Abstract
The assessment of postmortem degradation of skeletal muscle proteins has emerged as a novel approach to estimate the time since death in the early to mid-postmortem phase (approximately 24 h postmortem (hpm) to 120 hpm). Current protein-based methods are limited to a small number of skeletal muscle proteins, shown to undergo proteolysis after death. In this study, we investigated the usability of a target-based and unbiased system-wide protein analysis to gain further insights into systemic postmortem protein alterations and to identify additional markers for postmortem interval (PMI) delimitation. We performed proteomic profiling to globally analyze postmortem alterations of the rat and mouse skeletal muscle proteome at defined time points (0, 24, 48, 72, and 96 hpm), harnessing a mass spectrometry-based quantitative proteomics approach. Hierarchical clustering analysis for a total of 579 (rat) and 896 (mouse) quantified proteins revealed differentially expressed proteins during the investigated postmortem period. We further focused on two selected proteins (eEF1A2 and GAPDH), which were shown to consistently degrade postmortem in both rat and mouse, suggesting conserved intra- and interspecies degradation behavior, and thus preserved association with the PMI and possible transferability to humans. In turn, we validated the usefulness of these new markers by classical Western blot experiments in a rat model and in human autopsy cases. Our results demonstrate the feasibility of mass spectrometry–based analysis to discover novel protein markers for PMI estimation and show that the proteins eEF1A2 and GAPDH appear to be valuable markers for PMI estimation in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A precise estimation of the time since death or the postmortem interval (PMI) is a critical issue in forensic investigations, as it can have major implications on further case investigation or even provide decisive evidence in court [1]. Especially, biomedical analysis of the corpse can provide important, dependable data. The methodic spectrum for reliable PMI delimitation on this behalf includes the examination of postmortem body cooling, supravital functions such as electrical or pharmacological excitability of body tissues or organs, and the development of rigor mortis and hypostasis [2]. In certain cases, forensic entomology [3] or analysis of morphologic changes [4] can provide a minimum PMI. These methods, however, are limited to particular postmortem phases and often cannot be sensibly applied due to specific restrictions. Hence, additional approaches are required to complement the methodic spectrum and to provide a reliable set of tools for case specific time since death estimation.
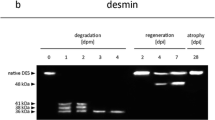
Especially, the postmortem decomposition of biomolecules has lately become of interest. In this aspect, several studies have investigated postmortem alterations of RNA [5, 6], DNA [7, 8], or proteins [9,10,11,12]. Recently, significant progress was achieved in research on postmortem degradation of skeletal muscle proteins. In standardized animal models, specific proteins were found to depict predictable degradation patterns when analyzed via SDS-PAGE and Western blotting. Postmortem changes, such as the loss of the native protein band or the occurrence of specific degradation products, were additionally shown to significantly correlate with the PMI. Among these proteins are calcineurin A, protein phosphatase 2A [13], titin, nebulin [14], desmin, troponin [15], vinculin [16], AMP-activated protein kinase, caspase 3, and glycogen synthase [17]. Furthermore, PMI-dependent desmin and cardiac troponin T degradation has been confirmed in humans [15] and was already successfully applied to obtain evidence about the succession of events in a criminal case [18]. However, to provide a broadly applicable method for time since death estimation, characterization of additional marker proteins, which undergo significant postmortem alterations, is required.
For this purpose, mass spectrometry (MS)–based proteomics approaches provide a modern technology for quantitative and qualitative assessment of proteins, enabling peptide sequencing and protein identification with high accuracy and sensitivity [28, 29]. Antibodies against GAPDH are thus commonly available, and the protein is easily detectable by the Western blot technique. In contrast to other common housekee** proteins, GAPDH is reported to be relatively stable in postmortem tissue of humans (brain tissue, 48 hpm) [30], which is in accordance with our qualitative evaluation of the results of GAPDH in H3 (PMI > 14 dpm). However, from the low number of human samples in this study, it cannot be concluded whether there is a statistical decrease of the native band in later postmortem stages, as it was the case in rat vastus lateralis muscle. The significant decomposition of GAPDH protein in rats over a PMI of 96 hpm is confirmed by the proteomic analysis and by a study reporting postmortem GAPDH decrease in rat psoas muscle [17] over a similar period of time. Despite the trend to decrease, the presence of the native band of GAPDH band at a PMI of 96 hpm and the occurring degradation products at a larger PMI in the human sample H3 indicate possible applicability as a marker in later postmortem stages. Especially, the degradation products depict a qualitative change of band pattern that, in other proteins, has been shown to be able to significantly contribute to estimate the time since death [27]. Additional experiments in wider time frames and a large sample size are required to determine whether there is a significant loss of the native band at later postmortem stages and whether the degradation products can be reproduced in human samples and may also appear in rat muscles in later stages. It has to be further evaluated whether their appearance is influenced by individual or environmental factors.
In contrast to the steadily decreasing character of GAPDH, the (early) loss of the translation factor eEF1A2 was found to be in strong correlation with the PMI, demonstrating the importance of this marker. Although eEF1A is found in high concentrations within body cells (3% of the total cellular protein) [31, 32], this is, to our knowledge, the first study on postmortem eEF1A2 decomposition. Proteomic investigations resulted in a successive decrease in the rat and mouse proteome, which was confirmed in Western blot experiments. Additionally, band intensities decreased below a 5% threshold from 48 hpm onwards and were, thus, considered a qualitative change (loss of a band). In contrast, in the human cases, we detected no obvious difference in eEF1A2 band intensity between H1 and H2, underlining the requirement of strict criteria for postmortem changes of protein band patterns for the use in forensic casework. Therefore, we generally suggest that qualitative changes (loss of a band, or appearance of a degradation product) should be preferred over quantitative measurements (e.g., merely a decrease in band intensities). However, additional experiments are required to characterize the precise timeframe in which the eEF1A2 band loss occurs in humans, and whether or how it is influenced by certain individual or environmental factors. Additional caution has to be taken when analyzing samples from cancer patients, as eEF1A2 can be overexpressed in such cases [33]. Additional research has to evaluate whether this might be an exclusion criteria for the use of this marker.
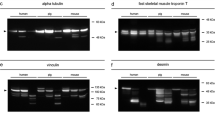
When comparing the results to already established markers (tropomyosin, desmin, and vinculin), it was detected that the loss of the native eEF1A2 band in rats depicted the strongest correlation values with the PMI. This resulting correlation could though be influenced by the specific temporal degradation characteristics of the native eEF1A2 band (initial band loss at 48 hpm, complete band loss at 72 hpm) and the selected statistic test in the present study. Comparable alterations, such as the loss of meta-vinculin, and the occurrence of the 84 kDa vinculin degradation product are, nevertheless, equally important markers for time since death estimation although resulting in lower correlation values. In the case of the 84 kDa vinculin band within the rat model, which was negative (−, no measureable band) in all 0-hpm samples and positive (+, measurable band) in all samples with larger PMI, lower correlation values resulted from a less balanced overall ± ratio. Still, in accordance with previous studies [16], all results of the animal model were found to significantly correlate with the PMI.
Changes, such as the transient appearance of the 41 kDa band in the cytoskeletal protein desmin, could not be described using Spearman correlations. However, if certain timeframes can be excluded in future application, such transiently appearing protein bands can be of potential interest. The transient character of the 41 kDa desmin degradation product in this experimental series was in accordance with earlier studies [16, 34]. Together with the loss of the native band and the appearance of the 34 kDa band (both significant correlations with the PMI), it could clearly be confirmed that desmin is a reliable marker for early phase time since death estimation. Tropomyosin results were as well in accordance with previous studies reporting postmortem stability of this protein until 240 hpm [15]. Although a real time passage (analysis of multiple sampling time points of one individual) is lacking for humans, selected cases with different PMI/ADD clearly demonstrated predictable degradation pattern of all tested standard proteins which are comparable to those in the rat model and also largely in accordance with previous studies on human muscle samples [35]. Notably, there were no tropomyosin bands detected in H3. Whether this represents a characteristic degradation pattern for larger PMIs remains to be determined.
It can be concluded that LC-MS/MS analysis of postmortem skeletal muscle samples is a valuable method for the identification of biomarkers for forensic time since death estimation by analysis of protein degradation. With the cost-effective method we presented here, we were able to characterize promising marker candidates for further research in an appropriate timeframe. By running additional routine experiments, we confirmed significant correlations of the new identified marker proteins GAPDH and eEF1A2 with the PMI in an animal model. Although the animal models used in this study are distinct to humans, we were able to demonstrate similar degradation pattern of these proteins in humans. With this, we prove their relevance in future forensic application and generally strengthen the applicability of animal models for basic research on PMI estimation as indicated by a previous work [34]. For future characterization of additional markers using proteomics, we recommend to consider additional experiments focusing as well on different species, extended time frames, or varying environmental conditions. Especially, investigations on the human postmortem proteome could, in future experiments, crucially contribute to the characterization of important additional biomarkers.
References
Madea B (2016) Methods for determining time of death. Forensic Sci Med Pathol 12:451–485. https://doi.org/10.1007/s12024-016-9776-y
Henssge C, Madea B (2004) Estimation of the time since death in the early post-mortem period. Forensic Sci Int 144:167–175. https://doi.org/10.1016/j.forsciint.2004.04.051
Amendt J, Campobasso CP, Gaudry E, Reiter C, LeBlanc HN, J. R. Hall M (2007) Best practice in forensic entomology--standards and guidelines. Int J Legal Med 121:90–104. https://doi.org/10.1007/s00414-006-0086-x
Megyesi MS, Nawrocki SP, Haskell NH (2005) Using accumulated degree-days to estimate the postmortem interval from decomposed human remains. J Forensic Sci 50:618–626
Sampaio-Silva F, Magalhães T, Carvalho F, Dinis-Oliveira RJ, Silvestre R (2013) Profiling of RNA degradation for estimation of post morterm interval. PLoS One 8:e56507. https://doi.org/10.1371/journal.pone.0056507
Bauer M, Gramlich I, Polzin S, Patzelt D (2003) Quantification of mRNA degradation as possible indicator of postmortem interval—a pilot study. Legal Med 5:220–227. https://doi.org/10.1016/j.legalmed.2003.08.001
Rhein M, Hagemeier L, Klintschar M, Muschler M, Bleich S, Frieling H (2015) DNA methylation results depend on DNA integrity-role of post mortem interval. Front Genet 6:182. https://doi.org/10.3389/fgene.2015.00182
Perry WL, Bass WM, Riggsby WS, Sirotkin K (1988) The autodegradation of deoxyribonucleic acid (DNA) in human rib bone and its relationship to the time interval since death. J Forensic Sci 33:144–153
Wehner F, Wehner H-D, Schieffer MC, Subke J (1999) Delimitation of the time of death by immunohistochemical detection of insulin in pancreatic β-cells. Forensic Sci Int 105:161–169. https://doi.org/10.1016/S0379-0738(99)00124-3
Wehner F, Wehner H-D, Schieffer MC, Subke J (2000) Delimitation of the time of death by immunohistochemical detection of thyroglobulin. Forensic Sci Int 110:199–206. https://doi.org/10.1016/S0379-0738(00)00177-8
Kumar S, Ali W, Singh US, Kumar A, Bhattacharya S, Verma AK, Rupani R (2016) Temperature-dependent postmortem changes in human cardiac troponin-T (cTnT): an approach in estimation of time since death. J Forensic Sci 61:S241–S245. https://doi.org/10.1111/1556-4029.12928
Kumar S, Ali W, Singh US, Kumar A, Bhattacharya S, Verma AK (2015) The effect of elapsed time on the cardiac troponin-T (cTnT) proteolysis in case of death due to burn: a study to evaluate the potential forensic use of cTnT to determine the postmortem interval. Sci Justice 55:189–194. https://doi.org/10.1016/j.scijus.2014.12.006
Poloz YO, O’Day DH (2009) Determining time of death: temperature-dependent postmortem changes in calcineurin a, MARCKS, CaMKII, and protein phosphatase 2A in mouse. Int J Legal Med 123:305–314. https://doi.org/10.1007/s00414-009-0343-x
Geesink GH, Koohmaraie M (1999) Postmortem proteolysis and calpain/calpastatin activity in callipyge and normal lamb biceps femoris during extended postmortem storage. J Anim Sci 77:1490–1501
Pittner S, Monticelli FC, Pfisterer A, Zissler A, Sänger AM, Stoiber W, Steinbacher P (2016) Postmortem degradation of skeletal muscle proteins: a novel approach to determine the time since death. Int J Legal Med 130:421–431. https://doi.org/10.1007/s00414-015-1210-6
Zissler A, Ehrenfellner B, Foditsch EE, Monticelli FC, Pittner S (2018) Does altered protein metabolism interfere with postmortem degradation analysis for PMI estimation? Int J Legal Med 132:1349–1356. https://doi.org/10.1007/s00414-018-1814-8
Lee D-G, Yang KE, Hwang JW, Kang HS, Lee SY, Choi S, Shin J, Jang IS, An HJ, Chung H, Jung HI, Choi JS (2016) Degradation of kidney and psoas muscle proteins as indicators of post-mortem interval in a rat model, with use of lateral flow technology. PLoS One 11:e0160557. https://doi.org/10.1371/journal.pone.0160557
Pittner S, Ehrenfellner B, Zissler A, Racher V, Trutschnig W, Bathke AC, Sänger AM, Stoiber W, Steinbacher P, Monticelli FC (2017) First application of a protein-based approach for time since death estimation. Int J Legal Med 131:479–483. https://doi.org/10.1007/s00414-016-1459-4
**e F, Liu T, Qian W-J, Petyuk VA, Smith RD (2011) Liquid chromatography-mass spectrometry-based quantitative proteomics. J Biol Chem 286:25443–25449. https://doi.org/10.1074/jbc.R110.199703
Hawkridge AM, Muddiman DC (2009) Mass spectrometry-based biomarker discovery: toward a global proteome index of individuality. Annu Rev Anal Chem Palo Alto Calif 2:265–277. https://doi.org/10.1146/annurev.anchem.1.031207.112942
Zhou W, Petricoin EF, Longo C (2017) Mass spectrometry-based biomarker discovery. Methods Mol Biol Clifton NJ 1606:297–311. https://doi.org/10.1007/978-1-4939-6990-6_19
Tavichakorntrakool R, Prasongwattana V, Sriboonlue P, Puapairoj A, Pongskul J, Khuntikeo N, Hanpanich W, Yenchitsomanus PT, Wongkham C, Thongboonkerd V (2008) Serial analyses of postmortem changes in human skeletal muscle: a case study of alterations in proteome profile, histology, electrolyte contents, water composition, and enzyme activity. Proteomics Clin Appl 2:1255–1264. https://doi.org/10.1002/prca.200800051
Procopio N, Williams A, Chamberlain AT, Buckley M (2018) Forensic proteomics for the evaluation of the post-mortem decay in bones. J Proteome 177:21–30. https://doi.org/10.1016/j.jprot.2018.01.016
Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26:1367–1372. https://doi.org/10.1038/nbt.1511
Kim J-Y, Welsh EA, Fang B, Bai Y, Kinose F, Eschrich SA, Koomen JM, Haura EB (2016) Phosphoproteomics reveals MAPK inhibitors enhance MET- and EGFR-driven AKT signaling in KRAS-mutant lung cancer. Mol Cancer Res MCR 14:1019–1029. https://doi.org/10.1158/1541-7786.MCR-15-0506
Chawade A, Alexandersson E, Levander F (2014) Normalyzer: a tool for rapid evaluation of normalization methods for omics data sets. J Proteome Res 13:3114–3120. https://doi.org/10.1021/pr401264n
Pittner S, Ehrenfellner B, Monticelli FC, Zissler A, Sänger AM, Stoiber W, Steinbacher P (2016) Postmortem muscle protein degradation in humans as a tool for PMI delimitation. Int J Legal Med 130:1547–1555. https://doi.org/10.1007/s00414-016-1349-9
Ferguson RE, Carroll HP, Harris A, Maher ER, Selby PJ, Banks RE (2005) Housekee** proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. PROTEOMICS 5:566–571. https://doi.org/10.1002/pmic.200400941
Kim HJ, Na JI, Min BW, Na JY, Lee KH, Lee JH, Lee YJ, Kim HS, Park JT (2014) Evaluation of protein expression in housekee** genes across multiple tissues in rats. Korean J Pathol 48:193–200. https://doi.org/10.4132/KoreanJPathol.2014.48.3.193
Blair JA, Wang C, Hernandez D, Siedlak SL, Rodgers MS, Achar RK, Fahmy LM, Torres SL, Petersen RB, Zhu X, Casadesus G, Lee HG (2016) Individual case analysis of postmortem interval time on brain tissue preservation. PLoS One 11. https://doi.org/10.1371/journal.pone.0151615
Abbas W, Kumar A, Herbein G (2015) The eEF1A proteins: at the crossroads of oncogenesis, apoptosis, and viral infections. Front Oncol 5:75. https://doi.org/10.3389/fonc.2015.00075
Li D, Wei T, Abbott CM, Harrich D (2013) The unexpected roles of eukaryotic translation elongation factors in RNA virus replication and pathogenesis. Microbiol Mol Biol Rev MMBR 77:253–266. https://doi.org/10.1128/MMBR.00059-12
Tomlinson VA, Newbery HJ, Wray NR et al (2005) Translation elongation factor eEF1A2 is a potential oncoprotein that is overexpressed in two-thirds of breast tumours. BMC Cancer 5:113. https://doi.org/10.1186/1471-2407-5-113
Baron CP, Jacobsen S, Purslow PP (2004) Cleavage of desmin by cysteine proteases: calpains and cathepsin B. Meat Sci 68:447–456. https://doi.org/10.1016/j.meatsci.2004.03.019
Ehrenfellner B, Zissler A, Steinbacher P, Monticelli FC, Pittner S (2017) Are animal models predictive for human postmortem muscle protein degradation? Int J Legal Med 131:1615–1621. https://doi.org/10.1007/s00414-017-1643-1
Funding
Open access funding provided by Austrian Science Fund (FWF). This work is supported by research fund of the Chungnam National University, National Research Foundation of Korea (2017R2014R1A6A9064166, 2016M3A9E1918321), the Korea Basic Science Institute under the R&D program (Project No. T38641) supervised by the Ministry of Science of Korea, and the Austrian Science Fund (FWF), grant P31490.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Choi, KM., Zissler, A., Kim, E. et al. Postmortem proteomics to discover biomarkers for forensic PMI estimation. Int J Legal Med 133, 899–908 (2019). https://doi.org/10.1007/s00414-019-02011-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-019-02011-6