Abstract
Purpose
To determine the frequency of fetal infection as well as adverse pregnancy outcomes following antenatal hyperimmunoglobulin (HIG) treatment for primary cytomegalovirus (CMV) infection in pregnancy.
Methods
In our observational cohort study, data from 46 women with a primary CMV infection during pregnancy were evaluated. Primary CMV infection was defined by seroconversion or the presence of CMV-IgM and low CMV-IgG avidity. All women received at least two or more infusions of HIG treatment (200 IU/kg). Congenital CMV infection (cCMV) was diagnosed by detection of CMV in amniotic fluid and/or neonatal urine. We compared the rate of maternal–fetal transmission from our cohort to data without treatment in the literature. The frequency of adverse pregnancy outcomes was compared to those of live-born infants delivered in our clinic.
Results
We detected 11 intrauterine infections in our cohort, which correlates to a transmission rate of 23.9%. Compared to the transmission rate found in cases without treatment (39.9%), this is a significant reduction (P = 0.026). There were no adverse pregnancy outcomes in our cohort. The mean gestational age at delivery was 39 weeks gestation in treatment and control group.
Conclusion
The administration of HIG for prevention of maternal–fetal CMV transmission during pregnancy seems safe and effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital cytomegalovirus infection (cCMV) is the main cause of hearing loss and mental retardation in infants without genetic disorder [1]. The rate of cCMV differs depending on whether the infection of the fetus results from a recurrence of an earlier CMV infection of the mother or if the mother is primarily infected during pregnancy. In recurrent infections, the intrauterine transmission rate is estimated to be 0.5–1.2% [2,3,4]. After cCMV due to recurrent infection, newborns are rarely symptomatic [5, 6], although severe cases are reported [3]. In the cases of primary infection during pregnancy, the rate of cCMV is approximately 40% [7, 8]. The rate of transmission after primary infection in the first trimester in a German and Belgian historic cohort is estimated to be 35.2% [9]. This paper addresses only primary CMV infection in pregnancy. The prevalence of cCMV varies between 0.6 and 6.1% in develo** countries [10] and 0.3% in Australia [11]. Since there is no general neonatal CMV screening in Germany, there is no available data on the prevalence for Germany. A retrospective data analysis in central Germany estimates the prevalence of clinically relevant Ccmv infection higher than 0.04% [12]. Approximately, 11% of congenitally infected infants have symptoms of cCMV [13]. The later the infection occurs in the mother during pregnancy, the higher the rate of transmission, but the lower the rate of symptomatic infants [14]. In a recent study of 138 children with cCMV, amniocentesis (AC) was performed in all pregnancies, predominantly at about 20–23 weeks gestation. In the cohort of infants with a negative CMV-DNA AC, thus with intrauterine infection later during the pregnancy, none of the children had long-term complications after birth. In contrast, in the cohort with a positive AC at about 20–23 weeks gestation, 14% suffered from long-term sequelae [15].
After fetal infection, in utero therapeutic options are limited [16]. In a non-randomized and non-controlled study, high-dose Valacyclovir was used for women with primary CMV infection during pregnancy which resulted in a better outcome for newborns with cCMV [17]. For in utero treatment with Valganciclovir, there are only case reports available [18].
For prevention of maternal–fetal transmission of CMV during pregnancy, hyperimmunoglobulin (HIG) treatment is controversially discussed and currently not generally recommended within international guidelines [19].
In Germany, since the publication of the guidelines on laboratory diagnostics of viral infections relevant in pregnancy [21], the number of voluntary CMV tests during pregnancy has increased. As a direct result, the consultation for positive tests in our outpatient clinic for infectious diseases in pregnancy rose accordingly [22]. In our outpatient clinic, we perform a thorough consultation for women with primary CMV infection during pregnancy, illustrating the limited and controversial data available. Women who requested an off-label HIG treatment for prevention of maternal–fetal transmission were offered a treatment in our clinic.
Material and methods
Patients and study design
All women who had received HIG between 01/2010 and 03/2017 in our outpatient clinic for infectious diseases in pregnancy at the Charité–Universitätsmedizin Berlin, Germany, a tertiary care hospital with the intention of prevention of maternal–fetal transmission, were contacted in January 2018 by mail. The study had local ethics committee (EA2/135/17) approval. Information was gathered from hospital charts/records, written questionnaires and partially telephone interviews. The women received information about the study purpose, an informed consent sheet for required signature and a data collection sheet about the pregnancy and infant’s outcome. Women were reminded about the study by phone and/or mail for a maximum of three times.
At the initial counselling, the option of HIG treatment was discussed with women with primary CMV infection during pregnancy. Primary infection of CMV was defined as either (a) confirmed seroconversion during pregnancy or (b) presence of CMV-IgM and low CMV-IgG avidity. CMV-IgGgB2 was registered if performed. Tests were inhomogeneous, as most women were referred from external gynecologists and presented their external laboratory values. In the cases of inconclusive data, the laboratory was contacted, or a control blood sample was taken in our center.
All women in the study collective received two or more infusions of HIG (200 IU/kg). HIG was given within a 2-week interval. After two infusions, avidity against CMV was tested. In the cases of increased avidity, no subsequent infusion was administered. For cases of similar avidity as was observed during the baseline measurements, a third infusion was administered. Each patient was informed about the possibility of AC to confirm or rule out intrauterine CMV infection. Congenital CMV infection was thus either diagnosed by detection of CMV in amniotic fluid and/or neonatal urine. According to our treatment protocol, all women had ultrasound follow-up during pregnancy.
Four women were lost to follow-up during pregnancy after AC was negative for CMV. One woman did not continue pregnancy after negative AC for other reasons. These five women were included in the transmission analysis, but not in the analysis on pregnancy outcome. Thus, our control group comprised of 82 women.
Control group
For comparison of the rate of adverse pregnancy outcomes (i.e. pregnancy-induced hypertension, preeclampsia, eclampsia, preterm birth, AND low birth weight), we took a matched random sample from all births in our center. The Charité University Medical Center in Germany is a tertiary care hospital offering specialized treatment for any risk pregnancies, for children with heart diseases as well as mothers with infectious diseases and their children. For each viable birth within our clinic in the HIG-group, to create the control group, we matched and accessed the births that occurred before and afterwards. When a woman from our study group did not give birth in our clinic, we matched the two births from the same day before and after noon. We accessed the following parameters: pregnancy-induced hypertension, preeclampsia, eclampsia, completed weeks of gestation at birth, mode of birth, birth weight, head circumference at birth, length at birth, umbilical cord pH at birth, and other complications.
Statistical analysis
The growth standard by Voigt et al., adjusting for gestational age at birth, was used to calculate age-adjusted and sex-adjusted z scores for weight, length, and head circumference at birth [23]. Results were given as mean or median [range or n (%)]. Groups were compared using the Fisher’s exact test with a significance level set at 0.05. Further testing was carried out by the Pearson Chi-square test for multiple division of outcomes. Statistical significance was set at P < 0.05.
The proportion of cases with transmission overall at any time during pregnancy was compared to the value of 39.9% (112/281) calculated by Feldman et al. [24]. Furthermore, transmission rates according to trimester of infection without HIG treatment for second trimester 42.0% (42/100) and third trimester 58.6% (17/29) were also taken from Feldman et al. The subgroup analysis of transmission before 20 weeks gestation and the start of HIG treatment before completed 15 weeks gestation was compared to the control group used by Kagan et al. [9] (35.2%) using the Fisher’s exact test with a significance level set at 0.05. Statistical analysis was performed using the software IBM® SPSS® Statistics Version 25.
Results
Study cohort
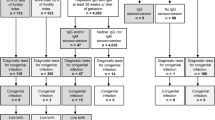
Between 01/2010 and 03/2017, a total of 62 women received HIG with the intention of prevention of maternal–fetal transmission in our outpatient clinic for infectious diseases. Most infections occurred in the first trimester (72%, 33 women). Mean interval between first suspicious lab test and HIG administration was 23 days. Mean gestational week at first HIG treatment was 17 weeks gestation. In the majority of cases, the HIG treatment was started within the second trimester, in nine cases within the first trimester and in three cases after 29 weeks gestation. Three of the women who received HIG were positive for IgG gB2. In those cases, the indication for HIG treatment was low avidity of IgG antibodies. HIG treatment was administered at least two times; however, six women received three doses. The third dosage was applied after 4 weeks in four cases and in two cases again after 2 weeks. Application was well tolerated, and no allergic reactions were reported. Data on maternal–fetal transmission were available for a total of 46 patients. Characteristics of the study population and their treatment course are summarized in Table 1.
Efficacy of transmission prophylaxis
In our cohort, 21 women decided for AC. Two ACs were positive for CMV in the amniotic fluid. Urine results after birth were only available for 34 neonates, of which ten were positive. In one case, a transmission occurred later in pregnancy after negative AC in 19 weeks gestation and the respective child was asymptomatic. The overall transmission rate was 23.9% (P = 0.026, in comparison to 39.9% in Israeli control group). When looking at transmissions before 23 weeks gestation confirmed via AC, the transmission rate is 7.1% with HIG treatment (P = 0.027, compared to 35.2% in the historic German Belgian control group). However, this subgroup is very small with only 14 ACs performed until 23 weeks gestation.
The transmission rate seems lower when HIG was administered within the first 21 days after the first suspicious lab result (19.2%, 5/26) compared to administration of HIG beyond 3 weeks (30.0%, 6/20), but this difference is not statistically significant (P = 0.307 in the Fisher’s exact test). The reduction in transmission of CMV from 39.9% in the Israeli cohort compared to 19.2% in our cohort remains significant in this subgroup analysis (P = 0.028). For an overview of transmission rates according to subgroup see Table 2.
Follow-up of the children was received for 39 children for a mean of 26 months (5–88 months). Of the 11 children born with cCMV, none had hearing impairments. Five children were treated postnatally for CMV. Only one child was symptomatic with microcephalus and development retardation.
Rate of adverse pregnancy outcomes
No pregnancy-induced hypertension, preeclampsia, or eclampsia were observed in the study group or in the control group. There was one case of HELLP syndrome in the control group but none in the study group. Median completed gestational weeks at birth was 39 in both groups. There was no significant difference regarding neonates’ growth characteristics compared to our HIG treatment group, with rather a tendency towards higher absolute values in the HIG treatment group. A lower vaginal birth rate in the control group was observed which is probably due sampling method and hospital’s characteristic. See Table 3 for a detailed overview of pregnancy and obstetric outcomes.
Discussion
Efficacy of HIG treatment for prevention of maternal–fetal transmission of CMV
The role of HIG for prevention of maternal–fetal transmission during primary CMV infection in pregnancy is controversially discussed [16, 19]. A non-randomized study by Nigro et al. reported a significant reduction of cCMV to 16% in comparison to 40% in the control group with no obstetric adverse events [8]. The subsequent randomized-controlled study by Revello et al. showed no significant effect of HIG (30% HIG group vs. 44% control group; P = 0.13) but raised concerns regarding a higher rate of obstetrical adverse events (preterm birth, preeclampsia and fetal growth retardation) in the HIG group compared to the placebo group (13% vs. 2%) [7]. Furthermore the study by Revello et al. could have reached significance level in a greater study collective according to van Leeuwen et al. [25]. The study by Revello et al. was criticized for various reasons [9]. First of all, a monthly HIG administration was performed although the half-life of HIG seems to be only 11 days [20]. Second, women with primary infection in the second trimester were treated and a median time interval between the diagnosis of infection and first HIG administration was five weeks. Third, the HIG dose administered was 100 IU per kg body weight. In a recent study by Kagan et al., these controversial points were addressed and only women with primary infection during first trimester of their pregnancy were included. Treatment was initiated within 3 weeks, the gestational age at first HIG administration was ≤ 14 weeks and HIG was administered at 200 IU per kg body weight biweekly until 20 weeks gestation. Within this study protocol, the transmission rate was significantly lower compared to the rate in a historic cohort (7.5% intervention group vs. 35.2% in the control group; P < 0.0001) [9]. The transmission rate of 23.9% in our study was higher despite having the same HIG-dose as used by Kagan et al. In comparison to the Israeli control group with a transmission rate of 39.9% (112 transmission in 281 pregnancies with no HIG treatment), this reduction is significant (P = 0.026).
The risk for the neonate is highest if the infection occurs in the first trimester [26,27,28,29,30]. A recent study even suggests that only infections up to 23 weeks gestation lead to sequelae in the infant [15]. Hence, specifically the prevention of early transmission in pregnancy should be of high priority. When doing a subgroup analysis among the women who underwent AC within the first 23 weeks gestation, we also saw a lower rate of transmission (7.1%) than 35.2% in the historic German Belgian control group. The rate of 7.1% is nonetheless higher than 2.5% described by Kagan et al. First of all, our subgroup of only 14 ACs is very small. Second of all, this can possibly be due to less courses of treatment. Most of the women in our collective only received two courses of HIG, while Kagan et al. continued treatment until a median gestational age of 16.6 weeks and applied up to six courses of treatment [9]. Furthermore, treatment in our cohort was initiated delayed compared to Kagan et al. The median time lag between primary infection of the mother, approximated in this study by first suspicious lab result, and first HIG treatment course was 23 days. Only 57% of our patients received HIG within the first 3 weeks after CMV infection. This can be explained due to clinical reality and administrative barriers. Because serologic testing was performed in external outpatient clinics, referral of patients was delayed. Furthermore, sometimes in the cases of inconclusive blood results additional laboratory parameters were ordered from the laboratory until decision on HIG treatment was taken. After the decision for HIG due to administrative necessities such as the refunding of off-label treatment, a waiting period of at least three full working days had to be kept in order for the insurance company to check the indication.
Safety of HIG treatment in pregnancy
The second controversially discussed aspect about HIG treatment during pregnancy are its potential side effects. Revello et al. reported obstetrical complications (preterm delivery, preeclampsia, and fetal growth restriction) in 7 of 53 women (13%) in the HIG group compared to 1 of 51 women (2%) in the placebo group (P = 0.06) [7]. Other authors found no increased rate of adverse events [9, 31, 32]. In our cohort, no preeclampsia occurred, and median completed gestational week was 39 weeks. Neonate growth parameters were not different in the HIG group compared to the control group. A lower c-section rate in the study group is most likely due to the sampling method where the time of birth was not known in the study group the control group was selected as the deliveries before and after noon in our center of tertiary care. This is typically the time for planned c-sections. Furthermore, the center of tertiary care is a referral center for women and children with illnesses necessitating c-sections as a mode of delivery. In conclusion, according to our data, no increased rate of adverse obstetric outcomes was detected and thus HIG application during pregnancy can be regarded as safe.
Limitations of this study
The limitations in our study is the retrospective character and the lack of randomization. Furthermore, the cohort is very diverse. Time between infection and initiation of treatment is often later than advised by Kagan et al. Moreover, HIG was not only administered to first trimester infections. We suggest that a national or international prospective study with faster treatment initiation should follow.
Strength of this study
We report results from the largest German cohort of HIG treatment during pregnancy, published so far. The results have implications for clinical management of CMV infection.
Conclusion
The administration of HIG for prevention of maternal–fetal CMV transmission during pregnancy seems safe and effective. If HIG is applied for prevention of vertical CMV transmission, it should be administered bi-weekly in an adequate dosage until AC is performed. Treatment should be initiated as soon as possible after detection of CMV infection in pregnancy.
References
Ludwig A, Hengel H (2009) Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Euro Surveill 14(9):26–32
Fowler KB et al (1992) The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med 326(10):663–667
Henrich W et al (2002) Recurrent cytomegalovirus infection during pregnancy: ultrasonographic diagnosis and fetal outcome. Ultrasound Obstet Gynecol 19(6):608–611
Kagan KO, Hamprecht K (2017) Cytomegalovirus infection in pregnancy. Arch Gynecol Obstet 296(1):15–26
Wang C et al (2011) Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis 52(2):e11–e13
Fowler KB, Stagno S, Pass RF (2003) Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 289(8):1008–1011
Revello MG et al (2014) A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 370(14):1316–1326
Nigro G et al (2005) Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 353(13):1350–1362
Kagan KO et al (2018) Prevention of maternal-fetal transmission of CMV by hyperimmunoglobulin (HIG) administered after a primary maternal CMV infectionin early gestation. Ultrasound Obstet Gynecol 78(10):FV43
Lanzieri TM et al (2014) Systematic review of the birth prevalence of congenital cytomegalovirus infection in develo** countries. Int J Infect Dis 22:44–48
Naing ZW et al (2016) Congenital cytomegalovirus infection in pregnancy: a review of prevalence, clinical features, diagnosis and prevention. Aust N Z J Obstet Gynaecol 56(1):9–18
Rutten H et al (2017) Congenital cytomegalovirus infection in Central Germany: an underestimated risk. Arch Gynecol Obstet 296(2):231–240
Kenneson A, Cannon MJ (2007) Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17(4):253–276
Daiminger A, Bader U, Enders G (2005) Pre- and periconceptional primary cytomegalovirus infection: risk of vertical transmission and congenital disease. BJOG 112(2):166–172
Bilavsky E et al (2016) Clinical implications for children born with congenital cytomegalovirus infection following a negative amniocentesis. Clin Infect Dis 63(1):33–38
Buxmann H et al (2017) Primary human cytomegalovirus (HCMV) infection in pregnancy. Dtsch Arztebl Int 114(4):45–52
Leruez-Ville M et al (2016) In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol 215(4):462e1–462e10
Seidel V et al (2017) Intrauterine therapy of cytomegalovirus infection with valganciclovir: review of the literature. Med Microbiol Immunol 206(5):347–354
Rawlinson WD et al (2017) Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis 17(6):e177–e188
Hamprecht K, Kagan K-O, Goelz R (2014) Hyperimmune globulin to prevent congenital CMV infection. N Engl J Med 370(26):2543
DVV and GfV (2014) Labordiagnostik schwangerschaftsrelevanter Virusinfektionen S2k-Leitlinie. http://www.awmf.org/uploads/tx_szleitlinien/093-001l_S2k_Labordiagnostik_schwangerschaftsrelevanter_Virusinfektionen_2014-05.pdf. 27 May 2016
Seidel V et al (2017) Auswirkung der zunehmenden CMV-Serodiagnostik in der Schwangerschaft. Zeitschrift Geburtshilfe Neonatol 221(S01):P04–5
Voigt M et al (2006) Analysis of the neonatal collective in the federal republic of Germany 12th report: presentation of detailed percentiles for the body measurement of newborns. Geburtsh Frauenheilk 66:956–970
Feldman B et al (2011) Pregestational, periconceptional, and gestational primary maternal cytomegalovirus infection: prenatal diagnosis in 508 pregnancies. Am J Obstet Gynecol 205(4):342.e1–6
van Leeuwen E, Oude Rengerink K, Pajkrt E (2014) Hyperimmune globulin to prevent congenital CMV infection. N Engl J Med 370(26):2543–2544
Adler SP (2012) Editorial commentary: primary maternal cytomegalovirus infection during pregnancy: do we have a treatment option? Clin Infect Dis 55(4):504–506
Enders G et al (2011) Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol 52(3):244–246
Lipitz S et al (2013) Risk of cytomegalovirus-associated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol 41(5):508–514
Picone O et al (2013) A series of 238 cytomegalovirus primary infections during pregnancy: description and outcome. Prenat Diagn 33(8):751–758
Faure-Bardon V et al (2018) Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis 69(9):1526–1532
Nigro G et al (2015) Primary maternal cytomegalovirus infections during pregnancy: association of CMV hyperimmune globulin with gestational age at birth and birth weight. J Matern Fetal Neonatal Med 28(2):168–171
Chiaie LD et al (2018) No evidence of obstetrical adverse events after hyperimmune globulin application for primary cytomegalovirus infection in pregnancy: experience from a single centre. Arch Gynecol Obstet 297(6):1389–1395
Acknowledgements
Open Access funding provided by Projekt DEAL.
Funding
None.
Author information
Authors and Affiliations
Contributions
VS: project development, data analysis, manuscript writing and editing; MH: data collection and management, manuscript editing; RCR: manuscript writing and editing; WH: Manuscript editing; JPS: project development and manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
VS, MH, RCR, and WH declare that they have no conflict of interest. JPS has served as local PI in a prospective randomized trial on Cytotect® conducted by Biotest AG, Dreieich Germany. In this position, he has received travel grants to a study meeting and a conference on CMV in 2016.
Ethical approval
The study had local ethics committee (EA2/135/17) approval. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All women who opted for HIG treatment gave informed written consent to off-label treatment. All women whose data were analyzed gave their written informed consent to study participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seidel, V., Hackelöer, M., Rancourt, R.C. et al. Fetal and maternal outcome after hyperimmunoglobulin administration for prevention of maternal–fetal transmission of cytomegalovirus during pregnancy: retrospective cohort analysis. Arch Gynecol Obstet 302, 1353–1359 (2020). https://doi.org/10.1007/s00404-020-05728-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05728-7