Abstract
Objectives
To investigate whether using the diffusion levels (DLs) proposed by the European Society of Breast Imaging (EUSOBI) improves the diagnostic accuracy of breast MRI.
Materials and methods
This retrospective study included 145 women who, between September 2019 and June 2020, underwent breast 1.5-T MRI with DWI. Reader 1 and reader 2 (R1-R2) independently assessed breast lesions using the BI-RADS on dynamic contrast-enhanced imaging and T2-weighted imaging. DWI was subsequently disclosed, allowing readers able to measure lesions ADC and subjectively express the overall risk of malignancy on a 1–5 Likert scale. ADCs were interpreted as a range of values corresponding to the EUSOBI DLs. The analysis evaluated the inter-reader agreement in measuring ADC and DLs, the per-DL malignancy rate, and accuracy for malignancy using ROC analysis against histological examination or a 3-year follow-up.
Results
Lesions were malignant and showed non-mass enhancement in 67.7% and 76.1% of cases, respectively. ADC was measurable in 63.2%/66.7% of lesions (R1/R2), with a minimal discrepancy on Bland–Altman analysis and 0.948 (95%CI 0.925–0.965)/0.989 (95%CI 0.988–0.991) intraclass correlation coefficient in measuring ADC/DLs. The malignancy rate (R1/R2) increased from 0.5/0.5% (“very high” DL) to 96.0/96.8% (“very low” DL), as expected. Likert categorization showed larger areas under the curve than the BI-RADS for both R1 (0.91 versus 0.87; p = 0.0208) and R2 (0.91 versus 0.89; p = 0.1171), with improved specificity (81.5% versus 78.5% for R1 and 84.4% versus 81.2% for R2).
Conclusion
Though ADC was not measurable in about one-third of lesions, DLs were categorized with excellent inter-reader agreement, improving the specificity for malignancy.
Clinical relevance statement
DLs proposed by the EUSOBI are a reproducible tool to interpret the ADC of breast lesions and, in turn, to improve the specificity of breast MRI and reduce unnecessary breast biopsies.
Key Points
• The European Society of Breast Imaging proposed diffusion levels for the interpretation of the apparent diffusion coefficient in diffusion-weighted imaging of the breast.
• Adding diffusion levels to the interpretation of magnetic resonance imaging improved the diagnostic accuracy for breast cancer, especially in terms of specificity.
• Diffusion levels can favor a more widespread and standardized use of diffusion-weighted imaging of the breast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dynamic contrast-enhanced imaging (DCE) represents the core of magnetic resonance imaging (MRI) of the breast, showing 81–100% sensitivity for malignancy [1]. Diffusion-weighted imaging (DWI) has progressively emerged as a valuable tool to complement DCE to improve the characterization of breast lesions [2,3,4] and, in turn, decrease the number of false positives of breast MRI and unnecessary biopsy recommendations [5]. Although DWI is widely used by most experienced breast radiologists [5], its routine implementation in breast MRI protocols has not been fully established. This is exemplified by the fact that the Breast Imaging Reporting and Data System (BI-RADS) does not provide specific criteria for interpreting DWI [6].
A major factor limiting the widespread adoption of DWI is the difficulty in standardizing the interpretation of the apparent diffusion coefficient (ADC), i.e., the DWI-derived metric quantitatively expressing the random motion of water molecules within normal and diseased breast tissues [7]. While ADC values are typically lower in malignant tumors than benign tumors [8], it is difficult to establish definite ADC thresholds for malignancy or different tumor types, mainly because of the variability in acquisition parameters and analysis methods across different centers and vendors [9]. In order to promote expanded and reproducible use and overcome the problem of defining a threshold for malignancy, a recent consensus and mission statement from the DWI working group of the European Society of Breast Imaging (EUSOBI) proposed a standard for technical acquisition of breast DWI and a classification of the diffusion level (DL) of breast lesions for interpretation [7]. Five different ranges of ADC values derived from a previous metanalysis [10] were assumed to correspond to as many DLs (i.e., very low, low, intermediate, and high) and were, in turn, supposed to be typical of different types of benign and malignant breast lesions [7].
In the EUSOBI proposal, DLs are meant to objectively describe DWI-related information rather than a stand-alone diagnostic tool. DLs should always be used in conjunction with all other MRI data for the purpose of lesion characterization. To our knowledge, no previous studies validated the DLs proposed by EUSOBI as a diagnostic tool. While Bickel et al [11] recently proposed DLs derived from a large multicentric dataset of ADC values rather than literature data, they did not evaluate their diagnostic performance in combination with DCE-based MRI. It is then still unclear how ADC categorization can influence lesion characterization when integrated with BI-RADS-based interpretation and whether the expected role of improving specificity can be achieved.
This study aimed to investigate the malignancy rate on a per-DL basis and the impact on the diagnostic performance of adding the information related to DLs to BI-RADS categorization.
Materials and methods
Study population and standard of reference
The referring Institutional Review Board approved this study. The acquisition of written informed consent from patients was waived because of the retrospective design.
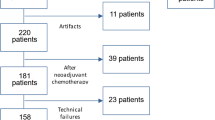
We identified all the women who underwent breast MRI between September 2019 and June 2020 in our Institution. In line with our activity as a tertiary referral center [12], the examination was performed for various indications, represented mainly by preoperative assessment and problem-solving imaging after inconclusive first-line imaging. After excluding 283 patients (Fig. 1), we finally included 145 women (mean age 53 years, range 21–81 years) showing 201 breast lesions (136 malignant and 65 benign). Factors supposed to limit the applicability of DWI were part of the exclusion criteria, i.e., lesion size < 6 mm or masking effect by post-biopsy hematoma [7].
Final diagnosis was available through biopsy in 39/201 lesions and pathological examination after surgery in 142/201 lesions. Breast biopsy was usually performed before MRI in the case of preoperative examinations and before or after MRI, depending on the indication of the examination and clinical scenario. On a per-patient basis, surgery included bilateral mastectomy (n = 2), unilateral mastectomy (n = 60), quadrantectomy (n = 31), lumpectomy (n = 6), surgical excisional biopsy (n = 8), and local excision of recurrent breast cancer after mastectomy (n = 2). Biopsy was performed under ultrasound (n = 36), tomosynthesis (n = 2), and MRI (n = 1) guidance by one of three breast radiologists with 10–20 years of experience, respectively. One of three pathologists with 6–26 years of experience evaluated biopsy and surgical specimens per the guidelines of the College of American Pathologists since 2020, using the version available when the analysis was performed [13, 14].
In the case of 20 remaining lesions categorized as benign and with no histopathological analysis, the standard of reference was represented by a 3-year follow-up with MRI and/or mammography and/or digital breast tomosynthesis and/or ultrasound. Imaging follow-up was established as the most reasonable standard of reference for this subset of lesions, given the impossibility of obtaining a histological sample [15].
MRI protocol
MRI examinations were performed on a 1.5-T magnet (Magnetom Aera, Siemens Healthineers) using a bilateral 16-channel coil. The acquisition parameters are reported in Table 1. The reported time of echo (TE) was set at the minimum possible, associated with a receiver bandwidth of 1602 Hz/pixel, an Echoplanar imaging factor of 102, and an echo spacing of 0.77 ms. The diffusion gradients were oriented along the three main space directions using the “3 scan trace modality”. The ADC map was generated by the vendor’s software (Leonardo Syngo.via, Siemens Healthineers), with ADC values calculated as ADC = ln(signal at b 0/signal at b 800)/(b800-b0). After DWI, dynamic contrast-enhanced imaging (DCE) was performed with intravenous administration of 0.1 mmol/kg of gadoteridol (Prohance, Bracco Imaging) at an injection rate of 2 ml/s.
Image analysis
A study coordinator organized independent reading sessions involving two radiologists with high (> 20 years; reader 1 [R1]) and low experience (< 2 years; reader 2 [R2]) in breast imaging, blinded to clinical, histopathological, or follow-up information. The study readings were preceded by a 1-week period of reading sessions in which R1 and R2 were recalled with the rules of ADC assessment established in the EUSOBI document [7] to achieve consistent measurements.
During each session, the coordinator initially showed readers T2-weighted imaging (T2WI) and DCE images only, asking them to identify any relevant finding (no upper limits in number) and categorize each of them according to the BI-RADS, fifth edition [6] (reading phase 1). Subsequently, the coordinator disclosed all the DWI images and the ADC map to apply EUSOBI criteria (reading phase 2) [7]. In particular, readers were asked to report any visible correlation of lesions found on DCE or T2WI imaging on b = 800 s/mm2 images. Second, they measured the ADC of lesions ≥ 6 mm in size, placing a circular region of interest (ROI) over the most hypointense region of the lesion on the ADC map, paying attention to avoid artifacts, hemorrhage, or necrosis, as well as checking that the region fell within the enhancing part of the lesion on DCE and hyperintense part on the b = 800 s/mm2 image to avoid the potential confounder of the blackout effect. Readers were allowed to adapt this strategy by placing a ROI over the whole lesion in the case of smaller observations.
In reading phase 3, R1 and R2 were left free to evaluate the MRI information as a whole, i.e., by adding DLs to DCE and T2WI in image interpretation. Readers were asked to assume that the DL of the lesion corresponded to the EUSOBI reference one within which the measured ADC lay and were left free to consider the ADC value relevant or not based on the per-patient combination of DCE and T2WI. Reference DLs were [7] “very low” for ADC values ≤ 0.9 × 10–3 mm2/s, “low” for ADC values 0.91–1.3 × 10–3 mm2/s, “intermediate” for ADC values 1.31–1.7 × 10–3 mm2/s, “high” for ADC values 1.71–2.1 × 10–3 mm2/s, and “very high” for ADC values > 2.1 × 10–3 mm2/s. Based on subjective interpretation, the comprehensive risk of malignancy of MRI findings was expressed with a Likert scale, as follows: 1 = highly unlikely; 2 = unlikely; 3 = indeterminate; 4 = likely; 5 = very likely. When the ADC was not measurable, e.g., because of no clear lesion on the ADC map, readers were asked to make the Likert category correspondent to the BI-RADS one.
Data analysis
ADC values were reported with median values with the interquartile range (IQR) as they were not normally distributed according to the Shapiro–Wilk test. However, we also reported mean values with ± standard deviation (SD) to facilitate the comparison with previous literature. The ADC values measured by R1 and R2 were compared with the Wilcoxon signed-rank test, while the differences between benign and malignant lesions were assessed with the u Mann–Whitney test. The inter-reader agreement in measurements was assessed with Bland–Altman analysis and the intraclass correlation coefficient (ICC). The results of the Bland–Altman analysis were expressed as dimensionless values given a preliminary logarithmic transformation of the data. ICC reference values were as follows [16]: 0.40 = poor; 0.40–0.59 = fair; 0.60–0.74 = good; 0.75–1.00 = excellent. ICC was also used to quantify the inter-reader agreement in assigning DLs corresponding to the measured ADC.
The diagnostic accuracy in assessing malignancy was evaluated with the receiver operating characteristics (ROC) analysis and expressed with an area under the curve (AUC). The sensitivity and specificity were calculated in correspondence with the cut-off of the BI-RADS category or Likert category with the highest Youden’s index. The AUCs were compared with the DeLong test. We did not run the ROC analysis concerning DLs because not all lesions showed measurable ADC. We then calculated the malignancy rate on a per-DL basis, defined as the percent ratio between the total number of cancers found and the total number of lesions assigned to a certain DL.
Analysis was performed with commercially available software (MedCalc Software version 19.8 Ltd).
Results
Histological and MRI features
Histological characteristics of 181/201 breast lesions referred to biopsy or surgery are summarized in Table 2. The remaining 20/201 findings included miscellaneous lesions categorized as benign on MRI, showing no evolution (n = 15) or disappearance (n = 5) during imaging follow-up. Overall, the prevalence of malignant lesions was 67.7% (136/201) (95%CI: 60.7–74.1). Nine high-risk B3 lesions were included among benign lesions after the results of excisional biopsy.
On MRI, lesions appeared as masses or non-mass enhancement in 153/201 (76.1%; 95%CI: 69.6–81.8) and 48/201 cases (23.8%; 95%CI: 18.2–30.4), respectively. The mean lesion size was 23 ± 18 mm (range 6–80 mm).
ADC values and inter-reader agreement
Lesion ADC was measurable in 127/201 cases for R1 (63.2%) and 134/201 cases for R2 (66.7%), with median (IQR)/mean ± SD values of 0.98 (0.83–1.26)/1.08 ± 0.35 and 0.92 (0.78–1.3)/1.04 ± 0.36 × 10–3 mm2/s, respectively (Fig. 2) (p = 0.0058). The ADC was significantly lower in malignant than benign lesions (p < 0.001) for both R1 (median [IQR]/mean ± SD 0.90 [0.78–1.02]/0.91 ± 0.22 versus 1.50 [1.25–1.62]/1.44 ± 0.31 × 10–3 mm2/s) and R2 (median [IQR]/mean ± SD 0.85 [0.75–0.96]/0.87 ± 0.24 versus 1.46 [1.27–1.58]/1.42 ± 0.31 × 10–3 mm2/s).
The mean difference in ADC values measured by R1 and R2 was minimal on Bland–Altman analysis, as R1 measured ADC values 0.02 times (i.e., 2%) higher than R2 on average. This corresponded to close limits of agreement, i.e., expected differences in R2 measurements ranging from -9% to + 0.12% compared to what measured by R1 (Fig. 3). The inter-reader agreement was excellent in measuring ADC (ICC = 0.948 [95%CI 0.925–0.965]) and assessing DLs (ICC = 0.989 [95%CI 0.988–0.991]) (Fig. 4). Table 3 shows the malignancy rate on a per-DL basis.
Bland–Altman graph plotting the mean (x-axis) versus the difference (y-axis) of any paired measurement of ADC values by reader 1 and reader 2, including the mean absolute difference (blue line) and 95% limits of agreement (dashed lines). ADC values are expressed as dimensionless values after logarithmic transformation
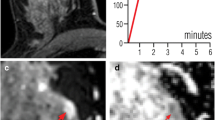
True-positive assessment of a ductal carcinoma in situ associated with invasive ductal carcinoma in a 46-year-old woman. The hypervascular left-sided lesion showed both mass and non-mass components on the first subtracted post-contrast image (a) while appearing relatively isointense to the fibroglandular background on T2-weighted imaging (b). The lesion signal was inhomogeneous on the high b-value image (c), with a markedly hyperintense area corresponding to contrast enhancement and a zone appearing as markedly hypointense compared to the surrounding tissue on the ADC map (arrow in d). Despite the inhomogeneous lesion appearance, both readers independently selected this zone for placing the ROI and obtained comparable ADC values of 0.75 e 0.72 × 10–3 mm2/s. The lesion was classified BI-RADS 5 and Likert 5 by both
Diagnostic accuracy
Table 4 summarizes which lesions were reclassified using Likert categorization, and whether reclassification was correct compared to the standard of reference (Fig. 5). Notably, most reclassifications concerned the same cases for both readers. As an overall balance, Likert categorization saved 2 false-positives for both R1 and R2, and 2 false-negatives and 1 false-negative for R1 and R2, respectively. While Likert categorization induced false-positives and avoided false-negatives in lesions with a “low” DL, saved false-positives cases showed lesion ADCs in the “low-to-intermediate” DL (Table 4). The single false-negative case induced by Likert categorization for R2 showed a “low” DL.
False-positive case avoided by Likert categorization in a 49-year-old woman with a histological diagnosis of fibrocystic disease. The left-sided breast lesion presented as a non-mass enhancement area on the first-subtracted post-contrast sequence (a), with isointensity compared to the fibroglandular tissue on T2-weighted imaging (b). On Diffusion-weighted imaging, the whole lesion appeared hyperintense on both the high b-value image (c) and ADC map (d). Reader 1 and reader 2 measured an ADC value of 1.90 and 1.46 × 10–3 mm.2/s, respectively, and downgraded the level of suspicion from BI-RADS 4 to Likert 2 (reader 1) and from BI-RADS 4 to Likert 3 (reader 2)
Overall, Likert categorization showed higher AUC (Fig. 6), sensitivity, and specificity than BI-RADS categorization (Table 5). Supplementary Table 1 shows the overview of correct and incorrect lesion classifications compared to the standard of reference, while Supplementary Table 2 reports the cases incorrectly assessed by both BI-RADS and Likert categorization.
Discussion
We compared breast lesion categorization using the BI-RADS on DCE and T2-weighted imaging versus Likert categorization on the whole examination including DLs [7], showing three main results. First, adding DLs to image interpretation improved the specificity for cancer from 78.5% to 81.5% in the case of R1 and 81.2 to 84.4% in the case of R2, translating into a significant improvement in the AUC for one out of two readers. Second, DLs were found to reliably reflect the risk of malignancy, as the large majority of cancers were associated with lower ADC values, with a malignancy rate in the “very low” and “low” DLs of 96.1% and 80%, respectively. Third, the use of a standardized method of measurement translated into an excellent inter-reader agreement in measuring both the ADC (ICC 0.948) and establishing the DLs (ICC 0.989). According to Bland–Altman analysis, ADC values showed minimum mean differences and close limits of agreement (i.e., minimal expected discrepancies) when measured by R1 and R2. Taken together, our results validate the DLs as a simple and reproducible means to include the quantitative information of the ADC in the interpretation of breast MRI and match the expected task of improving the specificity of the examination [17,18,19]. This also emphasizes the potential for DLs to reduce unnecessary biopsy procedures [20] and help to increase the use of DWI in clinical practice [7].
Several previous works investigated the diagnostic value of the ADC in assessing the malignancy of breast lesions [2,3,4, 21, 22]. However, as far as we know, only a recent study by Bickel et al [11] evaluated the potential of DLs to overcome the well-known problem of establishing absolute ADC thresholds in clinical practice. The Authors derived a system of six DLs ranging from 1 (“ADC not measurable”) to 5 (“very low”) from a multicentric population of 1625 women with 1736 pathologically confirmed lesions, showing that at an ADC threshold of < 1.0 × 10–3 mm2/s distinguishing between the “intermediate/low” from “low” DL, the positive predictive value for malignancy was 95.8%. Comparably to these authors, we found that the ADC values of malignant lesions were lower than benign ones, as expected [8]. Differently from them, we did not derive the DLs from the patient population under analysis but used the predefined ones prompted in an EUSOBI mission and consensus statement [7], which in turn originated from a metanalysis [10]. One might argue that using predefined values carried the risk of testing DLs obtained from a different distribution of histological subtypes of breast cancer compared to the study cohort. However, the added value we observed can be considered reasonable proof that the DLs we used can be successfully and reliably applied in an external cohort, thus being of potential clinical applicability. This is in line with the fact that the dataset of origin was large enough to reasonably adjust for fluctuations of lesions’ distribution and type (61 studies and 5205 breast lesions) [10], and the small differences between the DLs used by us and Bickel et al [11] (≤ 0.9 versus < 1.0 × 10–3 mm2/s for the “very low” level, 0.91 to 1.7 versus 1.0 to < 1.5 × 10–3 mm2/s for the “low-to-intermediate” level, 1.71–2.1 versus 1.5 to < 1.9 × 10–3 mm2/s for the “high” level, and > 2.1 versus ≥ 1.9 × 10–3 mm2/s for the “very high” level).
We believe there are two points of strength in our work. First, unlike the above Authors [11], we did not evaluate DLs as a stand-alone diagnostic tool but investigated the effect of adding them to breast MRI. We also found that there was no price to pay for increased specificity as the use of DLs was associated with a slight but measurable increase in sensitivity as well, which could be of special benefit in the preoperative setting. However, larger studies should investigate whether the overall balance between sensitivity and specificity favors the use of DWI in a certain clinical scenario. Second, we observed that ADC could not be provided in around 37% of lesions for both R1 and R2, thus making DLs unavailable. Rather than being a limitation, this result emphasizes that DWI is a complementary tool to the anatomical and functional information deriving from DCE and T2-weigthed imaging and that lesion characterization cannot be made upon DLs alone [7]. However, we acknowledge that further technical development in DWI sequences could improve our results by making more lesions measurable on the ADC map, e.g., because of increased spatial resolution. This line of research should be considered of primary importance to enhance the role of DWI in breast imaging.
This study is not devoid of limitations. Likert categorization was subjective, i.e., we did not provide predefined rules for the combined interpretation of DCE, T2WI, and DLs, e.g., as occurs with the Prostate Imaging Reporting and Data System (PI-RADS) [23]. Likert categorization induced false-positives and avoided false-negatives in lesions with a “low” DL (rate of malignancy 71.4–75.5% in our series), suggesting that readers trusted considerably the low ADC value as a problem-solving tool, despite this carries the risk of translating into errors. On the contrary, we do not have a definite explanation of why saved false-positives and one single induced false-negative at Likert categorization mainly showed “intermediate” DLs (rate of malignancy 9.1–9.5%) or even a “low” DLs of 0.92 10–3 mm2/s. One can hypothesize that, for reasons difficult to assess on a case-by-case basis, readers’ interpretation was influenced more by T2-weighted imaging, DCE, or even high b-values images than the ADC when making the overall assessment of these lesions. This emphasizes the need for further studies exploring how to weigh and combine the ADC value and individual MRI sequences into final lesion categorization. Second, while differing in experience in breast imaging, both readers came from the same institution, so the consistency we observed in Likert categorization could have been affected by comparable interpretation criteria. Finally, we did not investigate additional well-established tasks for DWI, such as differentiating between invasive versus noninvasive lesions [21, 24,25,26,27].
In conclusion, using predefined DLs prompted by EUSOBI in a mission and consensus statement for expanding the use of DWI in breast MRI, we observed that higher malignancy rates were associated with “very low” and “low” levels, as expected. Integrating DLs into the diagnostic process improved both the sensitivity and specificity for breast cancer compared to the BI-RADS-based interpretation of DCE and T2-weighted imaging alone, even using a subjective Likert scale. The improvement was greater for specificity, suggesting that, when measurable, DLs can avoid unnecessary biopsy recommendations as the main effect.
Change history
17 November 2023
Correspondence address has been changed.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- BI-RADS:
-
Breast imaging reporting and data system
- CI:
-
Confidence interval
- DCE:
-
Dynamic contrast-enhanced imaging
- DL:
-
Diffusion level
- DWI:
-
Diffusion-weighted imaging
- EUSOBI:
-
European Society of Breast Imaging
- IQR:
-
Interquartile range
- MRI:
-
Magnetic resonance imaging
- R1:
-
Reader 1
- R2:
-
Reader 2
- ROI:
-
Region of interest
- SD:
-
Standard deviation
References
Mann RM, Kuhl CK, Moy L (2019) Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging 50:377–390
Baxter GC, Graves MJ, Gilbert FJ, Patterson AJ (2019) A metaanalysis of the diagnostic performance of difusion MRI for breast lesion characterization. Radiology 291:632–641
Chen X, Li W-l, Zhang Y-l, Wu Q, Guo Y-m, Bai Z-l (2010) Metaanalysis of quantitative difusion-weighted MR imaging in the diferential diagnosis of breast lesions. BMC Cancer 10:693–693
McDonald ES, Romanof J, Rahbar H et al (2021) Mean apparent difusion coefcient is a sufcient conventional difusion-weighted MRI metric to improve breast MRI diagnostic performance: results from the ECOG-ACRIN Cancer Research Group A6702 Difusion Imaging Trial. Radiology 298:60–70
Lo Gullo R, Sevilimedu S, Baltzer P et al (2022) A survey by the European Society of Breast Imaging on the implementation of breast diffusion-weighted imaging in clinical practice. Eur Radiol 32:6588–6597
D’Orsi CJ SE, Mendelson EB, Morris EA et al (2013) ACR BI-RADS® atlas, breast imaging reporting and data system. American College of Radiology, Reston, VA
Baltzer P, Mann RM, Iima M et al (2020) Diffusion-weighted imaging of the breast – a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur Radiol 30:1436–1450
Iima M, Honda M, Sigmund EE et al (2020) Diffusion MRI of the breast: current status and future directions. J Magn Reson Imaging 52:70–90
Honda M, Iima M (2023) Is the breast ADC category system a useful addition to BI-RADS? Eur Radiol 33:5398–5399
Shi R, Yao Q, Wu L, Xu J (2018) Breast lesions: diagnosis using diffusion weighted imaging at 1.5T and 3.0T-systematic review and meta-analysis. Clin Breast Cancer 18:e305–e320
Bickel H, Clauser P, Pinker K et al (2023) Introduction of a breast apparent difusion coefcient category system (ADC-B) derived from a large multicenter MRI database. Eur Radiol 33:5400–5410
Clauser P, Mann R, Athanasiou A et al (2018) A survey by the European Society of Breast Imaging on the utilisation of breast MRI in clinical practice. Eur Radiol 28:1909–1918
Fitzgibbons PL, Connolly JL, Bose S, et al (2020) Protocol for the examination of resection specimens from patients with invasive carcinoma of the breast. College of American Pathologists (CAP). https://documents.cap.org/protocols/cp-breast-invasive-resection-20-4400.pdf (Last Access August 30, 2023)
Fitzgibbons PL, Connolly JL, Edgerton M, Simpson R (2020) Protocol for the examination of biopsy specimens from patients with invasive carcinoma of the breast. College of American Pathologists (CAP). https://documents.cap.org/protocols/cp-breast-invasive-biopsy-20-1100.pdf (Last Access August 30, 2023)
Cohen JF, Korevaar DA, Altman DG et al (2016) STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 6:e012799
Cicchetti DV (1994) Guidelines criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 6:284–290
Saccenti L, de Margerie Mellon C, Scholer M et al (2023) Combining b2500 diffusion-weighted imaging with BI-RADS improves the specificity of breast MRI. Diagn Interv Imaging 104:410–418
Meng L, Zhao X, Guo J et al (2023) Improved differential diagnosis based on BI-RADS descriptors and apparent diffusion coefficient for breast lesions: a multiparametric MRI analysis as compared to Kaiser score. Acad Radiol 30(Suppl 2):S93–S103
Goto M, Le Bihan D, Yoshida M, Sakai K, Yamada K (2019) Adding a model-free diffusion MRI marker to BI-RADS assessment improves specificity for diagnosing breast lesions. Radiology 292:84–93
Rahbar H, Zhang Z, Chenevert TL et al (2019) Utility of diffusion-weighted imaging to decrease unnecessary biopsies prompted by breast MRI: a trial of the ECOG-ACRIN Cancer Research Group (A6702). Clin Cancer Res 25:1756–1765
Bickel H, Pinker-Domenig K, Bogner W et al (2015) Quantitative apparent diffusion coefficient as a noninvasive imaging biomarker for the differentiation of invasive breast cancer and ductal carcinoma in situ. Invest Radiol 50:95–100
Partridge SC, Zhang Z, Newitt DC et al (2018) Diffusion-weighted MRI findings predict pathologic response in neoadjuvant treatment of breast cancer: the ACRIN 6698 Multicenter Trial. Radiology 289:618–627
Turkbey B, Rosenkrantz AB, Haider MA et al (2019) Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 76:340–351
Partridge SC, Mullins CD, Kurland BF et al (2010) Apparent diffusion coefficient values for discriminating benign and malignant breast MRI leisons: effects of lesion type and size. AJR Am J Roentgenol 194:1664–1673
Rahbar H, Partridge SC, Eby PE et al (2011) Characterization of ductal carcinoma in situ on diffusion weighted breast MRI. Eur Radiol 21:2011–2019
Iima M, Le BIhan D, Okumura R, et al (2011) Apparent diffusion coefficient as an MR imaging biomarker of low-risk ductal carcinoma in situ: a pilot study. Radiology 260:364–372
Ding JR, Wang DN, Pan JL (2016) Apparent diffusion coefficient value of diffusion-weighted imaging for differential diagnosis of ductal carcinoma in situ and infiltrating ductal carcinoma. J Cancer Res Ther 12:744–750
Funding
Open access funding provided by Università degli Studi di Udine within the CRUI-CARE Agreement. The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Rossano Girometti.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. Rossano Girometti is the Deputy Editor of European Radiology. As such, he had no role in handling this manuscript or finalizing decisions.
Statistics and biometry
Rossano Girometti has significant statistical expertise and performed statistical analysis. No advanced statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
The study has been approved by the Institutional Review Board of the Department of Medicine, University of Udine (approval number 149/2022).
Study subjects or cohorts overlap
Study subjects have not been previously reported in other works.
Methodology
• retrospective
• cross-sectional, observational study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zuiani, C., Mansutti, I., Caronia, G. et al. Added value of the EUSOBI diffusion levels in breast MRI. Eur Radiol 34, 3352–3363 (2024). https://doi.org/10.1007/s00330-023-10418-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10418-4