Abstract
Purpose
To evaluate the safety and efficacy of botulinum-A toxin injections into the bulbospongiosus muscle for cases of lifelong drug-resistant premature ejaculation (PE).
Methods
Ninety-eight outpatients diagnosed with lifelong PE were randomly assigned to two groups: the botulinum-A toxin group comprising forty-nine patients and the placebo (saline) group also consisting of forty-nine patients. A 100 U botulinum-A toxin was diluted into 10 cc of saline, with 5 cc injected into one side of the muscle (botulinum-A toxin group) guided by ultrasound to distribute across most muscle fibers. The same technique was applied using the same volume of saline injected into the bulbospongiosus muscle. Intravaginal ejaculatory latency time (IELT), scores from the premature ejaculation profile (PEP), Premature Ejaculation Diagnostic Tool (PEDT), International Index of Erectile Function (IIEF), and recording of any complications were obtained. Follow-ups occurred at 1-, 3-, and 6-month post-procedure.
Results
Cases receiving injections of botulinum-A toxin into the bulbospongiosus muscle showed notably extended intravaginal ejaculatory latency times compared to their initial performance after treatment. In addition, there were enhancements in PEP scores, and notably, no significant complications were reported. Conversely, the bilateral injection of saline into the bulbospongiosus muscle did not demonstrate any impact on ejaculation latencies.
Conclusion
Our study demonstrated that the injection of botulinum-A toxin into the bulbospongiosus muscle can serve as a safe and effective option for treating PE. Nonetheless, its clinical application warrants further studies involving larger sample sizes and longer follow-up periods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early ejaculation is very prevalent in males, as seen by reports [1], which place its incidence between 20 and 30%. Male sexual dysfunction known as lifelong premature ejaculation (PE) is characterized by recurrent or consistent ejaculation occurring within 1 min of vaginal penetration. This syndrome may lead to negative personal consequences such as distress, irritation, frustration, and even a reluctance to engage in sexual intimacy [2]. Although the exact etiology of PE remains unknown, it is thought to stem from a neurological pathway [3].
It is hypothesized that pharmacotherapy should be the treatment of choice for those with chronic PE [4, 5]. Unfortunately, at this time, the US Food and Drug Administration (FDA) has not granted approval to any drugs for the treatment of PE. On the other hand, dapoxetine, an SSRI characterized by its short half-life, has been authorized on-demand in over 50 countries for the management of PE [6, 7].
Botulinum-A toxin inhibits neuronal transmission via intramuscular administration by selectively blocking the release of acetylcholine from nerve terminals [8, 9]. Botulinum-A toxin has been shown to be an efficient and safe treatment for two urological disorders, namely Detrusor sphincter dysynergia and neurogenic detrusor overactivity [10, 11]. Involved in the ejaculatory reflex, local injection of botulinum-A can suppresses muscular contractions in the bulbospongiosus muscle [12, 13]. The concept of prescribing botulinum-A toxin to inhibit stereotyped rhythmic contractions of the bulbospongiosus muscle as a permanent treatment for PE was initially proposed in 2010 [13].
The aim of this study was to assess the safety and efficacy of botulinum-A toxin injection into the bulbospongiosus muscle for treating lifelong drug-resistant PE.
Methods
In this study, which was prospective, randomized, double-blind, and placebo-controlled, 98 individuals from Benha University Hospitals’ outpatient clinics participated between October 2022 and September 2023. These participants were split into two cohorts: the botox group comprising 49 cases and the saline (placebo) group with an equal number of 49 cases. Before the study commenced, all participants provided informed written consent in accordance with the Declaration of Helsinki. In addition, the study received approval from the local ethics committee at the Faculty of Medicine, Benha University, marked as ethically approved under “Ms 35-10-2022.”
Before the procedure, patients were assured and informed that BOTOX is a material under trial with no major complications. Inclusion criteria were heterosexually active men experiencing lifelong PE, aged 20–50, and having failed prior medical treatments (behavioral therapy, SSRIs, topical anesthetic agents). These patients were evaluated based on the intravaginal ejaculatory latency time (IELT) (less than 1 min) and International Index of Erectile Function (IIEF) (26 points or more) to ensure good erection status. Exclusion criteria include patients with erectile dysfunction (ED) (IIEF less than 26 points), previous urethral operations, prostatitis (LUTS), diabetes (DM), pelvic operations, neurological diseases, chronic psychological illness and antipsychotic medications. Each participant underwent a comprehensive medical and sexual history assessment.
Evaluation criteria consisted of the intravaginal ejaculatory latency time (IELT), where a normal duration ranged from 2.5 to 5 min; scores from the premature ejaculation profile (PEP) [14]; the Premature Ejaculation Diagnostic Tool (PEDT) [15], with scores of 11 or higher indicating common occurrence of PE, 9 or 10 indicating a “borderline” score, and 8 or lower suggesting the absence of PE; and erectile function assessed by the IIEF [16]. On the Visual Analog Scale (VAS), pain was rated from zero (indicating no discomfort) to ten (intolerable pain).
Throughout the research, two participants in the botox group and four in the saline group were lost to follow-up and subsequently excluded from the study. Prior to the procedure, random allocation took place using sealed envelopes containing either BOTOX or saline assignments, which were opened exclusively before the commencement of the procedure (Fig. 1).
The procedure
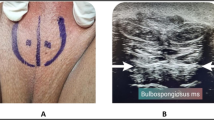
Patients were positioned in lithotomy position, the perineal area was disinfected with 70% alcohol, and the bulbospongiosus muscle was identified using ultrasound (6–13 Hz superficial probe with MAX depth 6 cm).
Botox group: Preparation for the BOTOX group involved diluting 100 U of botulinum-A toxin (Allergan, Ona botulinum toxin-A) into 10 mL of saline. Botox vial injection was administered as 5 mL into one side of the muscle, guided by ultrasound to distribute across most muscle fibers.
The placebo group: This group underwent a comparable procedure, differing only in the substance injected, as they were administered saline (10 mL) at identical injection sites.
To ensure blindness regarding the material used, syringes were concealed with plaster tape, maintaining this blindness for both the patient and the specialist involved. A specialist performed the injections while remaining unaware of the allocation.
Follow-up
Patients underwent evaluations at 1, 3, and 6 months following the injection. Assessments included the IELT, scores from the PEP, the PEDT, changes in erection (IIEF), and the recording of any complications.
Statistical analysis
The study employed Gpowersoft software version 3.1.7.9 to calculate the sample size based on Serefoglu et al.’s (2014) study, determining a minimum of 90 participants with a significance level of 0.05 and a type II error of 0.2. Subsequently, data collected were tabulated and statistically analyzed using SPSS software version 26.0 and Microsoft Excel 2016. Qualitative data were presented in numbers and percentages, while the Shapiro–Wilk test confirmed the normality of distribution. Quantitative data were described in terms of range, mean, standard deviation, median, and interquartile range (IQR), with a significance level set at 5%. Statistical tests included Friedman’s test for comparisons among non-parametric datasets across different time points and the Wilcoxon signed-rank test for analyzing related non-parametric samples. Interpretations were made based on significance levels: P ≥ 0.05 indicated non-significance (NS), P < 0.05 indicated significance (S), and P < 0.01 indicated high significance (HS).
Results
As illustrated in Table 1, all baseline characteristics, encompassing age, BMI, medical history, and premature ejaculation duration, demonstrated comparability across the studied groups (P value >0.05). In the BOTOX group, the IELT exhibited a significant increase (70.00 ± 47.64 s) after 1 month, as it was tripled compared to the pre-injection score (35.79 ± 14.23 s), and a onefold increase after 3 months (60.36 ± 40.08 s). However, insignificant changes were observed at 6 months (42.64 ± 30.35 s) in comparison to the pre-injection score. Although these fore mentioned improvement were statistically significant, yet these outcomes were clinically insufficient; however, the enrolled patients were happy enough to finally experience some increase in their latency time after years of trying other treatment options specially SSRIs with no obvious improve. Despite of this short time improvement, many patients were motivated to repeat the injection another time as they found these injection cycles more cost-effective than the long-term chronically used medication they have tried before without satisfactory results specially that we were concerned to evaluate the cost-effective aspects of this trail as our facility is dealing with patients of lower socioeconomic levels. Conversely, patients who received saline injections showed only a 0.5% insignificant improvement, possibly attributable to the placebo effect, and reverted to baseline satisfaction scores during subsequent follow-up visits, as depicted in Table 2.
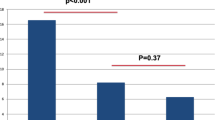
The scores from the PEDT questionnaire and the PEP significantly improved after 1 month (13.19 ± 3.84 and 8.36 ± 4.20) and also after 3 months ( 14.23 ± 2.88 and 7.19 ± 3.43) compared to the pre-injection score ( 16.11 ± 1.77 and 5.49 ± 2.17), respectively. However, there were no significant changes observed at 6 months (15.49 ± 2.87 and 6.11 ± 3.27) compared to the pre-injection score (16.11 ± 1.77 and 5.49 ± 2.17) for PEDT and PEP, respectively, as detailed in Table 2. Further analysis of the Botox effect revealed that 44.7% of patients showed improvement after 1 and 3 months, reducing to only 8.5% after 6 months, as indicated in Table 3. Regarding the IIEF score, no changes in erection were noted between pre- and post-injection IIEF (P value >0.05), as displayed in Table 2.
The saline group did not experience significant changes in IELT, PEP, or the score from the PEDT in comparison to baseline values (Table 2). In terms of treatment-induced pain, there was no notable difference in the VAS score between the botox and control groups (4.51 ± 1.46 vs. 4.11 ± 1.43, respectively), Table 3.
Post-injection complications were reported in four cases (8.5%) of the study group. Among these, two patients (4.3%) experienced post-micturition dribbling, one developed an infection managed with appropriate antibiotics, and one patient required NSAIDs for 1 day due to pain, detailed in Table 3.
Discussion
Premature ejaculation stands as one of the most prevalent sexual dysfunctions globally [17]. Around 30% of men between 18- and 59-years old report experiencing premature ejaculation issues, although some studies suggest a prevalence as high as 75% [18, 19]. Topical anesthetics applied to the penis have shown some success and offer an alternative to SSRIs, effectively avoiding potential systemic side effects [20]. SSRIs are typically the primary medical treatment for most premature ejaculation cases, even though they are commonly used off-label for treating both primary and secondary premature ejaculation cases [21]. As it is one of the theories proposed to explain the whole process of ejaculation, the primary role of bulbospongiosus and ischiocavernosus muscles were investigated as a part of a spinal cord reflex ending with rhythmic contractions of these muscles that facilitate semen propulsion throughout the urethra during the ejaculation phase [13, 22]. Botulinum-A toxin acts as a selective acetylcholine release blocker, impeding neural transmission upon injection into muscles and based on that, it has been used for years to treat a wide range of medical conditions related to muscle spasms and neurological insults such as blepharospasm, strabismus, detrusor overactivity, and detrusor sphincteric dyssynergia [13]. Based on that assumption, previous study was published to evaluate the potential role of BOTOX injection in theses muscles aiming to improve the ejaculation time [13]. Similarly, the hypothesis of BOTOX injection in bulbospongiosus muscles was proposed as a potential management option for patient with refractory PE [13, 23, 24].
Our study delves into the effect of BOTOX injections into the bulbospongiosus muscle in patients with treatment-resistant premature ejaculation. While animal studies have been conducted to assess the impact of BOTOX injections in this muscle on ejaculation latency [23, 24], there have been similar human studies limited by small group sizes and short-term outcomes [25]. For instance, Ongün et al. [23] reported significant improvements in ejaculatory latency in rats injected with botulinum toxin in the bulbospongiosus muscle, demonstrating longer ejaculation times compared to controls. Similar findings were observed by Serefoglu et al. [24], showing significantly longer ejaculatory latencies in rats treated with botulinum-A toxin.
Our recent study, a double-blind, randomized, placebo-controlled investigation guided by ultrasound during injection, demonstrated substantial improvement in IELT, PEP, and PEDT at 1- and 3-month post-BOTOX injection. However, there was no significant change observed at 6 months compared to pretreatment. Interestingly, only 0.5% of participants in the saline group exhibited negligible improvement, likely influenced by the placebo effect, reverting to baseline satisfaction scores in subsequent follow-up visits. Our findings align with Li et al.’s study [25], indicating longer IELT post-BOTOX injection compared to controls. In addition, our study revealed significant improvements in PEP and PEDT scores post-injection.
The effectiveness rate in our study after 1 month was 44.7%, akin to Li et al. [25], who demonstrated an effectiveness rate of 47.06%. However, our study showed a decline in effectiveness to 8% after 6 months, indicating a reduced effect over time. Regarding adverse reactions, Li et al. [25] reported complications in six cases (17.65%) in the trial group, including decreased erectile hardness and incomplete urination. Conversely, our study reported complications in four cases (8.5%) of the study population, such as post-micturition dribbling, infection managed with antibiotics, and transient pain requiring NSAIDs.
Nevertheless, limitations of this study, such as the small sample size, single-center approach, and short follow-up duration, need acknowledgment.
Conclusion
Administering botulinum-A toxin into the bulbospongiosus muscle demonstrates both safety and efficacy as a treatment for lifelong PE. However, its clinical implementation requires additional exploration via studies that encompass larger sample sizes and more extended follow-up periods.
Data availability
Data available upon reasonable request from the authors.
References
Giuliano F, Clement P (2005) Physiology of ejaculation: emphasis on serotonergic control. Eur Urol 48:408–417
Alwaal A, Breyer BN, Lue TF (2015) Normal male sexual function: emphasis on orgasm and ejaculation. Fertil Steril 104(5):1051–1060. https://doi.org/10.1016/j.fertnstert.2015.08.033
Lewis RW, Fugl-Meyer KS (2004) Definitions, classification, and epidemiology of sexual dysfunction. In: Lue TF, Basson R, Rosen R, Giuliano F, Khoury S, Montorsi F (eds) Sexual medicine: sexual dysfunctions in men and women. Health Publications, Paris, pp 37–72
McMahon CG, Althof SE, Waldinger MD et al (2008) An evidence-based definition of lifelong premature ejaculation: report of the International Society for Sexual Medicine (ISSM) ad hoc committee for the definition of premature ejaculation. J Sex Med 5:1590–1606
Waldinger MD (2008) Recent advances in the classification, neurobiology and treatment of premature ejaculation. Adv Psychosom Med 29:50–69
Waldinger MD, Schweitzer DH (2006) Changing paradigms from a historical DSM-III and DSM-IV view toward an evidence based definition of premature ejaculation. Part II—proposals for DSM-V and ICD-11. J Sex Med 3:693–705
Serefoglu EC, Yaman O, Cayan S et al (2011) Prevalence of the complaint of ejaculating prematurely and the four premature ejaculation syndromes: results from the Turkish Society of Andrology sexual health survey. J Sex Med 8:540–548
Gao J, Zhang X, Su P et al (2013) Prevalence and factors associated with the complaint of premature ejaculation and the four premature ejaculation syndromes: a large observational study in China. J Sex Med 10:1874–1881
Zhang X, Gao J, Liu J, **a L et al (2013) Distribution and factors associated with four premature ejaculation syndromes in outpatients complaining of ejaculating prematurely. J Sex Med 10:1603–1611
Serefoglu EC, Cimen HI, Atmaca AF, Balbay MD (2010) The distribution of patients who seek treatment for the complaint of ejaculating prematurely according to the four premature ejaculation syndromes. J Sex Med 7(2 Pt 1):810–815
Whelchel DD, Brehmer TM, Brooks PM et al (2004) Molecular targets of botulinum toxin at the mammalian neuromuscular junction. Mov Disord 19(suppl 8):S7–S16
Linsenmeyer TA (2013) Use of botulinum toxin in individuals with neurogenic detrusor overactivity: state of the art review. J Spinal Cord Med 36:402–419
Serefoglu EC, Silay MS (2010) Botulinum toxin-A injection may be beneficial in the treatment of life-long premature ejaculation. Med Hypotheses 74(1):83–84
Burbridge C, Symonds T, Osterloh IH et al (2019) Content validity of the premature ejaculation profile, original and per-event formats, in men with lifelong premature ejaculation. J Sex Med 16(4):569–576
Huang YP, Chen B, ** P et al (2014) The premature ejaculation diagnostic tool (PEDT): linguistic validity of the Chinese version. J Sex Med 11(9):2232–2238
Rosen RC, Riley A, Wagner G et al (1997) The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 49(6):822–830
Rosen RC (2000) Prevalence and risk factors of sexual dysfunction in men and women. Curr Psychiatry Rep 2(3):189–195
Rowland DL (2011) Psychological impact of premature ejaculation and barriers to its recognition and treatment. Curr Med Res Opin 27(8):1509–1518
Laumann EO, Paik A, Rosen RC (1999) Sexual dysfunction in the United States: prevalence and predictors. JAMA 281(6):537–544
Porst H (2011) An overview of pharmacotherapy in premature ejaculation. J Sex Med 8(Suppl 4):335–341
Waldinger MD, Zwinderman AH, Schweitzer DH, Olivier B (2004) Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta-analysis. Int J Impot Res 16(4):369–381
Gerstenberg TC, Levin RJ, Wagner G (1990) Erection and ejaculation in man. Assessment of the electromyographic activity of the bulbocavernosus and ischiocavernosus muscles. Br J Urol 65:395–402
Ongün Ş, Acar S, Koca P et al (2019) Can botulinum-A toxin be used to delay ejaculation: results of an ejaculation model in male rats. J Sex Med 16(9):1338–1343
Serefoglu EC, Hawley WR, Lasker GF et al (2014) Effect of botulinum-A toxin injection into bulbospongiosus muscle on ejaculation latency in male rats. Sex Med 11(7):1657–1663
Li ZT, Li YF, Zhang Y et al (2018) Injection of botulinum-A toxin into bulbospongiosus muscle for primary premature ejaculation: a preliminary clinical study. Zhonghua Nan Ke Xue 24(8):713–718
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
SHH: project development, data collection, manuscript writing. NK: data collection, manuscript writing. AM: project development, data collection. KW: data collection, manuscript editing. SI: project development, data collection and analysis. NI: manuscript writing, editing and revision.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Ethical approval
The study was approved by the Research Ethics Committee at Faculty of Medicine, Benha University (Ms.35.10.2022).
Informed consent
Written informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaher, H., Noah, K., Abdelzaher, M. et al. Is bulbospongiosus muscle botox injection safe and effective in treating lifelong premature ejaculation? Randomized controlled study. World J Urol 42, 218 (2024). https://doi.org/10.1007/s00345-024-04899-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-04899-1