuo Y-M et al (2017) α-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI insight 2:2
Bohórquez DV, Shahid RA, Erdmann A et al (2015) Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 125:782–786
Bohórquez DV, Samsa LA, Roholt A et al (2014) An enteroendocrine cell–enteric glia connection revealed by 3D electron microscopy. PLoS ONE 9:e89881
Reale M, Iarlori C, Thomas A et al (2009) Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun 23:55–63
Brodacki B, Staszewski J, Toczyłowska B et al (2008) Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFα, and INFγ concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci Lett 441:158–162
Sweeney MD, Zhao Z, Montagne A et al (2019) Blood-brain barrier: from physiology to disease and back. Physiol Rev 99:21–78
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14:133
Gray MT, Woulfe JM (2015) Striatal blood–brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab 35:747–750
Al-Bachari S, Naish JH, Parker GJ et al (2020) Blood-brain barrier leakage is increased in Parkinson’s disease. Front Physiol 2:2
Pisani V, Stefani A, Pierantozzi M et al (2012) Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson’s disease. J Neuroinflamm 9:1–4
Pienaar IS, Lee CH, Elson JL et al (2015) Deep-brain stimulation associates with improved microvascular integrity in the subthalamic nucleus in Parkinson’s disease. Neurobiol Dis 74:392–405
Kortekaas R, Leenders KL, Van Oostrom JCH et al (2005) Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57:176–179
Tang W, Zhu H, Feng Y et al (2020) The impact of gut microbiota disorders on the blood–brain barrier. Infect Drug Resist 13:3351
Banks WA, Gray AM, Erickson MA et al (2015) Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation 12:1–15
Barton SM, Janve VA, McClure R et al (2019) Lipopolysaccharide induced opening of the blood brain barrier on aging 5XFAD mouse model. J Alzheimer’s Dis 67:503–513
Banks WA, Erickson MA (2010) The blood–brain barrier and immune function and dysfunction. Neurobiol Dis 37:26–32
Elabi O, Gaceb A, Carlsson R et al (2021) Human α-synuclein overexpression in a mouse model of Parkinson’s disease leads to vascular pathology, blood brain barrier leakage and pericyte activation. Sci Rep 11:1–14
Varatharaj A, Galea I (2017) The blood–brain barrier in systemic inflammation. Brain Behav Immun 60:1–12
Sui Y-T, Bullock KM, Erickson MA et al (2014) Alpha synuclein is transported into and out of the brain by the blood–brain barrier. Peptides 62:197–202
Jangula A, Murphy EJ (2013) Lipopolysaccharide-induced blood brain barrier permeability is enhanced by alpha-synuclein expression. Neurosci Lett 551:23–27
Kim BJ, Hancock BM, Bermudez A et al (2015) Bacterial induction of Snail1 contributes to blood–brain barrier disruption. J Clin Invest 125:2473–2483
Haruwaka K, Ikegami A, Tachibana Y et al (2019) Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun 10:1–17
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285
Imamura K, Hishikawa N, Sawada M et al (2003) Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol 106:518–526
Ehrminger M, Latimier A, Pyatigorskaya N et al (2016) The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain 139:1180–1188
Knudsen K, Fedorova TD, Hansen AK et al (2018) In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol 17:618–628
Ferini-Strambi L, Oertel W, Dauvilliers Y et al (2014) Autonomic symptoms in idiopathic REM behavior disorder: a multicentre case–control study. J Neurol 261:1112–1118
Aguirre-Mardones C, Iranzo A, Vilas D et al (2015) Prevalence and timeline of nonmotor symptoms in idiopathic rapid eye movement sleep behavior disorder. J Neurol 262:1568–1578
Knudsen K, Fedorova TD, Hansen AK et al (2019) Objective intestinal function in patients with idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord 58:28–34
Heintz-Buschart A, Pandey U, Wicke T et al (2018) The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 33:88–98
Nishiwaki H, Hamaguchi T, Ito M et al (2020) Short-chain fatty acid-producing gut microbiota is decreased in Parkinson’s disease but not in rapid-eye-movement sleep behavior disorder. Msystems 5:e00797-e820
Kim R, Jun J, Kim H et al (2019) Peripheral blood inflammatory cytokines in idiopathic REM sleep behavior disorder. Mov Disord 34:1739–1744
Zhang H, Wang T, Li Y et al (2020) Plasma immune markers in an idiopathic REM sleep behavior disorder cohort. Parkinsonism Relat Disord 78:145–150
Stokholm MG, Iranzo A, Østergaard K et al (2017) Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case–control study. Lancet Neurol 16:789–796
Farmen K, Nissen SK, Stokholm MG et al (2021) Monocyte markers correlate with immune and neuronal brain changes in REM sleep behavior disorder. Proc Natl Acad Sci 118:e2020858118
Vijiaratnam N, Simuni T, Bandmann O et al (2021) Progress towards therapies for disease modification in Parkinson’s disease. Lancet Neurol 20:559–572
Dutta SK, Verma S, Jain V et al (2019) Parkinson’s disease: the emerging role of gut dysbiosis, antibiotics, probiotics, and fecal microbiota transplantation. J Neurogastroenterol Motil 25:363
Barichella M, Pacchetti C, Bolliri C et al (2016) Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology 87:1274–1280
Tamtaji OR, Taghizadeh M, Kakhaki RD et al (2019) Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr 38:1031–1035
van Kessel SP, Frye AK, El-Gendy AO et al (2019) Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun 10:1–11
Maini Rekdal V, Bess EN, Bisanz JE et al (2019) Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 364:6323
van Kessel SP, de Jong HR, Winkel SL et al (2020) Gut bacterial deamination of residual levodopa medication for Parkinson’s disease. BMC Biol 18:1–14
Lubomski M, Davis RL, Sue CM (2019) The gut microbiota: a novel therapeutic target in Parkinson’s disease? Parkinsonism Relat Disord 66:265–266
Acknowledgements
Dr. Ryman’s work is supported by the National Institutes of Health (P30 GM122734, R03 AG075408, UF1NS100598, P20 AG068077, R61 MH125126). Dr. Vakhtin’s work is supported by the National Institutes of Health (P30 GM122734). Dr. Pirio Richardson’s work is supported by the National Institutes of Health (R03 AG075408), Department of Defense, and industry (Pharma 2B, AEON, ADDEX, SCION). Dr. Lin’s work is supported by the Winkler Bacterial Overgrowth Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Drs. Ryman, Vakhtin, and Lin declare they have no competing financial interests. Dr. Pirio Richardson has received honoraria for lectures from the International Parkinson’s Disease and Movement Disorders Society and the American Academy of Neurology. Dr. Pirio Richardson serves on the Scientific Advisory Boards for private foundations including the Benign Essential Blepharospasm Research Foundation and the Dystonia Medical Research Foundation. She has received royalties from Springer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ryman, S., Vakhtin, A.A., Richardson, S.P. et al. Microbiome–gut–brain dysfunction in prodromal and symptomatic Lewy body diseases. J Neurol 270, 746–758 (2023). https://doi.org/10.1007/s00415-022-11461-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11461-9