Abstract
Purpose
Although lateral lymph node dissection has been performed to prevent lateral pelvic recurrence in locally advanced lower rectal cancer, the incidence of lateral pelvic recurrence after this procedure has not been investigated. Therefore, this study aimed to investigate the long-term outcomes of patients who underwent lateral pelvic lymph node dissection, with a particular focus on recurrence patterns.
Methods
This was a retrospective study conducted at a single high-volume cancer center in Japan. A total of 493 consecutive patients with stage II-III rectal cancer who underwent lateral lymph node dissection between January 2005 and August 2022 were included. The primary outcome measures included patterns of recurrence, overall survival, and relapse-free survival. Patterns of recurrence were categorized as lateral or central pelvic.
Results
Among patients who underwent lateral lymph node dissection, 18.1% had pathologically positive lateral lymph node metastasis. Lateral pelvic recurrence occurred in 5.5% of patients after surgery. Multivariate analysis identified age > 75 years, lateral lymph node metastasis, and adjuvant chemotherapy as independent risk factors for lateral pelvic recurrence. Evaluation of the recurrence rate by dissection area revealed approximately 1% of recurrences in each area after dissection.
Conclusion
We demonstrated the prognostic outcome and limitations of lateral lymph node dissection for patients with advanced lower rectal cancer, focusing on the incidence of recurrence in the lateral area after the dissection. Our study emphasizes the clinical importance of lateral lymph node dissection, which is an essential technique that surgeons should acquire.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Local recurrence reportedly develops in 4–15% of patients with locally advanced rectal cancer who undergo R0 tumor resection [1,2,3,4]. Preoperative chemoradiotherapy (CRT) has been widely used to reduce local recurrence, and favorable local control has been reported [5,6,7]. However, in some cases with lateral lymph node (LLN) metastasis, CRT has been proven insufficient for local control, and some studies have demonstrated that the addition of lateral lymph node dissection (LLND) to conventional total mesorectal excision (TME), even in cases of preoperative CRT, contributes to better local control [8, 9].
LLND, a systemic dissection of lymph nodes in the pelvic sidewall, such as those in the obturator or internal iliac areas, has been performed to reduce local recurrence as the standard therapy for locally advanced rectal cancer in Japan [10]. The concept and technique of LLND were originally developed in Western countries [11, 12]. However, in 1959, Stearns and Deddish reported discouraging results, noting high morbidity and mortality without oncological benefit [13]. Since then, LLND has been regarded as an ineffective procedure and was abandoned in Western countries. However, in recent years, the number of institutions performing LLND on patients with suspected LLN metastasis has increased even in Western countries, underscoring the growing importance of understanding the prognostic outcome after LLND. Nevertheless, the detailed incidence of lateral pelvic recurrence (LPR) after LLND has not yet been fully investigated.
In the present study, we aimed to investigate the long-term outcomes of patients who underwent LLND using a large retrospective cohort, with a particular focus on recurrence patterns.
Materials and methods
Patients
The medical records of 493 consecutive patients with clinical stage II–III rectal cancer who underwent TME and LLND between January 2005 and August 2022 were retrospectively reviewed. Tumor progression, location, and the presence of LLN metastasis were evaluated using imaging modalities such as colonoscopy, computed tomography (CT), and magnetic resonance imaging in some cases. The clinicopathological features of the patients were determined from their medical records, and tumor features and stages were classified according to the TNM classification system [14]. Previous cases were reclassified according to the latest edition of the TNM classification, the eighth edition. In cases where the lower edge of the tumor was located below the peritoneal reflection and invasion beyond the proper muscle was suspected, TME plus bilateral LLND was performed regardless of the presence or absence of lymph node metastases in the preoperative diagnosis. Unilateral lymph node dissection was performed if the distal edge of the tumor was above peritoneal reflection (PR) but LLN metastasis was suspected. There were also cases in the early years of the study in which unilateral dissection was performed based on the predominant location of the tumor, due to old age and poor general medical condition (e.g., right-sided dissection in the case of right wall lesions). This retrospective study was approved by the Institutional Ethics Committee of Komagome Hospital (no.3130), and consent was obtained from the participating patients in the form of an opt-out.
Procedure
This was a retrospective cohort study. The collected variables included age, sex, body mass index, Information on surgical procedures, tumor size, histological subtype, stage, chemotherapy, recurrence site, and survival time. Only data available for all the cohort were statistically analyzed to reduce the impact of information bias. The endpoint of this study was LPR. All the patients underwent radical surgery, including TME with bilateral or unilateral LLND without preoperative treatment. We defined LLND as complete dissection of the lymph nodes, at least in the internal iliac and obturator areas. The final stage was determined by examining the resected specimens according to the eight edition of the TNM classification for colorectal cancer [14]. Adjuvant chemotherapy was administered to patients with pathological stage III disease. Recurrence was monitored by regular examinations, including office visits and tumor marker assays every 3–6 months, colonoscopy every 1–2 years, and CT every 6 months. Postoperative surveillance was performed for 5 years according to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines [10]. Local recurrence was diagnosed radiologically or histologically. Local recurrence was categorized into LPR and central pelvic recurrences (CPR).
Statistical analysis
Continuous variables were presented as means and standard deviations or medians and ranges, whereas categorical variables were presented as frequencies and percentages. The Fisher’s exact test or t-test was used to compare differences between groups, as appropriate. Overall survival (OS) and relapse-free survival (RFS) were analyzed using the Kaplan–Meier method and compared between the groups using log-rank tests. RFS was defined as the time interval between LLND and disease recurrence or death. OS was defined as the interval between LLND and death from any cause. Univariate and multivariate analyses of the lateral pelvic wall recurrence were performed using the Cox proportional hazards model. Multivariate analysis was performed using all variables with a p-value of < 0.10 in univariate analysis. Statistical significance was set at p < 0.05. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, version 3.5.1). This interface is a modified version of R commander (version 2.5–1), which includes statistical functions that are frequently used in biostatistics.
This study adheres to the STROBE reporting recommendations of the Equator Network.
Results
Patient background
Patient characteristics are shown in Table 1. The edge of the tumor was below PR in 99.6% of the patients. LLND was performed bilaterally in 90.1% of the patients and unilaterally in 9.9%. There were 89 patients (18.1%) that had pathologically positive lateral lymph nodes. Postoperative adjuvant chemotherapy was administered to 51.4% of the patients.
Oncological outcome
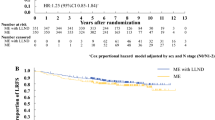
The median follow-up was 81.2 months (range 4–212.9 months). Figure 1 shows the RFS (a) and OS (b). The 5-year RFS rates were 33.1% and 72.3% in patients with and without pathological LLN metastases, respectively (p < 0.01). The 5-year OS rates were 50.0% and 84.9% for patients with and without pathological LLN metastases, respectively (p < 0.01).
LPR after LLND
Local recurrences developed in 97 patients, with a 5-year cumulative incidence of 20.1% (Fig. 1c), and LPR and CPR were observed in 27 and 85 patients after LLND, with 5-year cumulative incidence of 6.7% and 17.4%, respectively (Fig. 1d).
Univariate and multivariate analyses for the risk of LPR are shown in Table 2. In the univariate analysis, seven variables showed a significant correlation with LPR. Multivariate analysis showed that three of these variables were independently associated with LPR: age > 75 years, LLN metastasis, and adjuvant chemotherapy.
The locations and percentages of local recurrences are shown in Fig. 2. The internal iliac (A) and obturator (B) areas were routinely dissected because of the high frequency of metastases. Systematic complete lymph node dissection was performed in 93.4% of area A and 93.4% of area B, although not in all patients because of unilateral dissection in some cases (Fig. 2a). Recurrence rates after LLND in each area are presented in Fig. 2b. Because there were cases of unilateral dissection, the frequencies were calculated based on the number of cases with dissection on each side. On the right side, the incidence of recurrence was 1.3%, 0.9%, 0.9%, and 1.5% in areas A, B, C, and D, respectively. On the left side, the incidence of recurrence was 2.2%, 1.7%, 2.4%, and 1.5% in areas A, B, C, and D, respectively. A CPR of 17.2% was observed in all patients.
Percentage of the dissected lateral pelvic areas and incidence of recurrence in each area. a Proportion of the patients who underwent complete removal of the lymph nodes in each area. A: internal iliac, B: obturator, C: external iliac, and D: common iliac nodes. b The distribution and frequency of local recurrences are shown. In the lateral areas, the percentage of patients who developed recurrence in each area (area ABCD) among those who underwent a complete dissection of the area is presented. In the central pelvis, the percentage of patients who developed central pelvic recurrence is presented because all patients underwent total mesorectal excision
Case presentation
A representative case of local recurrence after LLND is shown (Fig. 3). A 68-year-old woman with lower rectal cancer underwent abdominoperineal resection and bilateral LLND without preoperative treatment. The final pathological diagnosis revealed stage I (T2N0M0) with the circumferential margin negative, and no metastasis was present in the dissected lateral lymph nodes on both the right and left sides. The preoperative CT image is shown in Fig. 3a, and the first postoperative CT image is shown in Fig. 3b. During surveillance without adjuvant chemotherapy, lymph node recurrence in the right obturator area was observed using CT at 24 months after surgery (Fig. 3c).
Discussion and conclusions
This study is the first comprehensive examination of recurrence after LLND without prior treatment, utilizing a substantial cohort of patients with advanced lower rectal cancer. In this study, TME plus bilateral LLND was performed in 90% of cases because this was the standard procedure in Japan during the study period and is recommended in the Japanese treatment guidelines for colorectal cancer published in 2005 [15]. Because preoperative treatments for rectal cancer, such as CRT or total neoadjuvant therapy, are not recommended in the guidelines, preoperative treatment was performed none of the patients in the present study.
We initially evaluated the prognosis of patients with LLND. Among patients who underwent LLND for Stage II/III rectal cancer, 20% had positive LLN metastases, and the 5-year RFS and OS rates were 33.1% and 50.0%, respectively. In previous studies, the 3-year OS and RFS rates in patients with rectal cancer and LLN metastasis were reportedly 37–60% and 37–39%, respectively, similar to our results. Although the prognosis of patients with LLN metastasis is poor, approximately half of the patients survived for 5 years without an impaired quality of life due to increased metastasis in the pelvic sidewall by the additional LLND.
In the large-scale randomized trial JCOG0212, which investigated the impact of LLND in a cohort without obvious LLN metastasis without pretreatment, recurrence in the pelvic sidewall decreased from 7 to 2% with the addition of LLND [13]. The lateral recurrence rate after LLND was 7% in the present study, which was higher than that reported by JCOG0212, probably because our cohort included many cases with suspected LLN metastasis in preoperative imaging modalities. In our multivariate analysis, the presence of LLN metastasis was a risk factor for lateral recurrence, probably due to residual micrometastasis in the dissected or undissected lateral area, such as the common iliac nodes. The results of JCOG0212 demonstrated that LLND decreased lateral recurrence but not central recurrence [16], whereas CRT without LLND has been reported to decrease local recurrence in total; however, lateral recurrence also reportedly remained a major local recurrence site even after CRT [17, 18]. To compensate for the drawbacks of these treatments, a combination of CRT and with LLND in selected patients has recently been considered a novel treatment option. Several studies have demonstrated favorable outcomes with this combination strategy [17, 19, 20].
This is the first study to investigate the site of lateral recurrence after LLND. As shown in Fig. 2, the recurrence rate in the dissected area was approximately 1%, regardless of the location. The overall lateral recurrence rate was 5%, and this discrepancy was partly due to the recurrence in undissected lateral areas. In this study, dissection of the internal iliac and obturator nodes was routinely performed. However, other lateral nodes, including the external and common iliac nodes, were dissected only in cases with suspected metastases in these areas. Considering the relatively low incidence of metastasis in these areas [21, 22], the complexity of dissecting these areas [23], and the fact that LLN metastasis in those area has a poor prognosis equivalent to systemic disease [24, 26,27,28]. The use of indocyanine green (ICG) fluorescence in robotic LLND has been reported to result in a 3-year cumulative lateral recurrence rate of 0% [29], suggesting that the outcomes after LLND in our series could have been improved by these techniques because our series included a relatively small number of robotic cases and no cases of ICG application.
The present study had several limitations. First, the study period was relatively long and, therefore, included a wide range of treatments, such as open, laparoscopic, or robotic surgery, and different indications for adjuvant chemotherapy. Because the majority of LLND are performed robotically, further updates of prognostic outcomes are necessary in the future. Second, the quality of LLND largely differs according to the technique used at each institution. As this study was conducted in a Japanese high-volume cancer center in which LLND was routinely performed, the outcomes in other institutions or countries that are not so accustomed to LLND could not be as good.
In conclusion, we demonstrated the prognostic outcomes and limitations of LLND for patients with advanced lower rectal cancer, focusing on the incidence of recurrence in the lateral area after LLND. LLND is an essential technique that surgeons should acquire.
Data availability
No datasets were generated or analysed during the current study.
References
Enker WE, Havenga K, Polyak T, Thaler H, Cranor M (1997) Abdominoperineal resection via total mesorectal excision and autonomic nerve preservation for low rectal cancer. World J Surg 21:715–720. https://doi.org/10.1007/s002689900296
Ishikura S, Ogino T, Ono M, Arai T, Sugito M, Kawashima M, Imai M, Ito Y, Ikeda H (1999) Preliminary results of pelvic autonomic nerve-preserving surgery combined with intraoperative and postoperative radiation therapy for patients with low rectal cancer. Jpn J Clin Oncol 29:429–433. https://doi.org/10.1093/jjco/29.9.429
Enker WE (1997) Total mesorectal excision—the new golden standard of surgery for rectal cancer. Ann Med 29:127–133. https://doi.org/10.3109/07853899709113698
MacFarlane JK, Ryall RDH, Heald RJ (1993) Mesorectal excision for rectal cancer. Lancet 341:457–460. https://doi.org/10.1016/0140-6736(93)90207-w
Swedish Rectal Cancer Trial (1993) Initial report from a Swedish multicentre study examining the role of preoperative irradiation in the treatment of patients with resectable rectal carcinoma. Br J Surg 80:1333–1336. https://doi.org/10.1002/bjs.1800801040
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC, EORTC Radiotherapy Group Trial 22921 (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123. https://doi.org/10.1056/NEJMoa060829
Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumbar M, Enker W, Paty PB, Weiser MR, Klimstra D, Saltz L, Minsky BD, Wong WD (2005) Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg 241:829–836; discussion 36–38. https://doi.org/10.1097/01.sla.0000161980.46459.96
Malakorn S, Yang Y, Bednarski BK, Kaur H, You YN, Holliday EB, Dasari A, Skibber JM, RodriguezBigas M, Chang GJ (2019) Who should get lateral pelvic lymph node dissection after neoadjuvant chemoradiation? Dis Colon Rectum 62:1158–1166. https://doi.org/10.1097/DCR.0000000000001465
Yang X, Yang S, Hu T, Gu C, Wei M, Deng X, Wang Z, Zhou Z (2020) What is the role of lateral lymph node dissection in rectal cancer patients with clinically suspected lateral lymph node metastasis after preoperative chemoradiotherapy? A meta-analysis and systematic review. Cancer Med 9:4477–4489. https://doi.org/10.1002/cam4.2643. Epub 2020 Apr 30
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanematsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K (2020) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25:1–42. https://doi.org/10.1007/s10147-019-01485-z. Epub 2019 Jun 15
Gerota D (1895) Die Lymphgefasse des Rectums und des Anus. Arch Anat Physiol 7:240–256
Sauer I, Bacon HE (1951) Influence of lateral spread of cancer of the rectum on radicability of operation and prognosis. Am J Surg 81:111–120. https://doi.org/10.1016/0002-9610(51)90196-1
Stearns MW Jr, Deddish MR (1959) Five-year results of abdominopelvic lymph node dissection for carcinoma of the rectum. Dis Colon Rectum 2:169–172. https://doi.org/10.1007/BF02616711
UICC (2017) TNM classification of malignant tumours, 8th edn. John Wiley & Sons Ltd, New York
Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishihara S, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Boku N, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K (2015) Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20:207–239. https://doi.org/10.1007/s10147-015-0801-z. Epub 2015 Mar 18
Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shinozawa M, Yamaguchi T, Bandou H, Katsumata K, Murata K, Akagi Y, Takiguchi N, Saida Y, Nakamura K, Fukuda H, Akasu T, Moriya Y (2017) Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): a multicenter, randomized controlled, noninferiority trial. Ann Surg 266:201–207. https://doi.org/10.1097/SLA.0000000000002212
Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee P, Putter H, Velde CJH, Beets GL, Rutten HJ, Kusters M (2019) Neoadjuvant (chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol 37:33–43. https://doi.org/10.1200/JCO.18.00032. Epub 2018 Nov 7
Ogura A, Konishi T, Beets GL, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee P, Putter H, Velde CJH, Rutten HJT, Tuynman JB, Kusters M (2019) Lateral nodal features on restaging magnetic resonance imaging associated with lateral local recurrence in low rectal cancer after neoadjuvant chemoradiotherapy or radiotherapy. JAMA Surg 154:e192172. https://doi.org/10.1001/jamasurg.2019.2172. Epub 2019 Sep 18
Kawai K, Shiratori H, Hata K, Nozawa H, Tanaka T, Nishizawa T, Murono K, Ishihara S (2021) Optimal size criteria for lateral lymph node dissection after neoadjuvant chemoradiotherapy for rectal cancer. Dis Colon Rectum 64:274–283. https://doi.org/10.1097/DCR.0000000000001866
Ishihara S, Kawai K, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Morikawa T, Watanabe T (2017) Oncological outcomes of lateral pelvic lymph node metastasis in rectal cancer treated with preoperative chemoradiotherapy. Dis Colon Rectum 60:469–476. https://doi.org/10.1097/DCR.0000000000000752
Chen Z, Sasaki K, Murono K, Kawai K, Nozawa H, Kobayashi H, Ishihara S, Sugihara K (2022) Oncologic status of obturator lymph node metastases in locally advanced low rectal cancer. Ann Surg Oncol. https://doi.org/10.1245/s10434-022-11372-z. Online ahead of print
Akiyoshi T, Watanabe T, Miyata S, Kotake K, Muto T, Sugihara K (2012) Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg 255:1129–1134. https://doi.org/10.1097/SLA.0b013e3182565d9d
Liang JT (2011) Technical feasibility of laparoscopic lateral pelvic lymph node dissection for patients with low rectal cancer after concurrent chemoradiation therapy. Ann Surg Oncol 18:153–159. https://doi.org/10.1245/s10434-010-1238-2. Epub 2010 Aug 13
Ishibe A, Watanabe J, Suwa Y, Suzuki S, Nakagawa K, Suwa H, Ozawa M, Ota M, Fujii S, Ike H, Ichikawa Y, Endo I (2021) Oncological outcomes of lateral lymph node dissection (LLND) for locally advanced rectal cancer: is LLND alone sufficient? Int J Colorectal Dis 36:293–301. https://doi.org/10.1007/s00384-020-03760-2. Epub 2020 Sep 23
Zhou S, Tang J, Liang J, Lou Z, Fu W, Feng B, Yang Y, **ao Y, Liu Q (2022) (2022) Effective dissecting range and prognostic significance of lateral pelvic lymph node dissection for middle-low rectal cancer patients with lateral pelvic lymph node metastasis: results of a large multicenter lateral node collaborative group in China. Front Oncol 12:916285. https://doi.org/10.3389/fonc.2022.916285.eCollection
Bae JH, Song J, Yoo RN, Kim JH, Kye BH, Lee IK, Cho HM, Lee YS (2023) Robotic lateral pelvic lymph node dissection could harvest more lateral pelvic lymph nodes over laparoscopic approach for mid-to-low rectal cancer: a multi-institutional retrospective cohort study. Biomedicines 11:1556. https://doi.org/10.3390/biomedicines11061556
Ishizaki T, Mazaki J, Kasahara K, Udo R, Tago T, Nagakawa Y (2023) Robotic versus laparoscopic approach for minimally invasive lateral pelvic lymph node dissection of advanced lower rectal cancer: a retrospective study comparing short-term outcomes. Tech Coloproctol 27:579–587. https://doi.org/10.1007/s10151-023-02818-x. Epub 2023 May 8
Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y, Furutani A, Manabe S, Yamaoka Y, Hino H (2018) Oncological outcomes of robotic-assisted laparoscopic versus open lateral lymph node dissection for locally advanced low rectal cancer. Surg Endosc 32:4498–4505. https://doi.org/10.1007/s00464-018-6197-x. Epub 2018 May 2
Watanabe J, Ohya H, Sakai J, Suwa Y, Goto K, Nakagawa K, Ozawa M, Ihibe A, Suwa H, Kunisaki C, Endo I (2023) Long-term outcomes of indocyanine green fluorescence imaging-guided laparoscopic lateral pelvic lymph node dissection for clinical stage II/III middle-lower rectal cancer: a propensity score-matched cohort study. Tech Coloproctol 27:759–767. https://doi.org/10.1007/s10151-023-02761-x. Epub 2023 Feb 11
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Misato Takao: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Writing – original draft. Kazushige Kawai: Methodology, Supervision, Writing – review & editing. Daisuke Nakano: Methodology, Writing – review & editing. Akira Dejima: Methodology, Writing – review & editing. Sakiko Nakamori: Methodology, Writing – review & editing, Soichiro Natsume: Methodology, Writing – review & editing, Ichiro Ise: Methodology, Writing – review & editing, Hiroki Kato: Methodology, Writing – review & editing, Tatsuro Yamaguchi: Methodology, Validation, Writing – review & editing. All authors approved the final version of the manuscript to be published and agreed to be accountable for all aspects of the study to ensure that questions related to the accuracy and integrity of any part of the study are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by an institutional review board.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takao, M., Kawai, K., Nakano, D. et al. Recurrence of rectal cancer on the pelvic sidewall after lateral lymph node dissection. Int J Colorectal Dis 39, 80 (2024). https://doi.org/10.1007/s00384-024-04650-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-024-04650-7