Abstract
Key message
Macroscopic, ultrastructural, and molecular features—like a ball shape, the presence of starch granules, and the up-regulation of genes involved in carbohydrate metabolism and secondary metabolite biosynthesis—distinguish PT regions within a callus.
Abstract
The modification of the mass of pluripotent cells into de novo shoot bud regeneration is highly relevant to developmental biology and for agriculture and biotechnology. This study deals with protuberances (PT), structures that appear during the organogenic long-term culturing of callus (OC) in kiwifruit. These ball-shaped regions of callus might be considered the first morphological sign of the subsequent shoot bud development. Sections of PT show the regular arrangement of some cells, especially on the surface, in contrast to the regions of OC beyond the PT. The cells of OC possess chloroplasts; however, starch granules were observed only in PTs’ plastids. Transcriptomic data revealed unique gene expression for each kind of sample: OC, PT, and PT with visible shoot buds (PT–SH). Higher expression of the gene involved in lipid (glycerol-3-phosphate acyltransferase 5 [GPAT5]), carbohydrate (granule-bound starch synthase 1 [GBSS1]), and secondary metabolite (beta-glucosidase 45 [BGL45]) pathways were detected in PT and could be proposed as the markers of these structures. The up-regulation of the regulatory associated protein of TOR (RAPTOR1) was found in PT–SH. The highest expression of the actinidain gene in leaves from two-year-old regenerated plants suggests that the synthesis of this protein takes place in fully developed organs. The findings indicate that PT and PT–SH are specific structures within OC but have more features in common with callus tissue than with organs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The formation of plant callus tissue, composed in large part of uniform cells, is not considered a regression in the developmental lineage but rather an opening to increase the developmental potency (Feher 2019). Reprogramming of the callus due to the culture conditions can induce plant regeneration through organogenesis or somatic embryogenesis. Investigation of the development of shoot meristems from a disorganized callus tissue is crucial for basic research and for applications in agriculture and biotechnology (Niazian et al. 2019). Adventitious shoot formation via a callus is a useful method for plant micropropagation and improvement of genotypes through the techniques used in plant biotechnology programs.
One of the most recently domesticated and popular fruit crops is Actinidia chinensis var. deliciosa A. Chev. (A. Chev.), formerly known as A. deliciosa (A. Chev.) C. F. Liang et A. R. Ferguson, and commonly called kiwifruit. A wide spectrum of interest in the Actinidia genus concern different levels of research—from the taxonomic concept to whole-genome sequencing (Ferguson 2016; Rey et al. 2020).
Previous histological studies revealed that the morphogenic events observed in endosperm-derived callus in kiwifruit led to adventitious shoot bud development (Popielarska et al. 2006; Popielarska-Konieczna et al. 2011). During the experiments each of endosperms isolated from an individual seed gave rise to induction and proliferation of the particular callus line. Further observations indicated that some callus lines maintain a capacity for long-term proliferation and the ability to form shoot buds even after a few years of culturing (unpublished). We suggested that a PT, a ball-shaped region of a callus, is the first morphological sign of shoot bud induction within a kiwifruit callus. The relationship between the appearance of PTs or outgrowths and the emergence of primordia-like structures or shoot buds has been observed during tissue culturing of different species (Nakano and Maeda 1974; Jarret et al. 1981; Fernando et al. 2007; Daffalla et al. 2011; Qiao et al. Full size image
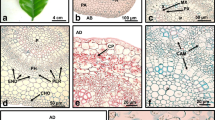
Histological analysis of sections of the bulgy and ball-shaped structures revealed that some cells on the surface and inside of these PT are arranged in a regular manner (Fig. 2a–d). The intercellular spaces were also clearly visible inside and near the surface of the PT. The clusters of cells originated from coordinated divisions were regularly arranged and closely attached (Fig. 2a–d). Numerous starch grains in cells located near the surface of the PT were observed (Fig. 2e, f). Phenolic deposits were observed in many cells as greenish (after TBO staining; Fig. 2b) or orange (after PAS reaction; Fig. 2f) vacuolar inclusions. The samples taken from the OC beyond PT were composed of loosely attached cells, and their appearance was irregular, especially on the surface (Fig. 2g). Some cells contained phenolic deposits (Fig. 2h). After the application of auramine O, the fluorescence of the cell wall was detected in the cells which composed the surface of the PT (Suppl. Figure 1a–c). Weak autofluorescence of the cell wall in cells located inside of the PT was observed. A similar weak fluorescence was found for all cells of the OC beyond PT (Suppl. Figure 1d, e). Staining with Sudan III revealed a reddish color in the outer cell layer of PT (Suppl. Figure 2), which corresponds with fluorescence after the application of auramine O.
Longitudinal sections of detached PTs (a–f) and OC (g, h) in Actinidia chinensis cv. deliciosa; TBO (a–d, g, h) and PAS (e, f) staining. a Bulgy PT with visible coordinated divisions (dv) and compact composition of cells (arrows) on the surface. b Magnification of the rectangle from a shows cells originated from coordinated divisions (dv) and cells with regular composition (arrows) on the surface; the deposition of phenols stained greenish (dotted arrows). c Ball-shaped PT with region of cells formed through coordinated divisions (dv); visible regular, composed cells on the surface (arrows); lineolate line indicates the site of dissection from the rest of the callus. d Magnification of the rectangle from c shows cells clusters formed after coordinated divisions (dv). e Part of the PT composed with the cells, which possess an abundance of blue-violet stained starch grains (open arrows). f Magnification of cells with the starch grains (open arrows) and the deposition of phenols stained orange (dotted arrows). g The surface composed of loosely attached cells (ce) was characteristic of the part of the OC beyond PT. h Phenolic deposits (dotted arrows) inside the cells. Arrows regular composition of cells on the surface; dv cells derived from coordinated divisions; dotted arrows phenolic deposits; open arrows starch grains; is intercellular spaces; sh adventitious shoot bud
The cells within PT were highly vacuolated with a large number of phenolic deposits (Fig. 3a). The nucleus, plastids, and other organelles were located peripherally, near the plasmalemma and the cell wall. Amyloplasts with large starch grains were observed (Fig. 3b). Chloroplasts contain thylakoids, plastoglobules, electron-dense inclusions, and starch grains (Fig. 3c, d). Some chloroplasts demonstrated an elongated shape. In PT cells, the mitochondria, lipid droplets, phenolic deposits, and numerous ribosomes were visible (Fig. 3c, d). Cells located near the surface of PT had a thick cell wall composed of the primary cell wall and the lamellate structure of the second cell wall (Fig. 3e). The cell middle lamella was partially highly extended. Cells of the callus beyond PT contained the mitochondria and chloroplasts without starch grains; however, no lipid droplets were found (Fig. 3f).
TEM images of cells, which form PT (a–e) and OC (f) in Actinidia chinensis cv. deliciosa. a Cells with an abundance of the deposition of phenols (open arrows) in vacuoles (v), laterally located nucleus (nu) and plastids (pl). b Amyloplasts (am) with numerous starch grains (arrows) and deposition of phenols (open arrows) in vacuole (v). c, d Chloroplasts (chl) with starch grains (arrows), plastoglobules (dotted arrows) and an electron-dense inclusions (stars); visible mitochondria (mt), lipid droplets (ld), dense cytoplasm with ribosomes (circles); note elongated chloroplasts (double arrows). e Cells from the surface of PT with thick cell wall composed of the primary cell wall (pw) and lamellate structure of the secondary cell wall (sw); note cell middle layer (cml) between cells (C1, C2). f Chloroplasts (chl) with plastoglobules (dotted arrows) and an electron-dense inclusions (stars); visible part of lobed nucleus (nu) with nucleolus (nc)
Comprehensive transcriptome analysis of OC, PT, and PT–SH
RNA sequencing of three biological replicates from the NOC, OC, PT, and PT–SH, a total of 24 samples, was performed on the Illumina HiSeq 4000 platform. The highest number of clean reads were generated in the OC samples (in total 99,694,899 clean reads and 9.96 Gbp, with an average of 3.32 Gbp per sample) (Table S2). For the PT samples, 15% fewer clean reads were obtained (in total 84,652,609 clean reads and 8.46 Gbp, with an average of 2.82 Gbp per sample). The average GC content was approximately 47% in the samples and the clean reads were of high quality (Q39 bases) (Table S2).
The clean reads from the all samples were subjected to assembly, resulting in 652,203 transcripts with an N50 of 944 bp. Among them, the longest transcript was 15,057 bp, and the average transcript length was 697 bp (Table S3). After identifying isoforms (different transcripts of a single unigene), a total of 260,372 unigenes with an N50 of 620 bp were generated. The average length of the unigenes was 514 bp, ranging from 201 to 14,092 bp (Table S3). Most of the unigenes (about 90%) were < 1000 bp in length.
To identify the putative function of the assembled unigenes of A. chinensis var. deliciosa, they were searched against the SwissProt/Uni-Prot, Pfam, EggNOG, GO, and KEGG databases using the BLASTP and BLASTX algorithms, with an E-value of less than 1.0 × 10–10. In total, 225,748 (86.7%) unigenes were found in at least one of these databases, of which 221,350 unigenes were aligned successfully to known proteins via BLASTX; BLASTP exhibited significant hits for 70,015 unigenes. Among these unigenes, 28,621 and 158,562 sequences matched successfully to records in the Pfam and eggNOG databases, respectively. The functions of the A. chinensis var. deliciosa unigenes were classified via GO analysis. In total, 215,195 unigenes were successfully categorized into 23,477 functional groups and they were classified into three major GO categories—biological processes, molecular function, and cell component—which were represented by 14,823, 2300, and 6354 GO terms, respectively (Suppl. data S1). The dominant subcategories of the classified genes in the category of biological processes included transcription DNA-templated (16,196), DNA integration (6729), DNA recombination (5181), translation (4646), cell division (4198), protein transport (3893), DNA repair (3626), cell cycle (3538), cell wall organization (3197), protein phosphorylation (2854), signal transduction (2827), carbohydrate metabolic process (2784), and multicellular organism development (2639) (Fig. 4a); in the molecular function category were ATP binding (42,842), metal ion binding (35,141), DNA binding (15,569), RNA binding (12,155), zinc ion binding (10,958), endonuclease activity (8471), protein serine/threonine kinase activity (6355), DNA-binding transcription factor activity (6099), and GTP binding (4371) (Fig. 4b); the cell component category included cytoplasm (44,239), integral component of membrane (40,217), nucleus (32,780), cytosol (16,532), chloroplast (8898), mitochondrion (8339), extracellular region (7609), Golgi apparatus (5557), endoplasmic reticulum membrane (5404), plasmodesma (3987), extracellular space (2710), and cell wall (2114) (Fig. 4c).
The KEGG pathway analysis provided annotations of 186,284 unigenes that were distributed to 519 KEGG pathways (Suppl. data S1). The most unigene sequences (59,389) exhibited matches to Arabidopsis thaliana pathways. Among the annotated pathways, the “chromosome and associated proteins,” “transfer RNA biogenesis,” “membrane trafficking,” “transporters,” “DNA repair and recombination proteins,” “amino acid related enzymes,” “mitochondrial biogenesis,” and “peptidases and inhibitors” pathways were the most abundant (Fig. 4d).
Based on the de novo assembled transcriptome as a reference, the genes expressed in the NOCs and three kinds of OC (OC, PT, and PT–SH) were identified. Differential expression analysis was based on fragments per kilobase of transcript per million (FPKM) and an FDR < 0.05. The DEG numbers are shown in Table S4.
Compared with the NOC, 3028 significant DEGs were identified in the OC; in the PT and PT–SH, the number of DEGs was 6.5 and 9.1 times higher, respectively. Among the DEGs detected in OC, the number of up-regulated genes was 2584 and there were 6 times fewer (444) down-regulated genes (Table S4 and Fig. 5a). We compared the three data sets from comparisons versus the NOC group (Fig. 5b). The Venn diagram analysis of DEGs revealed a total of 2455 commonly expressed genes in the OC, PT, and PT–SH and 252 genes exclusively expressed in OC, of which 203 were up-regulated.
Differentially expressed genes (DEGs) in OCs, PTs, and PT–SH presented in the volcano plots for OC vs. NOC, PT vs. OC, PT–SH vs. OC, and PT–SH vs. PT comparisons; the red dots represent DEGs (FDR < 0.05) in each comparison (a) and in Venn diagrams for OC vs. NOC, PT vs. NOC, and PT–SH vs. NOC (b) and PT vs. OC and PT–SH vs. OC comparisons (c)
Differential gene expression analysis among the three kinds of OC revealed 314 DEGs between PT and OC, 1521 DEGs between PT–SH and OC, and 885 DEGs between PT–SH and PT (Table S4). Among them, up-regulated genes were predominant, though the number of such genes is 0.6 times higher for PT vs. OC combination, 0.4 times for PT–SH vs. OC, and only 0.3 times higher for PT–SH vs. PT (Fig. 5a). The Venn diagram (Fig. 5c) revealed 216 commonly expressed DEGs in the PTs and PT–SH compared with the OC, and 98 and 1305 DEGs exclusively expressed in PTs and PT–SH. Detailed characteristics of common and specific DEGs for each combination are enclosed as Suppl. data S2.
To obtain insight into the functional categories of the DEGs induced in the transformation of the OC, a GO enrichment analysis was performed using TopGO (Fisher’s exact test; FDR ≤ 0.001). The GO enrichment analysis showed that the most significant GO DEGs in the biological process (BP) category were assigned to PT–SH vs. PT, and most DEGs were assigned to the molecular function (MF) and cellular component (CC) categories in the OC vs. NOC combination (Table S5). Among all combinations, the number of up-regulated DEGs was greater than the down-regulated ones. A total of 1851, 462, and 7778 DEGs were annotated by the BP terms derived from OC vs. NOC, PT vs. OC, and PT–SH vs. PT comparisons, respectively. For the biological process classification in the OC vs. NOC combination, the most significant enriched GO categories were photosynthesis, followed by oxylipin biosynthetic process, secondary metabolite biosynthetic process, oxidation–reduction process, lignin biosynthetic process, plant-type primary cell wall biogenesis, and response to oxidative stress (Table S6; Suppl. data S3). In the PT vs. OC comparison, the regulation of biological process showed 375 (81.2%) up-regulated genes (Table S5) and among all DEGs the most significant enriched GO categories sequentially were carbohydrate metabolic process, followed by plant-type primary cell wall biogenesis, cell wall organization, basic amino acid transport, multidimensional cell growth, and finally auxin catabolic process (Table S6; Suppl. data S3). In the aspect of enrichment of DEGs for the PT–SH vs. PT combination in the biological process category, the most significant enriched GO categories were DNA-templated transcription, water transport, response to abscisic acid, xyloglucan metabolic process, auxin catabolic process, hydrogen peroxide catabolic process, and urea transport (Table S6; Suppl. data S3).
The next stage was to select uniquely and commonly enriched GO terms in the BP category associated with each kind of OC. The Venn diagrams showed an overlap of BP GO terms relative to NOC among OC, PT, and PT–SH (Suppl. data S3:file 4). Thanks to this approach, in the OC, 26 uniquely enriched GO categories were indicated, involved in photosynthetic electron transport, stabilization and assembly of PSII, negative regulation of brassinosteroid biosynthetic process, positive regulation of development, somatic embryogenesis, regulation of cutin biosynthetic process, lignan biosynthetic process, 1-deoxy-d-xylulose 5-phosphate biosynthetic process, and stem vascular tissue pattern formation, among others. Venn diagrams showing an overlap of BP GO terms among PT and PT–SH relative to OC (Suppl. data S3) led to the identification of 12 unique GO terms for PT, i.e., beta-glucan biosynthetic and metabolic process, carbohydrate biosynthetic process, starch biosynthetic process, mitotic cytokinesis, and multidimensional cell growth and 70 specific BP GO categories for PT–SH, including response to abscisic acid and lipids, transport of water, urea, phosphate ion, hemicellulose metabolic process, suberin biosynthetic process, metabolic process of l-asparagine, phenylpropanoid, and brassinosteroid.
In the MF category classification, the most highly enriched MF terms by DEGs derived from the OC vs. NOC combination were binding and catalytic activity (Table S6; Suppl. data S4). In the binding subset, binding of pigment, iron ion, chlorophyll, and DNA transcription factor activity were significantly higher than the others. In catalytic activity, the most significant terms represented oxidoreductase activity, i.e., glyceraldehyde-3-phosphate dehydrogenase activity and linoleate 13S-lipoxygenase activity. In the PT vs. OC combination, the number of DEGs assigned to catalytic activity and binding was significantly higher than the other classifications (Table S6; Suppl. data S4). In the first subset were the activities of beta-galactosidase activity, cellulose synthase, and xyloglucan 1,6-alpha-xylosidase, and the binding MF category was represented by carbohydrate binding. For the PT–SH vs. OC combination, enrichment of DEGs in the MF category resulted in assignment to five subcategories: catalytic activity, transcription regulator activity, structural molecule activity, transporter activity, and binding (Table S6; Suppl. data S4:file 3). Venn diagrams presenting an overlap of MF GO terms among OC, PT, and PT–SH relative to NOC (Suppl. data S4) showed 29 unique GO terms for OC including the activity of: acylglycerol lipase, long-chain-(S)-2-hydroxy-long-chain-acid oxidase, carbohydrate transmembrane transporter, and 1-deoxy-d-xylulose-5-phosphate synthase. Venn diagrams for combinations of PT and PT–SH relative to OC (Suppl. data S4) indicted three unique GO terms for PT, i.e., glycerate dehydrogenase, xyloglucan 1,6-alpha-xylosidase, and alpha-d-xyloside xylohydrolase activity, and 59 unique MF GO categories for PT–SH, including activity of water transmembrane transporter, DNA binding transcription factor, xyloglucan:xyloglucosyl transferase, symporter, and asparagine synthase (glutamine-hydrolyzing).
In the CC classification, 42 GO terms were assigned to DEGs derived from the OC vs. NOC combination and 8 and 12 GO terms for DEGs from the PT vs. OC and PT–SH comparisons, respectively (Table S5). The most numerous group of downregulated genes was for PT–SH vs. PT (Table S4). Under the cellular component category, in the OC vs. NOC comparison most DEGs were assigned to chloroplast, PSI and PSII, integral component of membrane, and apoplast (Table S5; Suppl. data S5). In the PT vs. OC comparison, the highest number of DEGs were correlated with apoplast, cell wall, anchored component of membrane, and complex of cellulose synthase (Table S5; Suppl. data S5). In the PT–SH vs. PT combination, the most down-regulated DEGs were related with vacuole, primary cell wall, apoplast, and integral component of plasma membrane (Table S5; Suppl. data S5). Venn diagrams showing an overlap of CC terms among OC, PT, and PT–SH relative to NOC (Suppl. data S5:file 4) identified 16 unique GO terms for OC associated mainly with chloroplast and complexes of oxidoreductase and NAD(P)H dehydrogenase. Venn diagrams for combinations of PT and PT–SH relative to OC (Suppl. data S5:file 5) identified two unique CC GO terms for PT: anchored component of membrane and cellulose synthase complex. For the PT–SH vs. OC combination, enrichment of DEGs in the CC category resulted in assignment to 13 subcategories related with vacuole, plasma membrane, photosystem I, and etioplast (Suppl. data S3).
The pathways that displayed significant changes during transformation from OC to PT–SH were identified using the KEGG database. A total of 21 KEGG pathways were significantly enriched only for OC vs. NOC and PT–SH vs. PT combinations (Fig. 6), among which “biosynthesis of secondary metabolites” and “aminoacyl-tRNA biosynthesis” pathways were common. For the OC vs. NOC combination, the most highly represented pathways were “photosynthesis”, “plant hormone signal transduction”, and “terpenoid backbone biosynthesis” and for the PT–SH vs. PT combination they were “alanine, aspartate, and glutamate metabolism” and “biosynthesis of secondary metabolites”.
Pathway enrichment of DEGs based on KEGG during the transformation from OC to PT–SH for comparisons, i.e., OC vs. NOC (a) and PT–SH vs. PT (b); the area of bubbles indicates the number of enriched DEGs, the color of the bubbles indicates p adjusted value; up- and down-regulated DEGs are defined as activated and suppressed, respectively
Selection of OC, PT, and PT–SH-responsive genes
Among the 3028 DEGs identified for the OC vs. NOC combination, 252 DEGs were defined as uniquely expressed in OC (Fig. 5b, Suppl. data S2). The Venn diagram showing the overlap of DEGs among PT and PT–SH related to OC (Fig. 5c) identified 98 unique DEGs for PT vs. OC (Suppl. data S2) and 1305 unique DEGs for PT–SH vs. OC (Suppl. data S2). Among the 216 DEGs that were common in both combinations, i.e., PT vs. OC and PT–SH vs. OC, indicated genes that differed in the expression pattern (Suppl. data S2). The set of these common DEGs was subjected to hierarchical clustering (Fig. 7). The results showed that most of the DEGs presented a similar expression pattern between PT and PT–SH related to OC; however, DEGs with different expression levels are noticeable (Table 1). Among the DEGs whose expression was higher for the PT vs. OC combination than for PT–SH vs. OC were genes related to lipid transport (putative lipid-transfer protein DIR1), multicellular organism development (cytochrome P450 87A3), and regulation of growth (zeatin O-glucosyltransferase, calcium-binding protein PBP1), cell cycle (division) (protein ALP1-like, probable nicotinate-nucleotide adenylyltransferase), cell wall biogenesis (xyloglucan endotransglucosylase/hydrolase 2), and regulation of transcription (heat stress transcription factor A-3) (Table 1). The DEGs identified for the PT–SH vs. OC combination which characterized higher expression levels than for DEGs identified in the PT vs. OC combination were involved in multicellular organism development (protein light-dependent short hypocotyls, transcription factor MYB74, remorin 4,1), encoding actinidain (cysteine protease responsible for the cleavage of kiwellin), and the regulation of growth (3-ketoacyl-CoA synthase 6), lipid metabolism (linoleate 13S-lipoxygenase 2-1), and the carbohydrate metabolic process (uncharacterized protein RP505, beta-galactosidase 12) (Table 1). Finally, each type of examined callus—i.e., OC, PT, and PT–SH—was described by unique enriched GO terms for which were indicated unique genes expressed only in one type of callus (Table 2).
Heatmap plot using the hierarchical clustering method presenting the expression pattern of 216 common DEGs for PT vs. OC and PT–SH vs. OC; three main clusters are shown; expression values presented after being log-transformed; decreased and increased expression of DEGs are distinguished in blue and red, respectively
Among the pool of expressed genes in the OC were genes involved in photosynthesis and chloroplast metabolism. Eight up-regulated genes were involved in photosynthetic electron transport in PSI: photosynthetic NDH subunit of subcomplex B3 and B4 (LogFC = 6.2–8.3), encoding protein curvature thylakoid 1B (LogFC = 7.3), genes of PSI reaction center subunit V, and encoding protein proton gradient regulation 5 (LogFC = 4.3); six genes connected with the stabilization and assembly of PSII were significantly activated: PS II repair protein PSB27-H1 (LogFC = 10.3) and oxygen-evolving enhancer protein 1 (LogFC = 10.3), while gene encoded chaperone protein dnaJ C76 was strongly activated (LogFC = 9.1). In OC, we found that the expression of genes regulating the developmental process and transcription factors mainly dominated, i.e., TCP2-4 (LogFC = 2.1–5.7), nuclear transcription factor Y subunit A-1, A-5, and A-6 (LogFC = 3.3–9.5), but high expression (LogFC = 11.1) was also observed for ABC transporter G family member 14 gene, which is involved in stem vascular tissue pattern formation. Moreover, BR11 kinase inhibitor gene—related to the brassinosteroid biosynthetic process—was found to be up-regulated in relation to NOCs (LogFC = 6.7). In OCs, we identified DEGs that are associated with the lignan biosynthetic process: encoded secoisolariciresinol dehydrogenase (LogFC = 6.9) and momilactone A synthase (LogFC = 6.3). There were numerous DEGs associated with carbohydrate transmembrane transport, such as bidirectional sugar transporter SWEET12 (LogFC = 6.9), probable inositol transporter 2 (LogFC = 6.1), and ascorbate transporter (LogFC = 6.1). In OC we also identified down-regulated TF genes that were associated with the regulation of cutin biosynthetic process: ethylene-responsive TF WRI1 (LogFC = -7.4) or AP2-like ethylene-responsive TF (LogFC = − 4.3) (Table 2). In PT, unique DEGs were mostly related to carbohydrate metabolism and were associated with growth and developmental processes and cell wall structure. The beta-glucosidase 45 gene was found to be highly up-regulated (LogFC = 9.0), as were genes associated with starch biosynthetic process: granule-bound starch synthase 1 gene, glucose-1-phosphate adenylyltransferase genes, and genes which encode cellulose synthase subunits (Table 2). Among unique DEGs involved in cell wall modification, glycerol-3-phosphate acyltransferase 5 gene was activated in PT (LogFC = 7.6), while alpha-xylosidase 1 gene was down-regulated (LogFC = -4.7). In PT–SH the expression of genes involved in developmental processes and response to plant growth regulators was observed: ABA and lipids, transport of substances, cell wall modification, and secondary metabolites biosynthesis; numerous transcription factors were also activated (Table 2). Among unique DEGs for PT–SH, especially high expression levels were reported for genes such as regulatory-associated protein of TOR 1, which is associated with the maintenance of shoot apical meristem identity (LogFC = 9.1), and homeobox-leucine zipper protein, protodermal factor 2, which is involved in epidermal cell differentiation (LogFC = 7.5). Interestingly, among six genes annotated as xyloglucan endotransglucosylase/hydrolase protein, three of them were up-regulated (TRINITY_DN67175_c0_g, TRINITY_DN77522_c0_g1, and TRINITY_DN79364_c1_g3) while three were down-regulated (TRINITY_DN80529_c4_g2, TRINITY_DN92346_c1_g3, and TRINITY_DN78783_c3_g2) (Table 2).
RT-qPCR expression analysis of OC, PT, and PT–SH-responsive genes involved in carbohydrate, lipid and second metabolites metabolism
The results showed that, compared with NOC, all of the examined genes (Table S1) appeared to be up-regulated in different regions of OC–PT and PT–SH (Fig. 8). Genes related to carbohydrate metabolism, i.e., beta-glucosidase 45 (BGL45) and granule-bound starch synthase 1 (GBSS1), had differential expression patterns among the investigated developmental stages. An increase in the expression of the BGL45 gene was noted in OC; however, an expression three times higher was evidenced in PT–SH, though no significant differences in transcript abundance were detected between PT and PT–SH (Fig. 8a). Interestingly, the expression of this gene was not determined in regenerated plants. The greatest increase in GBSS1 gene activity was evidenced for PT with shoots (PT–SH), it was two times higher than in PT, although no significant differences in the expression of that gene were noted between NOC and OC or all REGs (Fig. 8b). For genes involved in the suberin biosynthesis process—glycerol-3-phosphate acyltransferase (GPAT5)—and lipid metabolism—linoleate 13 s-lipoxygenase 2-1 (LOX2.1)—differences in the expression levels of GPAT5 were observed in all probes, wherein the expression profile was as follows: PT > PT–SH > OC = REG > NOC (Fig. 8c). The greatest increase in LOX2.1 gene activity was observed in regenerated plants at the medium and elderly stages (REG-M, REG-E)—it was about 2.5 times higher than in OC and PT–SH (Fig. 8d). Differential expression patterns for homeobox-leucine zipper protein protodermal factor 2 (PDF2) gene, which are related to epidermal cell differentiation, were found across PT–SH and PT, wherein about a threefold increase in PT–SH was observed (Fig. 8e). The age of regenerated plants had a considerable influence on the expression of the PDF2 gene, i.e., a statistically significantly lower gene expression was observed in REG-E in comparison to REG-Y and REG-M. The increase in the expression of the actinidain (ACT1A) gene was evidenced in PT–SH (Fig. 8f) in relation to OC and PT, but the highest expression level was indicated in elderly regenerated plants (REG-E). The expression profile of the gene related to the organization of the apical meristem of shoots, i.e., regulatory associated protein of TOR1 (RAPTOR1), was interesting (Fig. 8g): an increase in transcript abundance was noted in OC and PT–SH, whereas no difference in expression was detected between PT and NOC, and the inhibition of RAPTOR1 expression was observed in all regenerants probes. To sum up, each region of OC could be characterized by the highest expression levels of specific genes evaluated in comparison to NOC—i.e., GPAT5 for PT and GBSS1, PDF2, and ACT1A for PT–SH—and in general, the expression profiles of BGL45, LOX2.1, and RAPTOR1 genes were common for OC, PT, and PT–SH.
Relative expression levels of BGL45 (a), GBSS1 (b), GPAT5 (c), LOX2.1 (d), PDF2 (e), ACT1A (f), and RAPTOR1 (g) genes for cultured explants—NOC, OC, PT, PT–SH, and regenerated plants at different ages: REG-Y, REG-M, REG-E in Actinidia chinensis cv. deliciosa. Error bars show the mean standard error calculated from three independent biological replicates. The same letters indicate a lack of differences in relative expression levels between developmental stages; p < 0.05; nd not determined in qRT-PCR