Abstract
In this study, it was aimed to improve the dielectric properties of ZnO nanoparticles with the addition of hBN, which was not previously available in the literature, and thus to expand their usage areas. Sol–gel synthesis method was used in this study to create pure and hexagonal boron nitride (hBN) doped zinc oxide (ZnO) nanoparticles. Zinc acetate dihydrate Zn(CH3COO)22H2O), sodium hydroxide NaOH, and hexagonal boron nitride (hBN), all from Sigma Aldrich, were used as starting reagents. The reagents were dissolved during the sol–gel synthesis by being heated to 90 °C for 4 h in a magnetic stirrer. FT-IR, XRD, FE-SEM, EDX characterization techniques, and impedance analyzer were used to find functional groups, structural, morphological, and chemical composition, and dielectric properties of the nanoparticles, respectively. The produced un-doped and hBN-doped ZnO particles consist of nano-sized structures. Changes occurred in the intensities and locations of the XRD diffraction peaks and FT-IR peaks with the addition of hBN. Characteristic peaks of both ZnO and hBN were observed in the diffraction peaks of the doped nanoparticles. All nanoparticles were of high purity and were successfully produced by the sol–gel method. It was shown that as the hBN do** level increased, there were more hBN nanoplates in the ZnO matrix, and the EDX results also showed an increase in hBN addition. The frequency stability of the dielectric properties improved after hBN do**. While the dielectric constant at 1 kHz frequency at room temperature is 12.07 in pure ZnO nanoparticles, the increase up to 55.21 is observed in 10% hBN doped nanocomposites. This situation is considered as a great potential for technological applications of this novel nanocomposite material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nano-sized materials are attracting a lot of attention today due to their potential to add new properties to existing materials and to apply them to different technologies [1,2,3,4,5]. During the production of nanomaterials, significant studies are carried out to control the structural and morphological properties [5,6,7,8,9].
Metal oxide semiconductors (MOS) are crucial in a variety of scientific disciplines, including chemistry, physics, medicine, and engineering, due to their unique electrical, optical, and magnetic characteristic [10, 11]. Due to its distinctive and new uses in optics, optoelectronics, catalysis, and piezoelectricity, research into functional oxide-based, one-dimensional nanostructures has rapidly grown since the discovery of oxide nanobelts of semiconductor oxides. An extensive range of materials with varied chemical compositions and crystallographic structures have been thoroughly studied for using a special class of semi-one-dimensional nanomaterials known as semiconductor oxide nanoparticles [10].
Zinc oxide (ZnO), a unique natural n-type semiconductor among semiconductor materials, has exceptional chemical stability, broad and direct band gap (3.37 eV), high exciton binding energy (60 meV) at room temperature and high exciton binding energy at low temperatures characteristic [10, 11]. ZnO has a hexagonal structure with cell parameters a = b = 3.250 and c = 5.206 Å, [4, 5]. Due to its properties, ZnO is used in a wide range of applications, including as ultraviolet (UV) light emitters, piezoelectric devices, chemical and gas sensors, transistors, solar cells, catalysts, and spintronics [7,8,9, 12,13,14,15]. To date, various methods have been used to create nanostructured ZnO powders with different morphologies and ionic additions for fundamental research. Numerous ions were identified to improve the optical, electrical, and catalytic properties of ZnO powder, increasing the material potential for use in technology [14,15,16,17,18,19,20].
Because of its essential application, nanotechnology has played a significant role in all areas of science for the past 20 years. The two-dimensional material (2D-mats) created in this period, graphene, has exceptional chemical and thermal properties, but more critically, exceptional solution workability to enhance dye degradation [21,22,23,24]. Hexagonal boron nitride (hBN), often known as “white graphene,” has a huge surface area and a polar B and N bond (B-N), which gives it outstanding adsorption characteristics against a variety of dyes, metallic ions, and organic contaminants [25, 26]. A 2-dimensional nanostructure with significant mechanical, electrical, and chemical characteristics is called hBN [27]. It is a substance based on hexagonal covalent bonds between nitrogen and boron atoms [28]. Between the layers, there are flimsy Van der Waals bonds [29]. It possesses exceptional qualities such as chemical inertness, high thermal conductivity, excellent thermal shock resistance, low thermal expansion, high electrical resistance, low dielectric constant, and non-toxicity [30,31,54,55,56,57].
It is well known that hBN has a strong thermal conductivity and a comparatively low dielectric constant. The crystalline structure of hBN is reflected in its plate-like shape, and both the dielectric and thermal characteristics are highly anisotropic [58,59,60]. When it comes to electrical resistance and dielectric loss, it was found that hBN nanocomposite materials outperform metallic fillers and carbon nanoparticles [58, 61].
The main purpose of this study is to expand the usage areas of ZnO semiconductor metal oxide, which is used in many electronic, optoelectronic and electrochemical technologies, by improving its dielectric properties. In current study, un-doped and doped ZnO nanocomposites with (1, 5, 10 wt%) hBN were fabricated by sol–gel method, and in our knowledge such composites were not produced before. When the literature studies of ZnO semiconductor nanoparticles are examined, it is seen that the improvement of dielectric and electrical properties can be improved by synthesizing with new reinforcing elements or materials. FT-IR, XRD, FE-SEM and EDX characterization techniques were used to find functional groups, structural, morphological and chemical composition properties of the nanoparticles, respectively. Also, the dielectric properties of the produced nanoparticles were analyzed and interpreted.
2 Materials and methods
2.1 Synthesis of nanoparticles
Sol–gel synthesis method was used in this study to create pure and hexagonal boron nitride (hBN) doped zinc oxide (ZnO) nanoparticles. Zinc acetate dihydrate Zn(CH3COO)22H2O), sodium hydroxide NaOH, and hexagonal boron nitride (hBN), all from Sigma Aldrich, were used as starting reagents. The production steps and production scheme of ZnO and hBN-reinforced ZnO nanoparticles are shown in Figs. 1 and 2, respectively. The notation and additive amounts of the produced nanoparticles are given in Table 1. Literature studies were also used during the synthesis of nanoparticles [62]. Detailed information about the production of pure ZnO nanoparticles was previously given in detail in the study carried out by researchers in our research team [35]. As a difference, nano-sized hBN particles were used as additive materials in this study and all other processes were carried out in the same way as in the related study. 1, 5, and 10 wt% were chosen as the contribution rate. Since hBN is chemically inert and insulating, the contribution rate was limited to 10% in order not to limit the working area too much [63, 64]. To ensure homogeneity, three samples were produced from each sample and analyzes were performed after the produced nanoparticleswere mixed with each other.
2.2 Characterization of nanoparticles
Detailed information about the sutructural, morphological and electrical characterizasions of un-doped and doped ZnO nanoparticles was previously given in detail in the study carried out by researchers in our research team [35]. Detailed information about the devices used is given in the relevant study and will be briefly mentioned here. The functional groups of un-doped and hBN-doped ZnO nanoparticles were investigated by FT-IR analysis at wavenumber range of 4000–500 cm−1. Structural properties of fabricated all nanoparticles were investigated by XRD technique. XRD diffraction patterns were recorded under CuKα (λ = 1.5406 Å) radiation over 2θ = 20 to 80° scanning range and 40 kV/40 mA. Important structural properties of the produced nanoparticles, such as the lattice parameters (a = b and c), volumes of unit cells (V), crystalline size (D) and average crystalline size, were calculated using XRD diffraction patterns and equations detailed in literature researches [22,23,24, 65,66,67,68,69,70,71]. Morphological properties and chemical composition of all nanoparticles were investigated by FE-SEM and EDX techniques, respectively. Impedance analyzers was used to analyze dielectric readings in the frequency range of 1000–20000 Hz. By using the capacitance, dielectric loss factor and conductance values recorded at room temperature, the dielectric properties were calculated with the help of equation given in literature researches [42, 46, 72].
3 Results and discussion
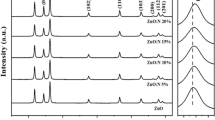
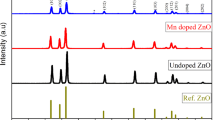
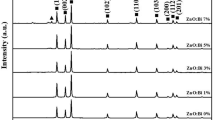
3.1 Characterization of synthesized ZnO-hBN nanoparticles
In the FT-IR spectra seen in Fig. 3, the peaks at 383, 407, 480, 512 and 582 cm−1 are correspondent to Zn–O bonding [73, 74]. The vibration at 886 cm-1 is thought to be a substituted hydrogen bond at the oxygen site (HO) attached to the lattice site of Zn (i.e., Zn-HO). For ZnO nanoparticles, these peaks correspond to the polar stretch vibration modes A1 (TO), E1 (TO), A1 (LO), and E1 (LO), respectively. Two core B-N peaks were found in FT-IR spectra at 786 and 1342 cm−1, and they were attributed to B-N-B bending vibrations and B-N stretched vibration. The last peak was associated with the bending vibration A2u mode (out-of-plane), while the previous peak was found to be well-matched with the stretching vibration E1u mode (in-plane) [75,76,
3.2 Dielectric properties
The dielectric constant, dielectric loss and conductivity values of the produced nanoparticles are shown in Figs. 10, 11, 12. While the dielectric constant values given in Fig. 8 show sudden decreases at low frequencies, they approach almost constant values at higher frequencies. This is due to the fact that dipoles tend to be more directional in the low frequency region. The dielectric test was replicated five different times at room temperature for each samples. The standard deviation and coefficient of variation of the dielectric parameters at 1 kHz for each composite were calculated and summarized in Table 5. The coefficient of variation for the dielectric constant ranges from 1.26 to 3.07, while for the dielectric loss it ranges from 1.36 to 6.25. In addition, while the dielectric constant at 1 kHz frequency at room temperature is 12.07 ± 0.224 for pure ZnO, it has increased to 55.21 ± 0.726 in 10% hBN added composite. This is thought to be due to the increased interfacial polarization between ZnO and hBN [92,97,98,99]. In studies investigating the dielectric properties of undoped or doped ZnO nanoparticles with different elements, dielectric constants varied between 7 and 10.26, and dielectric loss values varied between 0.04 and 0.212 [100,101,102,103]. In our study, these values are between 12.070–55.219 and 0.840–20.075 for dielectric constant and dielectric loss, respectively. While the dielectric constant at 1 kHz frequency at room temperature is 12.07 in pure ZnO nanoparticles, the increase up to 55.21 is observed in 10% hBN doped nanocomposites. The high dielectric constant shows that the nanocomposite particles produced have potential for use in the semiconductor electronics industry. The dielectric parameters indicate that, regardless of the contribution of the crystalline phases, perceived porosity might be the main factor in determining the relative dielectric constant values. But the hBN to ceramic bands' platelet-like crystal structure also contributes significantly to the dielectric response. It shows that, despite high apparent porosity, which lowers the material relative dielectric constant, high apparent porosity does not prevent the material from being employed as a high-frequency substrate. The latency of electromagnetic signals transmitted by high frequency devices is therefore decreased by integrating with hBN study [97,98,99, 104]. As a result, multilayer hBN doped ceramic tapes are considered to be a crucial high-frequency substrate for electronic transmission devices.
4 Conclusions
When the structural and morphological analyses of the nanoparticles produced by the sol–gel synthesis method were completed, it was observed that the ZnO nanoparticles and the hBN reinforced ZnO nanoparticles were combined at nanoscale and successfully synthesized. The distinctive peaks of ZnO and hBN nanoparticles were identified, and as the hBN contribution increased, the peaks shifted and grew in size. As a result of FE-SEM analysis of ZnO nanoparticles, it was observed that the hBN nanorods grew significantly with the increase of hBN do**. It was also seen in EDX results that Zn, O, B and N contributions were successfully reached. As the amount of hBN reinforcement in the nanocomposites increased, a rise in dielectric constant was seen. This rise is assumed to be a result of nanocomposites' shifting load centers. The hBN addition to ZnO nanoparticles contributed to the improvement of the dielectric response. It was observed that the increase in hBN content led to a significant reduction in dielectric loss, and it provides a great potential for technological applications. Thus, the frequency stability of the dielectric properties improved after adding. For example, the variation of dielectric constant is 0.91 for pure hBN in the frequency range of 102 to 20 kHz, while it is only 0.39 for 10% ZnO doped composite. The electrical differences between ZnO-hBN nanoparticles lead to charge transport and accumulation at the interface. The resulting interfacial polarization leads to an increase in dielectric constant, dielectric loss and AC conductivity values. these composites can be one of the most fundamental materials with the potential applications in energy storage devices, solar cell production and photovoltaic devices.