Abstract
Purpose
This study aimed to assess reliable options for bedside diagnosis of silent aspiration in the intensive care unit by examining the use of default grayscale images (DGI) obtained using a mobile, general-purpose, radiography system capable of dynamic digital radiography (M-DDR) and inverted grayscale images (IGI) of DGI.
Methods
This cohort study (exploratory and preliminary) involved 18 adult patients (mean age, 89.0 years) for whom a swallowing assessment request was received from their primary physicians. Fifty-six IGI videoclips were evaluated by three specialists using the penetration-aspiration scale (PAS), with the gold standard being the consensus reading of all three specialists. Another three speech-language pathologists (SLPs) assessed 56 DGI and IGI videoclips using the PAS. PAS scores 1 and 2 were classified as normal range, PAS scores 3–5 as pathological laryngeal penetration, and PAS scores 6–8 as aspiration. The correct rates with IGI and DGI were then determined, and the level of agreement of IGI and DGI evaluations was evaluated.
Results
The correct rate of all evaluators was 100% for normal range, 80–100% for pathological laryngeal penetration, and 83–100% for aspiration with IGI and 100% for normal range, 90% for pathological laryngeal penetration, and 83% for aspiration with DGI. The kappa coefficient for IGI and DGI showed almost complete agreement for abnormal conditions.
Conclusion
Dynamic imaging of swallowing 2–5 ml of liquid using M-DDR performed for elderly patients at the bedside showed that aspiration assessments by SLPs obtained from DGI videos immediately after imaging are acceptable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanisms underlying the occurrence of dysphagia in the intensive care unit (ICU) are not completely understood [1], but factors may include disuse muscle atrophy, sarcopenia, decreased muscle strength associated with malnutrition [2, 3], cognitive disorders that make following instructions difficult [4], gastroesophageal reflux, and lack of breathing and swallowing coordination [5], as well as direct injury from endotracheal and tracheostomy tubes. For endotracheal intubation tubes in particular, changes in sensation from post-extubation laryngeal injury or tubes have been reported [6]. Post-extubation dysphagia (PED) from long-term endotracheal intubation is reported to occur within a wide range, from 3 to 62% of cases [7]. The risk of occurrence increases with increasing duration of intubation [8] and larger diameter of intubation tubes [9, 10]. The rate of occurrence of post-extubation silent aspiration (SA) specifically is reported to be 17–25% [11,12,13], and the handling of PED may thus be considered important.
The bedside swallowing evaluation (BSE) [14] used by speech-language pathologists (SLPs), post-extubation dysphagia screening (PDS) [15] used by nurses, and the Yale Swallow Protocol (YSP) [16] that can be used by both SLPs and nurses are all well known internationally as non-instrumental assessments that can be performed soon after extubation in the ICU to screen for aspiration risk. These assessments emphasize evaluations using a 3-ounce water swallow test (3 oz. WST) [17], but much remains contentious about aspiration screening with the 3 oz. WST alone [18, 19]. Under- and overestimation of SA is an issue that needs to be addressed, and reliable means of detecting SA at the bedside are needed in the ICU. Instrumental assessments with a videofluoroscopic swallowing study (VFSS) or fiberoptic endoscopic examination of swallowing (FEES) are considered necessary for conclusive proof of SA.
However, performing these diagnostic imaging examinations for all ICU patients is impractical. Issues in the ICU include the burden on the patient of a series of manual procedures using a nasotracheal intubation tube and the risk of patient agitation or bleeding from FEES. With VFSS, multiple personnel including doctors are needed to move the patient to the fluoroscopy room and deal with problems that occur with the connected devices or in the general condition of the patient and other matters. FEES is considered inferior to VFSS for diagnosing SA, but has the advantage of being able to be performed at the bedside in the ICU.
This study therefore focused on elucidating the physiological mechanisms underlying dysphagia and making bedside assessments of aspiration with dynamic X-ray imaging of swallowing rather than VFSS to clarify treatment options based on that mechanism, in an attempt to introduce to the ICU reliable options for SA diagnosis in critically ill patients using dynamic X-ray imaging optimized for this purpose. Although this requires diagnostic imaging by physicians, we felt that image analysis by SLP as a diagnostic aid to the physician would facilitate rapid bedside aspiration assessment.
The purpose of this study was thus to explore the practicality of aspiration assessment from videos obtained at the bedside using a mobile, general-purpose, radiography system capable of dynamic digital radiography and measures to counter any problems that arise.
Methods
This cohort study (exploratory and preliminary) was approved by the university hospital’s research ethics committee (approval no. 22R-113). Written, informed consent was obtained from all participants prior to enrolment in this study.
The participants were 18 patients (8 men, 10 women) admitted to the hospital between November 1, 2022 and February 28, 2023 who satisfied all the inclusion criteria and none of the exclusion criteria. The inclusion criteria were: (1) age > 21 years; (2) request for swallowing evaluation from the primary care physician; (3) difficulty moving the patient to the fluoroscopy room and/or performing VFSS (e.g., inability to maintain a suitable sitting position in a wheelchair); and (4) stable respiratory status (respiratory rate < 30 breaths/min; saturation of percutaneous oxygen > 90% without supplemental oxygen). The exclusion criteria were: (1) exacerbation of medical condition; (2) severe dysphagia in which swallowing could not be induced with saliva or a small amount of water; (3) inability to maintain attention; or (4) nutritional management with a nasogastric tube or gastrostomy from before hospitalization.
The patients’ mean age was 89.0 (SD 7.3) years for the total cohort, 87.0 (SD 6.9) years for men, and 90.6 (SD 7.4) years for women. The mean body mass index was 20.5 (SD 3.0) kg/m2 for the total cohort, 20.5 (SD 2.7) kg/m2 for men, and 20.4 (SD 3.4) kg/m2 for women. The underlying pathology was pneumonia in 9 patients (aspiration pneumonia, n = 6; other, n = 3), cerebrovascular disease in 3 patients, renal/urological disease in 3 patients, heart disease in 1 patient, neuromuscular disease in 1 patient, and liver disease in 1 patient.
Imaging method
Digital dynamic radiography (DDR) was performed using a mobile DDR (M-DDR) system (AeroDR TX m01, Konica Minolta, Tokyo, Japan) and a flat-panel detector (FPD) (AeroDR fine motion, Konica Minolta). M-DDR can capture a maximum of 20 s of dynamic X-ray images at 15 frames per second (fps).
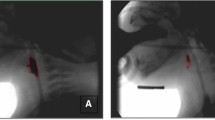
One radiologist, one doctor, and one SLP were involved in the imaging. The participant maintained an upright position in bed with the M-DDR tube facing the window side of the room, where no people were present. A lead partition was placed between the participant and the neighboring patient. An FPD affixed to a mobile detector holder (PLESiO, Obayashi Manufacturing, Saitama, Japan) was brought close to the side of the participant, and the oral cavity, pharynx, larynx, and part of the upper esophagus were included within the imaging range. The distance between the tube and detector was set at 120 cm (Fig. 1a). The initial settings for the imaging system were: 15 fps; tube voltage, 60 kV; tube current, 80 mA; and irradiation time, 5 msec. Tube voltage and tube current conditions were determined by test imaging of the patient. First, lateral imaging was conducted for swallowing 2 ml of liquid (barium sulfate, 38% w/v) using a 5-ml syringe (Fig. 1b). After imaging, videos were immediately reproduced on a 19-inch M-DDR monitor to confirm the presence or absence of aspiration (Fig. 1c). The default grayscale image (DGI) displayed on the M-DDR monitor showed bone and barium fluid as white. If no aspiration was identified, the amount of liquid was increased by 1-ml increments up to a maximum of 5 ml. In other words, data from a maximum of four DGIs were obtained from one patient.
Bedside diagnosis of silent aspiration using mobile dynamic digital radiography. (a) The tube is turned toward the window side where no people are present, and the detector is affixed to a holder at the side of the patient. (b) Barium swallow is conducted using a 5-ml syringe under lateral imaging. (c) Videoclips are reproduced on the M-DDR monitor immediately after imaging to confirm whether aspiration is present
Evaluation method
Conventional fluoroscopy used in VFSS shows bone and barium fluid as black, whereas DGI, which is standard on the M-DDR monitors, shows bone and barium fluid as white. It was thought that the differences between the two images on the M-DDR monitor should be examined for their potential to make a difference in the determination of pathological laryngeal penetration and aspiration.
A total of 59 DGI videos from 18 patients obtained with M-DDR were uploaded to analysis software dedicated to DDR (KINOSIS, Konica Minolta), then black and white were reversed for inverted grayscale image (IGI) videos to create two types of videoclips (Fig. 2). DGI videos that could be examined on the M-DDR monitor showed bone and barium fluid as white, whereas IGI videos showed bone and barium fluid as black, as in conventional X-ray fluoroscopy. All videoclips were saved in MPEG-4 format.
Two physicians and an SLP who specialized in swallowing assessments with more than 30 years of experience evaluated the IGI videoclips using the Penetration-Aspiration Scale (PAS) [20].
-
1:
Material does not enter the airway.
-
2:
Material enters the airway, remains above the vocal folds, and is ejected from the airway.
-
3:
Material enters the airway, remains above the vocal folds, and is not ejected from the airway.
-
4:
Material enters the airway, contacts the vocal folds, and is ejected from the airway.
-
5:
Material enters the airway, contacts the vocal folds, and is not ejected from the airway.
-
6:
Material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway.
-
7:
Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort.
-
8:
Material enters the airway, passes below the vocal folds, and no effort is made to eject.
The three assembled raters reviewed each videoclip frame by frame and excluded three videoclips in which laryngeal penetration and aspiration could not be determined from the images due to video problems. All 56 remaining videoclips from 18 patients were agreed upon by the two physicians and one SLP and were adopted as the gold standard. The 56 videoclips were classified as PAS score 1–8 (1, n = 31; 2, n = 9; 3, n = 4; 4, n = 1; 5, n = 5; 6, n = 2; 7, n = 1; and 8, n = 3). SA was considered to correspond to PAS score 8. PAS scores of 1 or 2 were classified as within the normal range (n = 40), PAS scores of 3–5 were classified as showing pathological laryngeal penetration (n = 10), and PAS scores of 6–8 were classified as showing aspiration (n = 6).
Fifty-six DGI videoclips were evaluated by three SLPs with at least five years of experience in PAS evaluation who were not involved in the gold standard evaluation. Each evaluator assessed the videoclips as PAS scores 1–8, including slow motion and stop motion with no time limit set for the assessment, and entered and submitted a judgment on a record sheet. At a later date, without revealing that the 56 DGI videoclips had been converted to IGI videoclips, the same SLPs evaluated the 56 randomly reordered IGI videoclips in the same manner, and their judgements were recorded on a recording sheet. Based on the PAS scores of each evaluator, videoclips were classified into three categories: normal range, pathological laryngeal penetration, and aspiration. Images were viewed on a 21.5-inch monitor with a resolution of 4096 × 2304.
-
1)
Correct rate with IGI and DGI
From the IGI and DGI assessments of each evaluator, correct rates for normal range, pathological laryngeal penetration, and aspiration were calculated.
-
2)
Level of agreement of IGI and DGI assessments
The kappa coefficient was used for the level of agreement of IGI and DGI in the evaluations from each of the evaluators.
Statistical analyses were performed using SPSS version 28 software, with kappa coefficients interpreted as follows: 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; 0.81–1, almost perfect [21].
Results
Correct rates with IGI and DGI
The correct rate for the normal range was 100% (40/40) for all three evaluators with both IGI and DGI. The correct rate for pathological laryngeal penetration was 80% (8/10) for Evaluator A, 100% (10/10) for Evaluator B, and 90% (9/10) for Evaluator C with IGI and 90% (9/10) for Evaluator A, 90% (9/10) for Evaluator B, and 90% (9/10) for Evaluator C with DGI. The correct rate for aspiration was 83% (5/6) for Evaluator A, 83% (5/6) for Evaluator B, and 100% (6/6) for Evaluator C with IGI, and 83% (5/6) for Evaluator A, 83% (5/6) for Evaluator B, and 83% (5/6) for Evaluator C with DGI.
Level of agreement of IGI and DGI assessments
The level of agreement with IGI and DGI assessments for the normal range was complete agreement for all three evaluators. The kappa coefficients with IGI and DGI for abnormal conditions (pathological laryngeal penetration and aspiration) were 0.885 for Evaluator A, 0.867 for Evaluator B, and 0.881 for Evaluator C, showing almost perfect agreement.
Discussion
In elucidating the physiological mechanisms underlying dysphagia and clarifying treatment options as a result, VFSS at ≥30 fps is recommended. The use of videos obtained with M-DDR at 15 fps for that same purpose is inappropriate [22].
In this study, the focus was narrowed to aspiration assessments at the bedside, and a preliminary experiment was performed with the idea of introducing to the ICU reliable options for SA diagnosis with dynamic X-ray imaging of swallowing optimized for that purpose.
In cases of laryngeal penetration, when the VFSS of adults is decreased from 30 fps to 15 fps, PAS score 2 may be misjudged as PAS score 1 [23]. In addition, children carry a risk of PAS score 4 being misjudged with the same reduction in frame rate [24]. However, in cases of aspiration, no reports have indicated the possibility that the information necessary for PAS scoring might be completely lost with the same reduction in frame rate, regardless of age. However, little is currently known about the effects of a decreased frame rate on PAS scoring. In the present study, PAS scores were classified into the three categories of “normal range”, “pathological laryngeal penetration”, and “aspiration” in adult patients, and analyses were limited to these categories.
Physicians and SLPs accustomed to VFSS image analysis may prefer IGI in black bone mode to the unfamiliar DGI in white bone mode, which is standard on the M-DDR monitors. Although there are a fair number of reports of the effect of inverting the black and white contrast on evaluations of still images, to the best of our knowledge, there are no reports of the diagnosis of aspiration with videos at 15 fps. Studies have shown that dark characters on a light background (positive polarity) are more legible than light characters on a dark background (negative polarity) [25,26,27]. In fact, better legibility with decreasing character size is achieved with positive polarity compared to negative polarity [28]. In this case, DGI corresponds to negative polarity and IGI to positive polarity.
The 18 patients for whom bedside evaluation was indicated had a mean age of 89 years, and evaluations by the three SLPs yielded a correct rate of ≥80% for all pathological laryngeal penetration and aspiration with both DGI and IGI. In all evaluated cases of pathological laryngeal penetration and aspiration, almost perfect agreement was obtained between DGI and IGI. A similarly high correct rate and agreement between DGI and IGI were also obtained for patients within the normal range. Thus, inverse processing from DGI to IGI does not seem to be essential when evaluating pathological laryngeal penetration. Furthermore, the SLPs’ determinations of “pathological laryngeal penetration” and “aspiration” appeared to be reliable.
One possible reason for the lack of difference in the diagnosis of aspiration with DGI and IGI is the difference in the black and white contrast for tissue including the larynx and liquid contrast agents, because clearly detecting both is essential for diagnosing aspiration. Though more detailed diagnosis of aspiration remains an issue, the possibility that diagnostic accuracy is heightened by making the diagnosis with a combination of DGI and IGI cannot be ruled out.
Given these findings, assessments by SLPs of the presence or absence of aspiration using DGI videos that can be reproduced immediately after M-DDR are acceptable. This finding suggests that SLPs’ assessments could serve as a diagnostic aid for physicians, and rapid bedside evaluation at the bedside may be feasible.
In our view, the option of this new instrumental swallowing assessment may be beneficial in standardizing transdisciplinary swallowing treatment protocols for critically ill patients.
Study limitations
Videos that can be checked on an M-DDR monitor can only be checked from recordings, not in real time. With respect to this, live-view images corresponding to 2 fps displayed on an M-DDR monitor after a delay of several seconds were used during imaging. When a judgment could not be made during imaging, 20 s was taken as the time limit for imaging. As a result, all elderly subjects were able to withstand the swallowing assessment with 2–5 ml of liquid.
When performing lateral dynamic X-ray imaging of swallowing in this study, the M-DDR tube was always directed toward the window side where no people were present, and a lead partition was always placed between the participant and the neighboring patient.
Naturally, the addition of an assessment of continuous swallowing of liquid from a cup necessitates an extension of maximum imaging time. Optimizing the imaging conditions for aspiration assessments at the bedside represents a key issue for the future.
Conclusions
Bedside imaging of elderly patients swallowing 2–5 ml of liquid using M-DDR appears feasible. A correct rate of ≥80% for assessment by SLPs in all cases of pathological laryngeal penetration and aspiration was obtained with DGI, which can be checked on M-DDR, and inverse-processed IGI. Almost perfect agreement was also obtained for assessments from DGI and IGI. Assessment of aspiration using DGI by SLPs obtained immediately after M-DDR appears acceptable.
Data availability
Not applicable.
Abbreviations
- SLP:
-
Speech-language pathologist
- ICU:
-
Intensive care unit
- DGI:
-
Default grayscale images
- IGI:
-
Inverted grayscale images
- PAS:
-
Penetration-Aspiration Scale
- SA:
-
Silent aspiration
- VFSS:
-
Videofluoroscopic swallowing study
- FEES:
-
Flexible endoscopic evaluation of swallowing
- PED:
-
Post-extubation dysphagia
- 3-oz WST:
-
3-ounce Water Swallow Test
- M-DDR:
-
Mobile-digital dynamic radiography
- FPD:
-
Flat-panel detector
- fps:
-
Frames per second
References
Macht M, Wimbish T, Bodine C, Moss M (2013) ICU-acquired swallowing disorders. Crit Care Med 41:2396–2405. https://doi.org/10.1097/CCM.0b013e31829caf33
Cha S, Kim WS, Kim KW et al (2019) Sarcopenia is an independent risk factor for dysphagia in community-dwelling older adults. Dysphagia 34:692–697. https://doi.org/10.1007/s00455-018-09973-6
Brodsky MB, De I, Chilukuri K et al (2018) Coordination of pharyngeal and laryngeal swallowing events during single liquid swallows after oral endotracheal intubation for patients with acute respiratory distress syndrome. Dysphagia 33:768–777. https://doi.org/10.1007/s00455-018-9901-z
Leder SB, Suiter DM, Warner HL (2009) Answering orientation questions and following single-step verbal commands: effect on aspiration status. Dysphagia 24:290–295. https://doi.org/10.1007/s00455-008-9204-x
Camargo FP, Ono J, Park M, Caruso P, Carvalho CRR (2010) An evaluation of respiration and swallowing interaction after orotracheal intubation. Clin (Sao Paulo) 65:919–922. https://doi.org/10.1590/S1807-59322010000900015
Borders JC, Daniel F, Levitt JE et al (2019) Relationship between laryngeal sensation, length of intubation, and aspiration in patients with acute respiratory failure. Dysphagia 34:521–528. https://doi.org/10.1007/s00455-019-09980-1
Skoretz SA, Flowers HL, Martino R (2010) The incidence of dysphagia following endotracheal intubation: a systematic review. Chest 137:665–673. https://doi.org/10.1378/chest.09-1823
Brodsky MB, Akst LM, Jedlanek et al (2021) Laryngeal injury and upper airway symptoms after endotracheal intubation during surgery: a systematic review and meta-analysis. Anesth Analg 132:1023–1032. https://doi.org/10.1213/ANE.0000000000005276
Krisciunas GP, Langmore SE, Gomez-Taborda S et al (2020) The association between endotracheal tube size and aspiration (during flexible endoscopic evaluation of swallowing) in acute respiratory failure survivors. Crit Care Med 48:1604–1611. https://doi.org/10.1213/ANE.0000000000005276
Plowman PK, Anderson A, York JD et al (2021) Dysphagia after cardiac surgery: prevalence, risk factors, and associated outcomes. J Thorac Cardiovasc Surg 165(2):737–746e3. https://doi.org/10.1016/j.jtcvs
Leder SB, Cohn SM, Moller BA et al (1998) Fiberoptic endoscopic documentation of the high incidence of aspiration following extubation in critically ill trauma patients. Dysphagia 13:208–212. https://doi.org/10.1007/PL00009573
Ajemian MS, Nirmul GB, Anderson MT et al (2001) Routine fiberoptic endoscopic evaluation of swallowing following prolonged intubation: implications for management. Arch Surg 136:434–437. https://doi.org/10.1001/archsurg.136.4.434
Hafner G, Neuhuber A, Hirtenfelder S et al (2008) Fiberoptic endoscopic evaluation of swallowing in intensive care unit patients. Eur Arch Otorhinolaryngol 265:441–446. https://doi.org/10.1007/s00405-007-0507-6
Lynch YT, Clark BJ, Macht M et al (2017) The accuracy of the bedside swallowing evaluation for detecting aspiration in survivors of acute respiratory failure. J Crit Care 39:143–148. https://doi.org/10.1016/j.jcrc
Johnson KL, Speirs L, Mitchell A et al (2018) Validation of a postextubation dysphagia screening tool for patients after prolonged endotracheal intubation. Am J Crit Care 27:89–96. https://doi.org/10.4037/ajcc2018483
Suiter DM, Sloggy J, Leder SB (2014) Validation of the Yale swallow protocol: a prospective double-blinded videofluoroscopic study. Dysphagia 29:199–203. https://doi.org/10.1007/s00455-013-9488-3
Suiter DM, Leder SB (2008) Clinical utility of the 3-ounce water swallow test. Dysphagia 23:244–250. https://doi.org/10.1007/s00455-007-9127-y
Leder SB, Suiter DM, Green BG (2011) Silent aspiration risk is volume-dependent. Dysphagia 26:304–309. https://doi.org/10.1007/s00455-010-9312-2
York JD, Leonard K, Anderson A et al (2022) Discriminant ability of the 3-ounce water swallow test to detect aspiration in acute postoperative cardiac surgical patients. Dysphagia 37:831–838. https://doi.org/10.1007/s00455-021-10333-0
Rosenbek JC, Robbins JA, Roecker JA et al (1996) A penetration-aspiration scale. Dysphagia 11:93–98. https://doi.org/10.1007/BF00417897
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174. https://doi.org/10.2307/2529310
Ingleby HR, Bonilha HS, Steele CM (2023) A tutorial on diagnostic benefit and radiation risk in videofluoroscopic swallowing studies. Dysphagia 38:517–542. https://doi.org/10.1007/s00455-021-10335-y
Bonilha HS, Blair J, Carnes B et al (2013) Preliminary investigation of the effect of pulse rate on judgments of swallowing impairment and treatment recommendations. Dysphagia 28:528–538. https://doi.org/10.1007/s00455-013-9463-z
Cohen MD (2009) Can we use pulsed fluoroscopy to decrease the radiation dose during video fluoroscopic feeding studies in children? Clin Radiol 64:70–73. https://doi.org/10.1016/j.crad.2008.07.011
Chan A, Lee P (2005) Effect of display factors on Chinese reading times, comprehension scores and preferences. Behav Inf Technol 24:81–91. https://doi.org/10.1080/0144929042000267073
Buchner A, Baumgartner N (2007) Text - background polarity affects performance irrespective of ambient illumination and colour contrast. Ergonomics 50:1036–1063. https://doi.org/10.1080/00140130701306413
Piepenbrock C, Mayr S, Mund I, Buchner A (2013) Positive display polarity is advantageous for both younger and older adults. Ergonomics 56:1116–1124. https://doi.org/10.1080/00140139.2013.790485
Piepenbrock C, Mayr S, Buchner A (2014) Positive display polarity is particularly advantageous for small character sizes. Hum Factors 56:942–951. https://doi.org/10.1177/0018720813515509
Funding
This work was supported by a Grant-in-Aid for Scientific Research (C), MEXT KAKENHI Grant Number 22K11348. This research was also supported by KONICA MINOLTA, Inc. The DDR imaging system was borrowed free of charge from KONICA MINOLTA, Inc.
Author information
Authors and Affiliations
Contributions
MAD: project development, data collection, and manuscript writing, MT: data collection and manuscript writing, MAD, MT: statistical analysis.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by Tokai University Hospital’s research ethics committee (approval no. 22R-113). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Written, informed consent was obtained from all individual participants included in the study.
Conflict of interest
This study was reviewed for conflicts of interest by the Tokai University Isehara Campus Conflicts of Interest Management Committee. The provision of the DDR imaging system was confirmed to have not adversely affected the transparency of study implementation or the reliability of the results, and approval was obtained.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presentations: This research was presented at the 60th Annual Meeting of the Japanese Association of Rehabilitation Medicine; Fukuoka; June 29 - July 2, 2023.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koyama, Y., R.A., Y.M., SLP, T. et al. Bedside diagnosis of silent aspiration using mobile dynamic digital radiography: a preliminary study. Eur Arch Otorhinolaryngol (2024). https://doi.org/10.1007/s00405-024-08785-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00405-024-08785-9