Abstract
Objectives
Computed tomography (CT) derived fractional flow reserve (FFRCT) decreases from the proximal to the distal part due to a variety of factors. The energy loss due to the bifurcation angle may potentially contribute to a progressive decline in FFRCT. However, the association of the bifurcation angle with FFRCT is still not entirely understood. This study aimed to investigate the impact of various bifurcation angles on FFRCT decline below the clinically crucial relevance of 0.80 in vessels with no apparent coronary artery disease (CAD).
Methods
A total of 83 patients who underwent both CT angiography including FFRCT and invasive coronary angiography, exhibiting no apparent CAD were evaluated. ΔFFRCT was defined as the change in FFRCT from the proximal to the distal in the left anterior descending artery (LAD) and left circumflex artery (LCX). The bifurcation angle was calculated from three-dimensional volume rendered images. Vessel morphology and plaque characteristics were also assessed.
Results
ΔFFRCT significantly correlated with the bifurcation angle (LAD angle, r = 0.35, p = 0.001; LCX angle, r = 0.26, p = 0.02) and vessel length (LAD angle, r = 0.30, p = 0.005; LCX angle, r = 0.49, p < 0.0001). In LAD, vessel length was the strongest predictor for distal FFRCT of ≤ 0.80 (β-coefficient = 0.55, p = 0.0003), immediately followed by the bifurcation angle (β-coefficient = 0.24, p = 0.02). The bifurcation angle was a good predictor for a distal FFRCT ≤ 0.80 (LAD angle, cut-off 31.0°, AUC 0.70, sensitivity 74%, specificity 68%; LCX angle, cut-off 52.6°, AUC 0.86, sensitivity 88%, specificity 85%).
Conclusions
In vessels with no apparent CAD, vessel length was the most influential factor on FFRCT, directly followed by the bifurcation angle.
Key Points
• Both LAD and LCX bifurcation angles are factors influencing FFR CT .
• Bifurcation angle is one of the predictors of a distal FFR CT of ≤ 0.80 and an optimal cut-off value of 31.0° for the LAD and 52.6° for the LCX.
• Bifurcation angle should be taken into consideration when interpreting numerical values of FFR CT .
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
FFRCT is a reliable method for detecting functional ischemia, but it can be difficult to interpret because it is affected by a variety of factors such as vessel length [1], plaque characteristics [2, 3], left ventricular mass [4, 5], ramus artery [6], or collateral circulation [7]. The left coronary artery bifurcation angle has been shown to affect wall shear stress and cause modifications to the bloodstream [8, 9]. Significant modifications of laminar flow can contribute to FFR values when taking into account well-established factors such as lumen narrowing and lesion length [10]. We previously reported that diverse FFRCT changes at distal segments occur due to the differences in the left coronary bifurcation angle even with the same vessel length and plaque characteristics [11]. The left coronary artery bifurcation angle contributes to hemodynamic changes in the left coronary arteries and may affect the FFRCT. However, the effect of the left coronary artery bifurcation angle on FFR (FFRCT and invasive FFR) has not been clarified. The present study aimed to identify the effect of atypical left coronary artery bifurcation angle on an FFRCT decline below the clinically relevant value of 0.80 in vessels with no apparent CAD.
Methods
Patient population
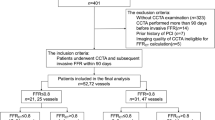
A total of 1278 outpatients with suspected coronary artery disease (CAD) and who had a CT angiography (CTA) with FFRCT analysis examined at the Universitair Ziekenhuis Brussel between January 2017 and March 2021 were evaluated. A retrospective case-control cohort analysis was conducted with approval from the research ethics board at the Universitair Ziekenhuis Brussel under protocol number B.U.N. 14320. The main inclusion criteria were normal vessels or vessels with no apparent CAD on invasive coronary angiography (ICA) and CTA in the left main trunk, left anterior descending artery (LAD) and left circumflex artery (LCX). FFRCT of other vessels, such as the right coronary artery or side branches (diagonal and obtuse side branches) were not considered. No apparent CAD was defined as vessels with < 20% coronary stenosis or luminal irregularities [12]. The severity of coronary stenosis was assessed by experienced interventional cardiologists (B.C., JF.A.) using ICA and by experts in cardiac radiology imaging (T.T., K.T.) using CTA. A total of 631 patients who had not undergone ICA and 463 patients who had coronary stenosis with ICA and CTA were excluded from the study. The following categories of patients were also excluded from the study: the large ramus artery that was large enough to be measured by FFRCT (34 patients), the interval between FFRCT and ICA > 90 days (25 patients), inappropriate image quality (motion artifact, blooming artifact, or noise artifact) (22 patients), post-trans-catheter aortic valve implantation (6 patients), post coronary artery bypass graft (6 patients), congenital heart disease (5 patients), post coronary artery stenting (3 patients), post aortic valve replacement (2 patients). Myocardial bridging leads to compression of vessels during the systolic phase, which is prolonged to the diastolic phase, resulting in hemodynamic changes and affecting FFR [13, 14]. Myocardial bridging was absent in our study. In total, 166 vessels including 83 LAD and 83 LCX from 83 patients were enrolled. The patient selection flowchart is presented in Figure 1.
Coronary computed tomography angiography acquisition
All coronary CT angiography (CTA) scans were acquired with the GE Revolution scanner (GE Healthcare) that had a spatial resolution of 230 μm, a rotational speed of 0.28 s, and a Z-axis coverage of 16 cm enabling to image the heart in one heartbeat. Beta-blockers were administered when necessary for targeting a heart rate of < 60 beats/min. Sublingual nitrates were administered before scanning in all patients. The images were reconstructed at 75 ± 10% of the R-R interval. Coronary arteries were classified based on the American Heart Association classification [15]. Only the major left coronary vessels were considered for analysis and each segment was classified into three categories (proximal, #6 and #11; middle, #7 and #13; distal, #8 and #15).
FFRCT
FFRCT was analyzed by HeartFlow Inc. Computational fluid dynamics and blood flow simulations were performed to calculate FFRCT available at any arbitrary point in the coronary artery. Coronary arteries were classified based on the American Heart Association classification [15] and each segment was divided into three equal segments, proximal, middle, and distal. FFRCT was measured at each point (Supplementary Figure 1A). The magnitude of change in FFRCT (ΔFFRCT) was measured from the proximal to the distal at each left coronary artery. A positive FFRCT was defined as a value ≤ 0.80 in accordance with previously published invasive and non-invasive literature [2, 4, 16, 17].
Left coronary artery bifurcation angle
As in previous studies [18, 19], each bifurcation angle was measured as the crossing angle between the center lines of the left main trunk and each coronary artery (LAD or LCX) on the three-dimensional volume-rendered image of the coronary artery tree. For the left coronary artery bifurcation angle both LAD angle and LCX were determined by assessing the crossing line with the left main trunk (Supplementary Figure 2: center panel).
Vessel morphology and plaque characteristics
Vessel length, lumen volume, and composition of each vessel were measured using GE AW server 3.2 software (GE Healthcare). Aligning the vessel to the region of interest on FFRCT and vessel length and lumen volume were obtained semi-automatically with Color Code Plaque (GE Healthcare) (Supplementary Figure 1B). Vessel constituents were characterized based on Hounsfield units (HU) into low-attenuation plaque (< 30 HU), intermediate-attenuation plaque (30–150 HU), and calcified plaque (> 150 HU) [20, 21].
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD). The 95% confidence interval (CI) was calculated as ± 1.96 SDs from the mean. Two-group comparisons were performed with unpaired Student’s t tests for means if the data were normally distributed or Mann–Whitney U-test if the data were not normally distributed. Correlation between continuous variables was performed using the Spearman correlation test. Multivariable linear regression analysis was performed to examine the independent correlations between FFRCT and baseline parameters. A hierarchical cluster analysis (Ward’s method) was performed to classify parameters related to bifurcation angles. Receiver operating characteristics curves were generated to determine the cut-off value that the highest diagnostic performance of an FFRCT ≤ 0.80. All statistical analyses were performed using JMP 11.0 statistical software (SAS Institute).
Results
Demographic data
Table 1 summarizes the demographic and CT image acquisition condition data of the study population. The time phase in the cardiac cycle was 75.2 ± 2.8%, and all CT images could be acquired at the mid-diastolic phase.
Distribution of distal FFRCT
The distribution of distal FFRCT for each vessel is presented in Supplementary Figure 3. Compared to LCX, a lower proportion of FFRCT > 0.80 was observed in LAD (54.2% vs. 89.2).
Changes in FFRCT
FFRCT showed a continuous gradual decline from the proximal to the distal in LAD and LCX vessels in both FFRCT > 0.80 and ≤ 0.80. LAD presented a more stepwise variation than LCX, and distal end of FFRCT was significantly lower in LAD (0.81 ± 0.08 vs. 0.88 ± 0.06, p < 0.01). In LAD, proximal FFRCT did not differ between FFRCT > 0.80 and ≤ 0.80 (0.96 ± 0.02 vs. 0.95 ± 0.04, p > 0.05), but FFRCT ≤ 0.80 showed a significant decline from the level of the middle segment 6 (0.94 ± 0.03 vs. 0.91 ± 0.07, p < 0.01), resulting in the distal end of FFRCT to be significantly lower in FFRCT ≤ 0.80 (0.86 ± 0.04 vs. 0.74 ± 0.07, p < 0.01). In LCX, FFRCT ≤ 0.80 was significantly decreased at the proximal and distal end of FFRCT. ΔFFRCT was significantly higher in LAD (0.14 ± 0.07) than in LCX (0.09 ± 0.05) (Table 2 and Figure 2).
Changes in FFRCT according to vascular characteristics
Vessel length, lumen volume, and plaque characteristics including, low-attenuation plaque, intermediate-attenuation plaque, and calcified plaque volume were significantly higher in LAD. The bifurcation angle was significantly lower in the LAD angle (31.1 ± 6.6° vs. 42.8 ± 14.9°, p < 0.01). Both in LAD and LCX, bifurcation angle and vessel length were significantly higher in FFRCT ≤ 0.80 (Table 3).
Univariate and multivariate analysis of the relationship between FFRCT and vessel characteristics
In LAD, ΔFFRCT significantly correlated with the bifurcation angle (r = 0.35, p = 0.001) and vessel length (r = 0.30, p = 0.005). With each 10° increase in bifurcation angle, FFRCT changed by 0.063. In LCX, ΔFFRCT also significantly correlated with the bifurcation angle (r = 0.26, p = 0.02), vessel length (r = 0.49, p < 0.0001). With each 10° increase in bifurcation angle, FFRCT changed by 0.047 (Figure 3).
Both angles of LAD- and LCX-related parameters were classified into three groups. The angles of LAD parameters were classified into the following groups: (vessel length and IAP volume) (LAP volume, ΔFFRCT, LAD angle, and CP volume) (lumen volume). The angles of LCX parameters were classified into the following groups: (vessel length, IAP volume, and LCX angle) (LAP volume, CP volume, and ΔFFRCT) (lumen volume) (Supplementary Figure 4). For LAD, multivariable analysis showed that angle (β-coefficient = 0.24, p = 0.02) and vessel length (β-coefficient = 0.55, p = 0.0003) had a predictive value for ΔFFRCT. For LCX, vessel length (β-coefficient = 0.46, p = 0.02) and calcified plaque volume (β-coefficient = 0.34, p = 0.001) were predictive for ΔFFRCT (Table 4). Receiver operating characteristic curve revealed that both LAD and LCX angle had a predictive value for FFRCT ≤ 0.80 at the distal aspect of the vessel (LAD; cut-off 31.0°, AUC 0.70, 95% CI 0.58–0.83, sensitivity 74%, specificity 68%, p = 0.007; LCX, cut-off 52.6°, AUC 0.86, 95% CI 0.76–0.96, sensitivity 88%, specificity 85%, p = 0.0006) (Figure 4).
Discussion
In patients with an ICA and CTA showing essentially vessels with no apparent CAD, our study highlighted the following: (1) Both LAD and LCX angles correlated with ΔFFRCT; (2) Bifurcation angle was one of the predictors of a distal FFRCT ≤ 0.80 and an optimal cut-off value of 31.0° for the LAD and 52.6° for the LCX were demonstrated in our investigation.
To the best of our knowledge, few investigations have studied the role of the bifurcation angle on FFRCT despite the fact that the bifurcation angle may even be one of the most important factors contributing to an unexpected FFRCT decline in vessels with no apparent CAD. This has significant clinical implications.
There was some accordance between our study of bifurcation angles and previous articles. In previous studies that enrolled patients with suspected CAD, bifurcation angles of LAD and LCX assessed by CTA were 34.2 ± 13.4° and 44.3 ± 13.4° [8] and 37 ± 13° and 59 ± 21° [22], and assessed by ICA were 34.1 ± 18.5° and 42.8 ± 20.1° [19]. These bifurcation angles were larger than in our study where they were 31.1 ± 6.6° and 42.8 ± 14.9°, respectively. This difference might be due to selection bias. LAD angle was greater in patients with advanced calcification (33.8 ± 11.6°) than in those without (40.3 ± 10.0°) [14] LCX angle was also higher in patients with coronary stenosis ≥ 50% (39.5 ± 27.0°) than < 50% (46.9 ± 24.6°) [8].
An advantage of our investigation was that our results of the LAD and LCX angles were studied in patients with no apparent CAD. This is valuable as it shows that even with no apparent CAD the bifurcation angle has an influence on FFRCT. In the previous studies, many confounding factors could potentially have influenced the results.
The finding in our work that vessel morphology was the most important factor influencing FFRCT decline was in line with previous studies [3]. However, we showed that the bifurcation angle also had a considerable influence on FFRCT decline in vessels with no apparent CAD. We believe our findings are of clinical interest. As shown by different studies the important FFRCT value should be placed in perspective and many factors should be taken into account when interpreting it. Both in LAD and LCX, vessel length was the strongest factor influencing FFRCT, followed by bifurcation angle in LAD, and CP volume in LCX. Experimental studies indeed showed that a wide bifurcation angle was closely associated with modifications of the bloodstream in vivo [23] as well as in vitro [22,23,24]. Fluid dynamically, a disturbance of laminar flow generates thermal energy, which is consumed, resulting in FFRCT decline in not only atherosclerotic vessels but also normal vessels [10]. Turbulence may be an important factor in this setting. Previous investigations in patients with CAD with a wider bifurcation angle assessed by CTA [25], intravascular ultrasound [26], or angiography [19] showed a higher prevalence of CAD, high-risk plaque, or restenosis after stent implantation.
Our study is original in that it investigates the bifurcation angle in patients without CAD. The present study included only patients with no apparent CAD. In contradistinction, other studies have investigated the relationship between bifurcation angle and plaque formation. In these reports, bifurcation angle was shown to affect the shear stress and consequently influence plaque formation [27]. A wider bifurcation angle was shown to be related to higher turbulence and low shear stress which might cause plaque formation in the areas of bifurcation [24, 28]. Our study showed that plaque volume did not affect FFRCT dynamics. We suggest that there was only limited total plaque volume to affect FFRCT because of the enrolled patients with no apparent CAD. However, our results offer important additional information even in vessels with no apparent CAD the bifurcation angle has a significant effect on FFRCT and erroneous clinical decisions could occur. Whether at a later phase of the disease, the bifurcation angle caused CAD was beyond the scope of our study.
In our study, the use of a cut-off value of 31.0° for LAD and 52.6° for LCX bifurcation after corrections for confounding factors, was still significant, suggesting that LAD and LCX angles were a reliable predictor of a distal vessel FFRCT of ≤ 0.80.
To the best of our knowledge, this is the first work to investigate the effects of the left coronary bifurcation angle on FFRCT in vessels with no apparent CAD. The present study included vessels with no apparent CAD to exclude confounding factors such as coronary stenosis. In common coronary artery disease, the effect of the bifurcation angle may be more important than the results of our study suggest. Our findings indicate that a large bifurcation angle can affect hemodynamics, resulting in the overestimation of FFRCT. FFRCT should be interpreted more carefully and a correction may be required depending on the left coronary artery bifurcation angle for assessment of myocardial ischemia. Since the bifurcation angle has an effect, the addition of an angulation measurement to conventional FFRCT evaluation may have clinical value and should be considered.
Limitations
Our study has several limitations. First, this is a single-center study. Still, the number of patients included was sufficient for statistical analysis. Second, according to the American Heart Association [29] and European Society of Cardiology guidelines [30], FFR is recommended to assess angiographically intermediate-grade coronary stenosis. The present study included patients with vessels with < 20% coronary stenosis. Therefore, invasive procedures (measurement of FFR or bifurcation angle) were not performed. Previous studies have shown that FFRCT can be an alternative test to invasive FFR due to the high concordance between FFRCT and invasive FFR [31]. FFR values [31] and bifurcation angle [9, 32] assessed by CTA correlate well with invasive measurements. However, it is indeed uncertain whether distal FFR changes related to the bifurcation angle occur in invasive FFR as well as FFRCT. Third, despite the fact that only vessels with no apparent CAD were selected, plaque burden was still observed. Plaque burden observed with no apparent CAD may be due to the following factors: (1) Plaque formation generates at the bifurcation angle due to higher turbulence and low shear stress. The vessel diameter immediately after the bifurcation angle is large, resulting in higher absolute plaque volume even with no apparent CAD; (2) Diffuse plaque deposits throughout the vessel. (3) Plaque deposits on the extravascular side. Our plaque analysis software could not differentiate between intravascular and extravascular plaque. However, it is controversial and difficult to investigate FFRCT in vessel models that completely eliminate the effects of plaque characteristics. Simulated vessel models are assumed to have a rigid wall rather than the elastic wall; therefore, such simulation does not reflect the physiological situation [23]. Plaque burden caused an impaired vasodilator capacity due to oxidative stress and inflammation [33, 34]. This vasodilator dysfunction could interfere with accurate assessment of FFRCT. Fourth, this study did not include ‘no obvious CAD’, thus minimal atherosclerotic lesions were present. Because vessels with advanced atherosclerosis also may show a complex morphology due to curvature or tortuosity in the actual clinical setting, measurement of the bifurcation would optimally be better performed by three-dimensional analysis. However, similar to our experience in previous studies [9, 18, 19, 25], it was quite difficult to assess the bifurcation angle by three-dimensional analysis. Moreover, complex vessel morphology may affect blood flow leading to paradoxical FFRCT changes. Blood flow velocity is accelerated at the stenotic lesion, resulting in potential energy being converted into kinetic energy. A small amount of turbulent eddies generated at the stenotic lesion leads to less thermal energy loss, thus kinetic energy is reconverted into potential energy (pressure recovery phenomenon), which can cause FFRCT increase In order to explore the influence of vessel morphology on FFRCT changes, further investigations of various types of lesions in a larger number of patients may be needed. Fifth, the relationship between bifurcation angle, turbulence, and FFRCT is not clear because turbulence is present not quantifiable [35].
Conclusions
FFRCT decline was dependent on each left coronary bifurcation angle. Vessel length most influences FFRCT, but also the bifurcation angle in vessels with no apparent CAD plays a significant role. As such, a correction may be required depending on the left coronary artery bifurcation angle when interpreting numerical values of FFRCT for assessment of myocardial ischemia. We provide cut-off values at which this effect occurs.
Abbreviations
- CAD:
-
Coronary artery disease
- CTA:
-
Computed tomography angiography
- FFR:
-
Fractional flow reserve
- FFRCT :
-
Computed tomography derived fractional flow reserve
- LAD:
-
Left anterior descending artery
- LCX:
-
Left circumflex artery
References
Cami E, Tagami T, Raff G et al (2018) Assessment of lesion-specific ischemia using fractional flow reserve (FFR) profiles derived from coronary computed tomography angiography (FFRCT) and invasive pressure measurements (FFRINV): importance of the site of measurement and implications for patient referral for invasive coronary angiography and percutaneous coronary intervention. J Cardiovasc Comput Tomogr 12:480–492
Gaur S, Taylor CA, Jensen JM et al (2017) FFR derived from coronary CT angiography in nonculprit lesions of patients with recent STEMI. JACC Cardiovasc Imaging 10:424–433
Tsugu T, Tanaka K, Belsack D et al (2021) Impact of vascular morphology and plaque characteristics on computed tomography derived fractional flow reserve in early stage coronary artery disease. Int J Cardiol 343:187–193
Fairbairn TA, Dobson R, Hurwitz-Koweek L et al (2020) Sex differences in coronary computed tomography angiography-derived fractional flow reserve: lessons from ADVANCE. JACC Cardiovasc Imaging 13:2576–2587
Tsugu T, Tanaka K, Belsack D et al (2022) Effects of left ventricular mass on computed tomography derived fractional flow reserve in significant obstructive coronary artery disease. Int J Cardiol. S0167-5273(22)00324-2 [pii] 355:59–64. https://doi.org/10.1016/j.ijcard.2022.03.005
Tsugu T, Tanaka K, Nagatomo Y, Belsack D, De Maeseneer M, De Mey J (2022) Paradoxical changes of coronary computed tomography derived fractional flow reserve. Echocardiography 39:398–403
Tsugu T, Tanaka K, Belsack D, Jean-Francois A, Mey J (2021) Impact of collateral circulation with fractional flow reserve derived from coronary computed tomography angiography. Turk Kardiyol Dern Ars 49:694–695
Cui Y, Zeng W, Yu J et al (2017) Quantification of left coronary bifurcation angles and plaques by coronary computed tomography angiography for prediction of significant coronary stenosis: a preliminary study with dual-source CT. PLoS One 12:e0174352
Sun Z, Xu L, Fan Z (2016) Coronary CT angiography in calcified coronary plaques: Comparison of diagnostic accuracy between bifurcation angle measurement and coronary lumen assessment for diagnosing significant coronary stenosis. Int J Cardiol 203:78–86
Johnson NP, Kirkeeide RL, Gould KL (2013) Coronary anatomy to predict physiology: fundamental limits. Circ Cardiovasc Imaging 6:817–832
Tsugu T, Tanaka K (2022) Differences in fractional flow reserve derived from coronary computed tomography angiography according to coronary artery bifurcation angle. Turk Kardiyol Dern Ars 50:83–84
Maddox TM, Stanislawski MA, Grunwald GK et al (2014) Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 312:1754–1763
Yoshino S, Cassar A, Matsuo Y et al (2014) Fractional flow reserve with dobutamine challenge and coronary microvascular endothelial dysfunction in symptomatic myocardial bridging. Circ J 78:685–692
Escaned J, Cortes J, Flores A et al (2003) Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J Am Coll Cardiol 42:226–233
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–542
Tonino PA, De Bruyne B, Pijls NH et al (2009) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 360:213–224
Achenbach S, Rudolph T, Rieber J et al (2017) Performing and interpreting fractional flow reserve measurements in clinical practice: an expert consensus document. Interv Cardiol 12:97–109
Konishi T, Funayama N, Yamamoto T, Hotta D, Tanaka S (2018) Relationship between left main and left anterior descending arteries bifurcation angle and coronary artery calcium score in chronic kidney disease: a 3-dimensional analysis of coronary computed tomography. PLoS One 13:e0198566
Konishi T, Yamamoto T, Funayama N, Nishihara H, Hotta D (2016) Relationship between left coronary artery bifurcation angle and restenosis after stenting of the proximal left anterior descending artery. Coron Artery Dis 27:449–459
Motoyama S, Sarai M, Harigaya H et al (2009) Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 54:49–57
Nadjiri J, Hausleiter J, Jahnichen C et al (2016) Incremental prognostic value of quantitative plaque assessment in coronary CT angiography during 5 years of follow up. J Cardiovasc Comput Tomogr 10:97–104
Kawasaki T, Koga H, Serikawa T et al (2009) The bifurcation study using 64 multislice computed tomography. Catheter Cardiovasc Interv 73:653–658
Chaichana T, Sun Z, Jewkes J (2011) Computation of hemodynamics in the left coronary artery with variable angulations. J Biomech 44:1869–1878
Kaazempur-Mofrad MR, Isasi AG, Younis HF et al (2004) Characterization of the atherosclerotic carotid bifurcation using MRI, finite element modeling, and histology. Ann Biomed Eng 32:932–946
Sun Z, Cao Y (2011) Multislice CT angiography assessment of left coronary artery: correlation between bifurcation angle and dimensions and development of coronary artery disease. Eur J Radiol 79:e90–e95
Papadopoulou SL, Brugaletta S, Garcia-Garcia HM et al (2012) Assessment of atherosclerotic plaques at coronary bifurcations with multidetector computed tomography angiography and intravascular ultrasound-virtual histology. Eur Heart J Cardiovasc Imaging 13:635–642
Rodriguez-Granillo GA, Rosales MA, Degrossi E, Durbano I, Rodriguez AE (2007) Multislice CT coronary angiography for the detection of burden, morphology and distribution of atherosclerotic plaques in the left main bifurcation. Int J Cardiovasc Imaging 23:389–392
Rodriguez-Granillo GA, Garcia-Garcia HM, Wentzel J et al (2006) Plaque composition and its relationship with acknowledged shear stress patterns in coronary arteries. J Am Coll Cardiol 47:884–885
Levine GN, Bates ER, Blankenship JC et al (2011) 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 124:2574–2609
Neumann FJ, Sousa-Uva M, Ahlsson A et al (2019) 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 40:87–165
Ko BS, Cameron JD, Munnur RK et al (2017) Noninvasive CT-derived ffr based on structural and fluid analysis: a comparison with invasive FFR for detection of functionally significant stenosis. JACC Cardiovasc Imaging 10:663–673
Pflederer T, Ludwig J, Ropers D, Daniel WG, Achenbach S (2006) Measurement of coronary artery bifurcation angles by multidetector computed tomography. Invest Radiol 41:793–798
Ahmadi A, Stone GW, Leipsic J et al (2016) Association of coronary stenosis and plaque morphology with fractional flow reserve and outcomes. JAMA Cardiol 1:350–357
Lavi S, Yang EH, Prasad A et al (2008) The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension 51:127–133
Chu M, von Birgelen C, Li Y et al (2018) Quantification of disturbed coronary flow by disturbed vorticity index and relation with fractional flow reserve. Atherosclerosis 273:136–144
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Johan De Mey.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 780 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsugu, T., Tanaka, K., Nagatomo, Y. et al. Impact of coronary bifurcation angle on computed tomography derived fractional flow reserve in coronary vessels with no apparent coronary artery disease. Eur Radiol 33, 1277–1285 (2023). https://doi.org/10.1007/s00330-022-09125-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09125-3