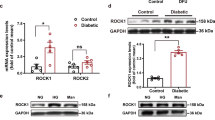
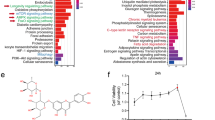
Abstract
The aim of this study is to delineate the expression patterns of prolyl cis-trans isomerase NIMA-interacting protein 1 (Pin1), Glial cell-derived neurotrophic factor (GDNF), and Angiotensin II (ANG II) during the process of wound repair, and to ascertain the effects of Pin1, GDNF, and ANG II on the healing of wounds in a rat model. A total of 18 rats were allocated into three groups—sham (control), DMSO (vehicle control), and Pin1 inhibitor (treatment with juglone)—with six animals in each group. An animal model of wound healing was established, followed by the intraperitoneal administration of juglone. Tissue samples from the wounds were subsequently collected for histopathological evaluation. Expression levels of Pin1, GDNF, and Ang II were quantified. In addition, an in vitro model of wound healing was created using human umbilical vein endothelial cells (HUVEC), to assess cell proliferation, migration, and tube formation under conditions of juglone pre-treatment. The expression levels of Pin1, GDNF, and ANG II were notably elevated on 7-, and 10- days post-wound compared to those measured on 3-day. Contrastingly, pre-treatment with juglone significantly inhibited the expression of these molecules. Histological analyses, including HE (Hematoxylin and Eosin), Masson’s trichrome, and EVG (Elastic van Gieson) staining, demonstrated that vascular angiogenesis, as well as collagen and elastin deposition, were substantially reduced in the juglone pre-treated group when compared to the normal group. Further, immunohistochemical analysis revealed a considerable decrease in CD31 expression in the juglone pre-treatment group relative to the normal control group. Pin1 serves as a pivotal facilitator of wound repair. The findings indicate that the modulation of Pin1, GDNF, and ANG II expression impacts the wound healing process in rats, suggesting potential targets for therapeutic intervention in human wound repair.







Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Dissemond J, Romanelli M (2022) Inflammatory skin diseases and wounds. Br J Dermatol 187:167–177
Mascharak S, Talbott HE, Januszyk M et al Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell Stem Cell 29: 315–327 e316, 2022.
Zhang M, Xu S, Du C et al (2023) Novel PLCL nanofibrous/keratin hydrogel bilayer wound dressing for skin wound repair. Colloids Surf B Biointerfaces 222:113119
Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ (2018) Wound healing. J Chin Med Assoc 81:94–101
Kogan S, Sood A, Garnick MS (2017) Zinc and Wound Healing: a review of Zinc Physiology and clinical applications. Wounds 29:102–106
Kumari A, Kumar C, Pergu R et al (2021) Phosphorylation and Pin1 binding to the LIC1 subunit selectively regulate mitotic dynein functions. J Cell Biol 220
Tonnus W, Belavgeni A, Beuschlein F et al (2021) The role of regulated necrosis in endocrine diseases. Nat Rev Endocrinol 17:497–510
Koikawa K, Kibe S, Suizu F et al Targeting Pin1 renders pancreatic cancer eradicable by synergizing with immunochemotherapy. Cell 184: 4753–4771 e4727, 2021.
Ranganathan R, Lu KP, Hunter T, Noel JP (1997) Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell 89:875–886
Ryo A, Liou YC, Lu KP, Wulf G (2003) Prolyl isomerase Pin1: a catalyst for oncogenesis and a potential therapeutic target in cancer. J Cell Sci 116:773–783
Wulf G, Finn G, Suizu F, Lu KP (2005) Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat Cell Biol 7:435–441
Feng D, Yao J, Wang G et al (2017) Inhibition of p66Shc-mediated mitochondrial apoptosis via targeting prolyl-isomerase Pin1 attenuates intestinal ischemia/reperfusion injury in rats. Clin Sci (Lond) 131:759–773
Risal P, Shrestha N, Chand L, Sylvester KG, Jeong YJ (2017) Involvement of prolyl isomerase PIN1 in the cell cycle progression and proliferation of hepatic oval cells. Pathol Res Pract 213:373–380
Lin CH, Li HY, Lee YC et al (2015) Landscape of Pin1 in the cell cycle. Exp Biol Med (Maywood) 240:403–408
Lu KP, Hanes SD, Hunter T (1996) A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380:544–547
Liou YC, Zhou XZ, Lu KP (2011) Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem Sci 36:501–514
Nakatsu Y, Matsunaga Y, Yamamotoya T et al Physiological and pathogenic roles of Prolyl Isomerase Pin1 in metabolic regulations via Multiple Signal Transduction Pathway Modulations. Int J Mol Sci 17: 2016
Chen M, **a Y, Tan Y, Jiang G, ** H, Chen Y (2018) Downregulation of microRNA-370 in esophageal squamous-cell carcinoma is associated with cancer progression and promotes cancer cell proliferation via upregulating PIN1. Gene 661:68–77
Zeng L, Luo S, Li X et al (2017) Functional PIN1 promoter polymorphisms associated with risk of nasopharyngeal carcinoma in Southern Chinese populations. Sci Rep 7:4593
Lee TH, Pastorino L, Lu KP (2011) Peptidyl-prolyl cis-trans isomerase Pin1 in ageing, cancer and Alzheimer disease. Expert Rev Mol Med 13:e21
Lv L, Ye M, Duan R et al (2018), : Downregulation of Pin1 in human atherosclerosis and its association with vascular smooth muscle cell senescence. J Vasc Surg 68: 873–883 e875,
Rizzolio F, Lucchetti C, Caligiuri I et al (2012) Retinoblastoma tumor-suppressor protein phosphorylation and inactivation depend on direct interaction with Pin1. Cell Death Differ 19:1152–1161
Rizzolio F, Caligiuri I, Lucchetti C et al (2013) Dissecting Pin1 and phospho-pRb regulation. J Cell Physiol 228:73–77
Tong Y, Ying H, Liu R, Li L, Bergholz J, **ao ZX (2015) Pin1 inhibits PP2A-mediated rb dephosphorylation in regulation of cell cycle and S-phase DNA damage. Cell Death Dis 6:e1640
Toko H, Konstandin MH, Doroudgar S et al (2013) Regulation of cardiac hypertrophic signaling by prolyl isomerase Pin1. Circ Res 112:1244–1252
Wu Y, Zhang M, Xu C, Chai D, Peng F, Lin J (2020) Anti-diabetic atherosclerosis by inhibiting high glucose-Induced Vascular smooth muscle cell proliferation via Pin1/BRD4 pathway. Oxid Med Cell Longev : 4196482, 2020
Mesalam AA, El-Sheikh M, Joo MD et al (2020) Induction of oxidative stress and mitochondrial dysfunction by Juglone affects the development of bovine oocytes. Int J Mol Sci 22
Moore JD, Potter A (2013) Pin1 inhibitors: pitfalls, progress and cellular pharmacology. Bioorg Med Chem Lett 23:4283–4291
Meng X, Lindahl M, Hyvonen ME et al (2000) Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287:1489–1493
Chen C, Ouyang W, Grigura V et al (2005) ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature 436:1030–1034
Meir M, Flemming S, Burkard N et al (2015) Glial cell line-derived neurotrophic factor promotes barrier maturation and wound healing in intestinal epithelial cells in vitro. Am J Physiol Gastrointest Liver Physiol 309:G613–624
Akwii RG, Sajib MS, Zahra FT, Mikelis CM (2019) Role of Angiopoietin-2 in vascular physiology and pathophysiology. Cells 8
Suri C, Jones PF, Patan S et al (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87:1171–1180
Maisonpierre PC, Suri C, Jones PF et al (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60
Matin M, Morgelin M, Stetefeld J et al (2020) Affinity-enhanced multimeric VEGF (vascular endothelial growth factor) and PlGF (placental growth factor) variants for specific adsorption of sFlt-1 to restore angiogenic balance in Preeclampsia. Hypertension 76:1176–1184
Kant V, Gopal A, Kumar D et al (2015) Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res 193:978–988
Zhou J, Zhang X, Liang P et al (2016) Protective role of microRNA-29a in denatured dermis and skin fibroblast cells after thermal injury. Biol Open 5:211–219
Hodde JP, Johnson CE (2007) Extracellular matrix as a strategy for treating chronic wounds. Am J Clin Dermatol 8:61–66
Shyu KG, Wang BW, Pan CM, Fang WJ, Lin CM (2019) Hyperbaric oxygen boosts long noncoding RNA MALAT1 exosome secretion to suppress microRNA-92a expression in therapeutic angiogenesis. Int J Cardiol 274:271–278
Mahmoud AA, Salama AH (2016) Norfloxacin-loaded collagen/chitosan scaffolds for skin reconstruction: Preparation, evaluation and in-vivo wound healing assessment. Eur J Pharm Sci 83:155–165
Acknowledgements
We thank Professor Chen Gang for the financial support of this study and Li **ang for technical support.
Ethics declarations
Ethical approval
Approval for the study was granted by the Ethics Committee of the First Affiliated Hospital of Soochow University, ensuring adherence to ethical guidelines for animal research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, QX., Zhuang, QS. & Shen, GL. Expression and significance of pin1 in the wound healing. Arch Dermatol Res 316, 235 (2024). https://doi.org/10.1007/s00403-024-03030-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00403-024-03030-z