Abstract
Population dynamics and evolutionary genetics underly the structure of ecosystems, changing on the same timescale for interacting species with rapid turnover, such as virus (e.g. HIV) and immune response. Thus, an important problem in mathematical modeling is to connect ecology, evolution and genetics, which often have been treated separately. Here, extending analysis of multiple virus and immune response populations in a resource—prey (consumer)—predator model from Browne and Smith (2018), we show that long term dynamics of viral mutants evolving resistance at distinct epitopes (viral proteins targeted by immune responses) are governed by epistasis in the virus fitness landscape. In particular, the stability of persistent equilibrium virus-immune (prey-predator) network structures, such as nested and one-to-one, and bifurcations are determined by a collection of circuits defined by combinations of viral fitnesses that are minimally additive within a hypercube of binary sequences representing all possible viral epitope sequences ordered according to immunodominance hierarchy. Numerical solutions of our ordinary differential equation system, along with an extended stochastic version including random mutation, demonstrate how pairwise or multiplicative epistatic interactions shape viral evolution against concurrent immune responses and convergence to the multi-variant steady state predicted by theoretical results. Furthermore, simulations illustrate how periodic infusions of subdominant immune responses can induce a bifurcation in the persistent viral strains, offering superior host outcome over an alternative strategy of immunotherapy with strongest immune response.








Similar content being viewed by others
References
Ahmed SF, Quadeer AA, Morales-Jimenez D, McKay MR (2019) Sub-dominant principal components inform new vaccine targets for hiv gag. Bioinformatics 35(20):3884–3889
Althaus CL, Boer RD (2008) Dynamics of immune escape during HIV/SIV infection. PLoS Comput Biol 4:e1000103
Barton JP, Goonetilleke N, Butler TC, Walker BD, McMichael AJ, Chakraborty AK (2016) Relative rate and location of intra-host HIV evolution to evade cellular immunity are predictable. Nat Commun 7:1–10
Bascompte J, Jordano P, Melián CJ, Olesen JM (2003) The nested assembly of plant-animal mutualistic networks. Proc Natl Acad Sci 100(16):9383–9387
Beerenwinkel N, Pachter L, Sturmfels B (2007) Epistasis and shapes of fitness landscapes. Stat Sinica pp 1317–1342
Bobko N, Zubelli JP (2015) A singularly perturbed HIV model with treatment and antigenic variation. Math Biosci Eng MBE 12(1):1–21
Bratus AS, Novozhilov AS, Semenov YS (2019) Rigorous mathematical analysis of the quasispecies model: from Manfred Eigen to the recent developments. Adv Math Methods Biosci Appl pp 27–51
Browne, C.: Global properties of nested network model with application to multi-epitope HIV/CTL dynamics. J Math Biol pp 1–22 (2017)
Browne CJ, Smith HL (2018) Dynamics of virus and immune response in multi-epitope network. J Math Biol 77(6–7):1833–1870
Chakraborty AK, Barton JP (2017) Rational design of vaccine targets and strategies for HIV: a crossroad of statistical physics, biology, and medicine. Rep Progress Phys 80(3):032601
Champagnat N, Méléard S (2011) Polymorphic evolution sequence and evolutionary branching. Probab Theory Relat Fields 151(1–2):45–94
Costa M, Hauzy C, Loeuille N, Méléard S (2016) Stochastic eco-evolutionary model of a prey-predator community. J Math Biol 72(3):573–622
Crona K (2020) Rank orders and signed interactions in evolutionary biology. Elife 9:e51004
Crona K, Gavryushkin A, Greene D, Beerenwinkel N (2017) Inferring genetic interactions from comparative fitness data. Elife 6:e28629
Deutekom HV, Wijnker G, Boer RD (2013) The rate of immune escape vanishes when multiple immune responses control an HIV infection. J Immunol 191:3277–3286
Eble H, Joswig M, Lamberti L, Ludington W (2020)Higher-order interactions in fitness landscapes are sparse. ar**v preprint ar**v:2009.12277
Ferretti L, Schmiegelt B, Weinreich D, Yamauchi A, Kobayashi Y, Tajima F, Achaz G (2016) Measuring epistasis in fitness landscapes: the correlation of fitness effects of mutations. J Theor Biol 396:132–143
Ganusov VV, De Boer RJ (2006) Estimating costs and benefits of CTL escape mutations in SIV/HIV infection. PLoS Comput Biol 2(3):e24
Ganusov VV, Goonetilleke N, Liu MK, Ferrari G, Shaw GM, Borrow AJMP, Korber BT, Perelson AS (2011) Fitness costs and diversity of the cytotoxic t lymphocyte (CTL) response determine the rate of CTL escape during acute and chronic phases of HIV infection. J Virol 85(20):10518–10528
Goh B (1978) Sector stability of a complex ecosystem model. Math Biosci 40(1–2):157–166
Gould AL, Zhang V, Lamberti L, Jones EW, Obadia B, Korasidis N, Gavryushkin A, Carlson JM, Beerenwinkel N, Ludington WB (2018) Microbiome interactions shape host fitness. Proc Natl Acad Sci 115(51):951–960
Gurney J, Aldakak L, Betts A, Gougat-Barbera C, Poisot T, Kaltz O, Hochberg ME (2017) Network structure and local adaptation in co-evolving bacteria-phage interactions. Mol Ecol 26(7):1764–1777
Hallgrímsdóttir IB, Yuster DS (2008) A complete classification of epistatic two-locus models. BMC Genet 9(1):1–15
Hofbauer J, Sigmund K (1998) Evolutionary games and population dynamics. Cambridge University Press, Cambridge
Jover LF, Cortez MH, Weitz JS (2013) Mechanisms of multi-strain coexistence in host-phage systems with nested infection networks. J Theor Biol 332:65–77
Kessinger TA, Perelson S, Neher RA (2015)Inferring HIV escape rates from multi-locus genotype data. Immune system modeling and analysis p 348
Korytowski DA, Smith HL (2015) How nested and monogamous infection networks in host-phage communities come to be. Thyroid Res 8(1):111–120
Kraut A, Bovier A (2019) From adaptive dynamics to adaptive walks. J Math Biol 79(5):1699–1747
Leviyang U, Ganusov VV (2015) road CTL response in early HIV infection drives multiple concurrent CTL escapes. PLoS Comput Biol 11(10):e1004492
Liu MK, Hawkins N, Ritchie AJ, Ganusov VV, Whale V, Brackenridge S, Li H, Pavlicek JW, Cai F, Rose-Abrahams M et al (2013) Vertical t cell immunodominance and epitope entropy determine HIV-1 escape. J Clin Investig 123(1):380–393
Mani R, Onge RPS, Hartman JL, Giaever G, Roth FP (2008) Defining genetic interaction. Proc Natl Acad Sci 105(9):3461–3466
McMichael AJ (2006) HIV vaccines. Annu Rev Immunol 24:227–255
Nowak MA, Bangham CR (1996) Population dynamics of immune responses to persistent viruses. Science 272(5258):74–79
Pantaleo G, Lévy Y (2013) Vaccine and immunotherapeutic interventions. Curr Opin HIV AIDS 8(3):236–242
Rife Magalis, B., Autissier, P., Williams, K.C., Chen, X., Browne, C., Salemi, M.: Predator-prey dynamics of intra-host simian immunodeficiency virus evolution within the untreated host. Front Immunol p 4191 (2021)
Seifert D, Di Giallonardo F, Metzner KJ, Günthard HF, Beerenwinkel N (2015) A framework for inferring fitness landscapes of patient-derived viruses using quasispecies theory. Genetics 199(1):191–203
Stadler PF (2002) Fitness landscapes. Biological Evolution and Statistical Physics. Springer, Berlin, pp 183–204
Walker BD, Xu GY (2013) Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol 13(7):487–498
Weinreich DM, Lan Y, Wylie CS, Heckendorn RB (2013) Should evolutionary geneticists worry about higher-order epistasis? Curr Opin Genet Dev 23(6):700–707
Weitz JS, Poisot T, Meyer JR, Flores CO, Valverde S, Sullivan MB, Hochberg ME (2013) Phage-bacteria infection networks. Trends Microbiol 21(2):82–91
Wolkowicz GS (1989) Successful invasion of a food web in a chemostat. Math Biosci 93(2):249–268
Acknowledgements
We would like to thank the editor and anonymous reviewers for comments which have led to an improved manuscript. We also thank Hal Smith for insightful discussions.
Funding
CJB and FY acknowledge support by a U.S. National Science Foundation grant (DMS-1815095).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
1.1 Proofs of Theorems
Proof of Proposition 2
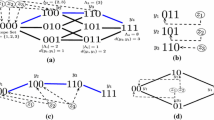
Let \({\mathcal {C}}\subset \left\{ 0,1\right\} ^n\) be a circuit and suppose by way of contradiction that \(\mathcal E^*=(x^*,\textbf{y}^*,\textbf{z}^*)\) is an equilibrium of (4) with \(y_{{\textbf{k}}}^*>0\) for corresponding sequences \({\textbf{k}}\in {\mathcal {C}}\). Adding up the relative growth rates of these nonzero components at equilibrium in differential Eq. (4), we find the following:
because \(\sum _{{\textbf{k}}\in {\mathcal {C}}} \alpha _{{\textbf{k}}} \textbf{k}=0\) and \(\sum _{{\textbf{k}}\in {\mathcal {C}}} \alpha _{{\textbf{k}}} =0\) for a circuit. This contradicts assumption \(\sum _{{\textbf{k}}\in {\mathcal {C}}} \alpha _{{\textbf{k}}} {\mathcal {R}}_{{\textbf{k}}} \ne 0\) and thus proves the first statement. The next statement follows from Proposition 1 upon assuming \(\sum _{{\textbf{k}}\in {\mathcal {C}}} \alpha _{{\textbf{k}}} R_{{\textbf{k}}}=0\). Indeed, uniqueness of equilibrium in a certain positivity class is equivalent to \(\textrm{Ker}(A')^T\cap \mathbf {{\mathcal {R}}}'^{\perp }=\left\{ {\textbf{0}}\right\} \), which is equivalent to the condition that the augmented matrix \(C=\begin{pmatrix} A'&\mathbf {{\mathcal {R}}'}\end{pmatrix}^T\) has trivial kernel (Browne and Smith 2018). Here \(A'\) is the \(m'\times n'\) interaction matrix consisting of the \(m'\) strains comprising the circuit and \(n'\) (positive component) immune responses. Consider the vector \( \varvec{\alpha }\) consisting of the circuit weights. Then from the previous points, we find that \(C \varvec{\alpha }={\textbf{0}}\). Thus, there cannot be a unique equilibrium with \(y^*_{{\textbf{k}}}>0\) for all \({\textbf{k}}\in \mathcal C\). If such an equilibrium exists, then the virus component vector denoted by \(\bar{{\textbf{y}}}\) which satisfies \(\bar{\textbf{y}}-{\textbf{y}}^*\in \textrm{Ker}(A')^T\cap \mathbf {{\mathcal {R}}}'^{\perp }\), also satisfies equilibrium equations, and thus there are infinitely many equilibria with component \(\bar{{\textbf{y}}}= {\textbf{y}}^* + \beta \varvec{\alpha }\) for \(\beta \in {\mathbb {R}}\). \(\square \)
Proof I of Theorem 2
In order to prove the theorem, we show that the general saturated equilibria inequalities (11) reduce to vanishing linear forms of invasion circuits (Definition 3) when evaluated at the nested network equilibria (14). Then we will apply Theorem 1 to show stability of nested equilibrium and uniform persistence of associated positive component strains.
Let \({\mathcal {R}}_0> {\mathcal {Q}}_0:=1\) and k be the largest integer in [1, n] such that \({\mathcal {R}}_{k-1} > {\mathcal {Q}}_{k}\). First, we look at the case \(k=n\) and \({\mathcal {R}}_n>{\mathcal {Q}}_n\) when \(\widetilde{{\mathcal {E}}}_{n}\) is non-negative equilibrium with positive components at the \(n+1\) nested strains \(y_0,y_1,\dots ,y_n\). Consider a given missing viral strain \(y_{i}\) (\(i\in [n+1,2^n-1]\)) with sequence \({\textbf{i}}\). We define a linear form, \({\mathcal {A}}_i\), based on it’s invasion rate as follows:
The telesco** sum above is determined by the following sequence: \(\left( \alpha _{j}\right) , \ j=0,1,\dots ,n\), where \(\alpha _{0}=1-i_{1}\), \(\alpha _{j}=i_{j}-i_{j+1}\) for \(j=2,\dots ,n-1\), \(\alpha _{n}=i_{n}\). In this way, \(-{\mathcal {A}}_{i}:= {\mathcal {R}}_{i}-\sum _{j=0}^{n}\ \alpha _j {\mathcal {R}}_j\). In order to prove that this is a vanishing linear form of a circuit, we show that it is the linear form of a minimally linearly dependent collection of extended binary sequences. Denote the binary sequences of nested network as \({\textbf{k}}_0,\dots ,{\textbf{k}}_n\) corresponding to ordered strains \(y_0,\dots , y_n\). Let \(\mathcal N\subset \left\{ 0,1\right\} ^{n+1}\) denote the subset of nested extended binary sequences, where \(\hat{{\textbf{i}}}={\textbf{i}}1 \in \left\{ 0,1\right\} ^{n+1}{\setminus } {\mathcal {N}}\) and \(\hat{\textbf{k}}={\textbf{k}}1\in {\mathcal {N}}\) represent binary sequences extended by digit 1. Notice that \({\mathcal {N}}\) forms a basis of \(\mathbb R^{n+1}\) (since the \(n+1\times n+1\) matrix \(\left( \textbf{k}_n,{\textbf{k}}_{n-1},\dots ,{\textbf{k}}_0 \right) \) has a triangular row reduced eschelon form with values \(\pm 1\) on diagonal). Thus for \(\hat{{\textbf{i}}} \in \left\{ 0,1\right\} ^{n+1}{\setminus } {\mathcal {N}}\), there is a unique set of coefficients \(\alpha _{j}\), \(j=0,1,2,\dots ,n\), yielding \(\hat{{\textbf{i}}}\) as a linear combination of the nested network vectors:
The above linear system resolves as follows:
which leads us to the set of coefficients \(\alpha _{k}\) where \(k=0,1,\dots ,n\) defined by the following:
Therefore the set \(\hat{{\textbf{i}}}\cup \left\{ \hat{\textbf{k}}\right\} _{\hat{{\textbf{k}}}\in {\mathcal {N}}}\) is linearly dependent. Let \(\alpha _i\) be the nonzero terms in sequence \((\alpha _j)\), i.e. \(\varTheta _i:=\left\{ j\in [0,n]: \alpha _j\ne 0\right\} \), where \(\alpha _j=\pm 1\) for \(\alpha _j\in \varTheta _i\). Since \((\alpha _j)\) is unique linear combination with respect to basis \({\mathcal {N}}\), the set \(\hat{{\textbf{i}}}\cup \left\{ \hat{\textbf{k}}\right\} _{\hat{{\textbf{k}}}\in \varTheta _i}\) is a minimal linearly dependent set. Thus we obtain the following circuit and corresponding vanishing linear form:
Furthermore, if \({\mathcal {A}}_i>0\), then \(\frac{\dot{y}_i}{\gamma _i y_i} <0\) for \(i\in [n+1,2^n-1]\). For this case \(k=n\), the nested equilibrium (14) include all immune response \(z_1,\dots ,z_n\) as positive components. If \({\mathcal {R}}_n>\mathcal Q_n\), then all missing species of non-negative equilibrium, \(\widetilde{{\mathcal {E}}}_{n}\) are the \(y_{i}\) with index \(i\in [n+1,2^n-1]\). Thus, inequalities (11) hold strictly and the conclusions of Theorem 1 follow, in particular, \(\widetilde{{\mathcal {E}}}_{n}\) is stable, \(y_0,\dots ,y_n\) are uniformly persistent, and missing strains \(y_{i}\), \(i\in [n+1,2^n-1]\), go extinct. Notice in this case of \(\mathcal R_n>{\mathcal {Q}}_n\), that \(\widetilde{{\mathcal {E}}}_{n}\) becomes unstable if any of the invasion circuits’ linear forms become non-positive. In particular, if \({\mathcal {A}}_i\le 0\), then \(\frac{\dot{y}_i}{\gamma _i y_i} \ge 0\), yielding the necessary condition of stability of \(\widetilde{{\mathcal {E}}}_{n}\) based on sign of \({\mathcal {A}}_i\) which is stated in Corollary 1. Furthermore, by Proposition 2, in the critical case of \({\mathcal {A}}_i=0\), there exists a continuum of equilibria with positive components including the circuit elements consisting of invading strain, \(y_i\) and subset of nested strains \({\mathcal {S}}_i\), \({\mathcal {C}}_i=\left\{ y_i\right\} \cup {\mathcal {S}}_i\), which connects to nested equilibrium \(\widetilde{{\mathcal {E}}}_{n}\).
Next, consider the case \(k=n\) but \({\mathcal {R}}_n\le {\mathcal {Q}}_n\), then we consider non-negative equilibrium \(\overline{\mathcal E}_{n}\). Notice that
where \({\mathcal {A}}_{i}\) is the same vanishing linear form (22) with corresponding circuit \({\mathcal {C}}_i\) as prior case. Therefore, if \({\mathcal {A}}_{i}>0\) (invasion circuit has positive epistasis), then \(\frac{\dot{y}_i}{\gamma _i y_i}\le -\frac{\mathcal A_{i}}{{\mathcal {Q}}_n}<0\). The only other missing species additional to \(y_{i}\), \(i\in [n+1,2^n-1]\), is \(y_n\). It is not hard to see that \(\frac{\dot{y}_n}{\gamma _n y_n}=\frac{{\mathcal {R}}_n -\mathcal Q_n}{{\mathcal {Q}}_n} \le 0\). Thus, all saturated inequalities (11) are satisfied strictly (except when \(\mathcal R_n={\mathcal {Q}}_n\)), and stability of \(\overline{{\mathcal {E}}}_{n}\) (and other conclusions) from Theorem 1 follow. In the case \({\mathcal {R}}_n={\mathcal {Q}}_n\), arguments from Browne (2017) give same result. Finally, suppose \(k<n\), so there are k positive component immune responses, \(z_1,\dots ,z_k\), in feasible nested equilibrium \( \widetilde{{\mathcal {E}}}_{k}\) or \( \bar{{\mathcal {E}}}_k\). Consider the missing strains \(y_{i}\), \(i\in [n+1,2^n-1]\), which are non-nested strains. Let \(y_i\) be contained on the k dimensional hypercube. It necessarily has binary sequence where \(i_{k+1}=\dots =i_n=0\). Observe from above that the invasion circuit involving \(y_i\) will contain nested strains on the k dimensional (sub-)hypercube; \(y_0,\dots ,y_k\). It follows that if \({\mathcal {A}}_i>0\), \(\frac{\dot{y}_i}{\gamma _i y_i}\le -\frac{{\mathcal {A}}_{i}}{ C_k}<0\), where \(C_k={\mathcal {R}}_k\) when \({\mathcal {R}}_k>{\mathcal {Q}}_k\) and \(C_k={\mathcal {Q}}_k\) \({\mathcal {R}}_k\le {\mathcal {Q}}_k\). For a strain \(y_i\) not on k dimensional hypercube, then we can find another strain \(y_{\ell }\) with same sequence in first k bits, and less mutations overall, so that \({\mathcal {R}}_{\ell }>{\mathcal {R}}_k\). Then it is not hard to see that
Arguments from Browne (2017) show that \(\frac{s_j\dot{z}_j}{\sigma _jz_j}_{|_{{\mathcal {E}}^*}}<0\) for \(j>k\). Applying Theorem 1 gives the desired result. \(\square \)
Proof II of Theorem 2
We prove the case where \({\mathcal {R}}_n>{\mathcal {Q}}_n\), so that \(\widetilde{{\mathcal {E}}}_{n}\) is feasible equilibrium and, by same arguments in Proof I above, the other cases follow. Consider a given missing viral strain \(y_{i}\) (\(i\in [n+1,2^n-1]\)) with sequence \({\textbf{i}}\). Define the following linear form based on it’s invasion rate:
and \(C_n={\mathcal {R}}_n\) when \({\mathcal {R}}_n>{\mathcal {Q}}_n\) and \(C_n={\mathcal {Q}}_n\) \({\mathcal {R}}_n\le {\mathcal {Q}}_n\). We claim that \({\mathcal {A}}_{i}=0\) in additive case, and furthermore \(\mathcal A_{i}\ne 0\) if any (non-zero) viral fitness is removed from \({\mathcal {A}}_{i}\) in the resulting sum. In other words we claim that \({\mathcal {A}}_{i}\) defines a circuit \({\mathcal {C}}\) containing strain i and other strains on nested network. To test additivity, it suffices to consider the linear form on the binary sequences:
Since \({\textbf{i}}\) is not in nested network (\(i\in [n+1,2^n-1]\)), there exists \(p\in [1,n-1]\) such that \(i_p=0,i_{p+1}=1\). In other words, there exists a 01 string in the binary sequence \(\textbf{i}\). We prove that \({\mathcal {A}}_i\) defines a circuit by induction on the number of 01 strings, s. First suppose that \(s=1\). Let \(0\le m_1 <p\) be maximal such that \(i_{p}=1\) and \(p+1\le m_2\le n\) be maximal such that \(i_{m_2}=0\). With these conditions, \({\textbf{i}}=1^{m_1}0^{p-m_1}1^{m_2-p}0^{n-m_2}\). Then
Furthermore \(f_i= {\textbf{i}} - 1^{m_1}0^{n-m_1}+1^{p}0^{n-p} - 1^{m_2}0^{n-m_2}\) contains the viral sequences corresponding the non-zero fitness quantities in \({\mathcal {A}}_i\). Thus \({\mathcal {A}}_i\) defines a circuit since the minimal circuit size is 4. Now for the induction step, consider \(s>1\). Assume that \({\mathcal {A}}_{\ell }\) defines a circuit for any sequence \(\mathbf {\ell }\) with \(s-1\) or less (01) strings, and suppose the sequence \({\textbf{i}}\) has s (01) strings. Let \(p_1<p_2<\dots <p_s\) be locations of the 01 strings (with \(i_{p_j}=0,i_{p_j+1}=1\)). Let \(0\le m_1 <p_1\) be maximal such that \(i_{m_1}=1\) and \(p_1+1\le m_2\le p_2\) be maximal such that \(i_{m_2}=1\). So \(\textbf{i}=1^{m_1}0^{p_1-m_1}1^{m_2-p_1}i_{p_2}\dots i_n\). Then
where \({\tilde{\textbf{i}}}=1^{m_1}0^{p_2-m_1}1i_{p_2+2}\dots i_n\) has \(s-1\) (01) strings. Thus by induction hypothesis, we obtain \(f_i=f_{{\tilde{i}}}=0\). Let \({\mathcal {C}}_i\) denote the collection of viral sequences corresponding the non-zero fitness quantities in \({\mathcal {A}}_i\). Notice that it is not hard to ascertain from the above calculations that
Consider an arbitrary proper subset \({\mathcal {B}}\) of \({\mathcal {C}}_i\). First, we claim that there can not be a circuit consisting solely of sequences in the nested network. Suppose by way of contradiction that there exists a linear form with \(g:=\sum _{j=1}^{n+1} b_j {\textbf{j}}=0\). Let \(k=\max \left\{ 1\le j\le n+1 | b_j\ne 0 \right\} \). Then for the \(k^{th}\) digit in the binary sequence of \(g_N\), we find \((g)_k\ne 0\). So there are no vanishing linear forms on the nested network. Thus it suffices to consider the case where \({\textbf{i}}\in {\mathcal {B}}\). Motivated from calculations above, define
where \({\tilde{\textbf{i}}}\) is not in nested network since \(\mathcal B\ne \emptyset \). Furthermore because \({\mathcal {C}}_i{\setminus } {\mathcal {B}}\ne \emptyset \), we obtain that \({\tilde{\textbf{i}}}\) has less than s (01) strings. By induction hypothesis, \(\mathcal A_{{\tilde{i}}}\) defines a circuit \({\mathcal {C}}_{{\tilde{i}}}\) for the sequence \({\tilde{\textbf{i}}}\), where \({\mathcal {C}}_{\tilde{i}}=\left\{ {\tilde{\textbf{i}}}\right\} \cup \mathcal B{\setminus }\left\{ \textbf{i}\right\} \). Denote the vanishing linear form as \(f_{{\tilde{i}}}=\sum _{\ell \in {\mathcal {C}}_{{\tilde{i}}}} a_{\ell } \mathbf {\ell }\). Now for arbitrary coefficients \(b_j\),
The above sum consists solely of sequences in the nested network and thus there are no vanishing linear forms. This implies that the above sum is zero only if \(b_i=0\), which further leads to conclusion that \(b_j=0\) for \(j\in {\mathcal {B}}{\setminus }\left\{ \textbf{i}\right\} \). Thus the proper subset \({\mathcal {B}}\) can not be a circuit for any linear form. \(\square \)
Proof of Theorem 3
By Proposition 6 in Browne and Smith (2018), equivalent conditions for stability of (i) \({\mathcal {E}}^{\dagger }_n\) or (ii) \(\mathcal E^{\ddagger }_{n+1}\) are the following:
-
i.
\({\mathcal {R}}_{n+1}\le {\mathcal {P}}_n\) and \(\left( |\varLambda _{i}| - 1 \right) {\mathcal {P}}_n + {\mathcal {R}}_{i} \le \sum \limits _{j\in \varLambda _{i}} {\mathcal {R}}_j \quad \forall i \in [n+2, 2^n]\), in which case \(\varOmega _y=\varOmega _z= [1,n]\).
-
ii.
\({\mathcal {R}}_{n+1}> {\mathcal {P}}_n\) and \(\left( |\varLambda _{i}| - 1 \right) {\mathcal {R}}_{n+1} + {\mathcal {R}}_{i} \le \sum \limits _{j\in \varLambda _{i}} {\mathcal {R}}_j \quad \forall i \in [n+2, 2^n]\), in which case \(\varOmega _y=[1,n+1]\) and \(\varOmega _z= [1,n]\).
Furthermore, (i) and (ii) are the only possible stable equilibria \({\mathcal {E}}^*\) with a strain-specific subgraph, i.e. \(\varOmega _y\subseteq [1,n+1]\), and strains \(y_1,\dots ,y_n\) (\(y_{n+1}\) also for (ii)) are uniformly persistent. We remark that these prior results follow from Theorem 1. Now to prove Theorem 3, we will show that positive epistasis of corresponding invasion circuits are sufficient (also necessary in case (ii)) for inequalities in (i) and (ii) to hold. Fix an invading strain \(y_i\), \(i\in [n+2, 2^n]\), with binary sequence. First, note that a sufficient condition for inequalities in cases (i) and (ii) to be satisfied is \(\frac{{\mathcal {A}}_{i}}{K_n}\ge 0\) where \({\mathcal {A}}_i= -{\mathcal {R}}_i -\left( |\varLambda _{i}| - 1 \right) {\mathcal {R}}_{n+1} + \sum _{j\in \varLambda _i} {\mathcal {R}}_{j}\), and \(K_n={\mathcal {P}}_n\) if \({\mathcal {R}}_{n+1}\le {\mathcal {P}}_n\) and \(K_n={\mathcal {R}}_{n+1}\) if \({\mathcal {R}}_{n+1}> {\mathcal {P}}_n\). In particular, inequality in case (ii) is equivalent to \(\frac{{\mathcal {A}}_{i}}{K_n}\ge 0\). To show that \({\mathcal {C}}_i=y_i \cup \left\{ y_j \right\} _{j\in \varLambda _i}\) is a circuit with linear form \({\mathcal {A}}_i\), we proceed with a similar approach to our first proof of Theorem 2. Denote the binary sequences of one-to-one network as \({\textbf{k}}_1,\dots ,\textbf{k}_{n+1}\) corresponding to ordered strains \(y_1,\dots , y_{n+1}\). Let \({\mathcal {S}}\subset \left\{ 0,1\right\} ^{n+1}\) denote the subset of strain-specific extended binary sequences, where \(\hat{\textbf{i}}={\textbf{i}}1 \in \left\{ 0,1\right\} ^{n+1}{\setminus } {\mathcal {S}}\) and \(\hat{{\textbf{k}}_j}={\textbf{k}}_j1\in {\mathcal {S}}\) represent binary sequences extended by digit 1. Notice that \({\mathcal {S}}\) forms a basis of \({\mathbb {R}}^{n+1}\). Indeed, it is not hard to show the row reduced echelon form of \(n+1 \times n+1\) matrix is triangular. Thus for \(\hat{{\textbf{i}}} \in \left\{ 0,1\right\} ^{n+1}{\setminus } {\mathcal {N}}\), there is a unique set of coefficients \(\alpha _{j}\), \(j=1,2,\dots ,n+1\), yielding \(\hat{{\textbf{i}}}\) as a linear combination of the nested network vectors:
The above linear system resolves as follows:
which leads us to the set of coefficients \(\alpha _{k}\) where \(k=1,\dots ,n+1\) defined by the following:
Thus, with analogous argument as before, we obtain the indicated circuit \({\mathcal {C}}_i\) and corresponding linear form \({\mathcal {A}}_i\). \(\square \)
Proof of Proposition 3
Let \({\textbf{i}} \in \left\{ 0,1\right\} ^n \setminus {\mathcal {S}}\) with integer coordinates \(\left( \alpha _{{\textbf{k}}}\right) _{{\textbf{k}}\in {\mathcal {S}}}\) with respect to \({\mathcal {S}}\times \left\{ 1\right\} \) as a basis of \({\mathbb {R}}^{n+1}\) and addended binary sequence \({\textbf{i}}1\in \left\{ 0,1\right\} ^n\times \left\{ 1\right\} \). Clearly \({\mathcal {C}} \times \left\{ 1\right\} = {\mathcal {S}}\times \left\{ 1\right\} \cup \left\{ {\textbf{i}} 1\right\} \) is a linearly dependent set \({\mathbb {R}}^{n+1}\) with linear form on fitnesses given by \({\mathcal {A}}_{{\textbf{i}}}=-{\mathcal {R}}_{{\textbf{i}}}-\sum _{{\textbf{k}}\in {\mathcal {S}}} \alpha _{{\textbf{k}}} {\mathcal {R}}_{{\textbf{k}}}\). Furthermore any proper subset is linearly independent since \({\mathcal {S}}\times \left\{ 1\right\} \) is a basis of \({\mathbb {R}}^{n+1}\). Thus \({\mathcal {C}}\) is a circuit with linear form \({\mathcal {A}}_{{\textbf{i}}}\). By proof of Prop 2,
Thus, by Theorem 1, the stability of \({\mathcal {E}}^*\) is determined by the sign of \({\mathcal {A}}_{{\textbf{i}}}\). \(\square \)
Proof of Proposition 4
We apply Proposition 3. Consider the network with at most one mutation, \(\tilde{{\mathcal {S}}}_1\), consisting of wild-type and 1-mutation viral strains \(y_0,y_1,\dots ,y_n\) where the sequence of \(y_j\) is \({\textbf{j}}=\left( \delta _{\ell j}\right) _{\ell =1}^{n}\) for \(j=1,\dots ,n\). First, notice that binary sequences of \(y_0,y_1,\dots ,y_n\) form basis of \({\mathbb {R}}^{n+1}\). For missing strain, \(y_i\), \(i=n+1,\dots 2^n-1\), \({\textbf{i}} \in \left\{ 0,1\right\} ^n {\setminus } \tilde{{\mathcal {S}}}_1\), the coordinates of addended binary sequence \({\textbf{i}}1\) with respect to this basis can be seen to be \(\alpha _j=i_j, \ j=1,\dots ,n, \ \alpha _0=1-\sum _{j=1}^n i_j\). Thus, by Proposition 3, \(\frac{\dot{y}_i}{\gamma _i y_i} = -x^*{\mathcal {A}}_{{\textbf{i}}}\), where \({\mathcal {A}}_{{\textbf{i}}}=-{\mathcal {R}}_{{\textbf{i}}}-\sum _j=1^n i_j {\mathcal {R}}_j - \left( 1-\sum _{j=1}^n i_j\right) {\mathcal {R}}_0= -{\mathcal {R}}_{{\textbf{i}}} -\left( n- |\varLambda _{i}| - 1 \right) \mathcal R_{0} + \sum _{j\notin \varLambda _i} {\mathcal {R}}_{j}\). \(\square \)
Proof of Theorem 4
First assume that pairwise interaction matrix B is positive and consider the stability of the nested equilibrium, \(\widetilde{{\mathcal {E}}}_{n}\) (or \(\overline{{\mathcal {E}}}_{n}\)), as characterized by circuits in Corollary 1 (ii). We proceed by induction on the number of (01) strings denoted by s for the invading strain. Suppose \(s=1\) and the invading strain is written as in prior proof as \(\textbf{i}=1^{m_1}0^{p-m_1}1^{m_2-p}0^{n-m_2}\) and the collection of strains in the circuit is given by \({\mathcal {C}}_i={\textbf{i}} \cup \left\{ 1^{m_1}0^{n-m_1},1^{p_1}0^{n-p_1}\right\} \cup 1^{m_{2}}0^{n-m_{2}}\). Then since the additive elements will sum to zero in the linear form \({\mathcal {A}}_i\), the only remain terms come from pairwise interactions in B and can be calculated as:
Now for the induction step, suppose that \({\textbf{i}}\) has s (01) strings. It is not hard to see that \({\mathcal {A}}_i=\mathcal A_{{\tilde{i}}}\), for invading strain \({\tilde{\textbf{i}}}\), where \({\tilde{\textbf{i}}}=1^{m_1}0^{p_2-m_1}1i_{p_2+2}\dots i_n\) has \(s-1\) (01) strings. Thus by induction hypothesis \(-{\mathcal {A}}_i<0\), or \({\mathcal {A}}_i>0\) giving positive epistasis and stability of nested network.
Next suppose that matrix B is negative and consider the stability of \({\mathcal {E}}^{\dagger }_n\) and \({\mathcal {E}}^{\ddagger }_{n+1}\) consisting of strains \(y_1,\dots ,y_n,y_{n+1}\) with binary sequences \({\textbf{k}}_1,\dots ,{\textbf{k}}_{n+1}\), where \(\varLambda _j=\left\{ j\right\} \) for \(j=1,\dots ,n\) and \(\varLambda _{n+1}=\emptyset \). We inspect the invasion circuit of a strain with sequence \({\textbf{i}}\) outside the one-to-one network. Let s be the number of 1s in sequence \({\textbf{i}}\), located at loci \(\ell _1,\dots ,\ell _s\), where \(0\le s\le n-2\). Again the additive terms in \({\mathcal {A}}_{{\textbf{i}}}\) are zero and thus we have:
Finally, for the network with at most one mutation, only the invading strain \({\textbf{i}}\) will have \(\ge 2\) mutations, so
\(\square \)
Proof of Theorem 5
Let \(0<f_j<1, j=1,\dots ,n\) represent the multiplicative fitness costs for each epitope. Similar to proof of Proposition 4, we use stability formulation by circuits in Corollary 1 (ii) and prove by induction on the number of (01) strings denoted by s for the invading strain. Suppose \(s=1\) and the invading strain is written as in prior proof as \({\textbf{i}}=1^{m_1}0^{p-m_1}1^{m_2-p}0^{n-m_2}\) and the collection of strains in the circuit is given by \({\mathcal {C}}_i={\textbf{i}} \cup \left\{ 1^{m_1}0^{n-m_1},1^{p_1}0^{n-p_1}\right\} \cup 1^{m_{2}}0^{n-m_{2}}\). Then the linear form \({\mathcal {A}}_i\) can be calculated as:
Now for the induction step, suppose that \({\textbf{i}}\) has s (01) strings. It is not hard to see that \({\mathcal {A}}_i=\mathcal A_{{\tilde{i}}}\), for invading strain \({\tilde{\textbf{i}}}\), where \({\tilde{\textbf{i}}}=1^{m_1}0^{p_2-m_1}1i_{p_2+2}\dots i_n\) has \(s-1\) (01) strings. Thus by induction hypothesis \(-{\mathcal {A}}_i<0\), proving nested network is stable. \(\square \)
1.2 The “at most one mutation” network equilibria
Consider the network with at most one mutation, \(\tilde{\mathcal S}_1\), consisting of wild-type and 1-mutation viral strains \(y_0,y_1,\dots ,y_n\) where the sequence of \(y_j\) is \(\textbf{j}=\left( \delta _{\ell j}\right) _{\ell =1}^{n}\) for \(j=1,\dots ,n\). First it is simpler to look at the n strain equilibrium \(\mathcal E^{1*}\) containing positive components for \(y_1^*,\dots ,y_n^*\), where \(y_0^*=0\), i.e. leaving out the wild-type strain. By Proposition 1 and (10), such a positive equilibrium \({\mathcal {E}}^{1*}=(x^*,y^{1*},z^*)\) of system (4) satisfies
with \(I_n\) is the \(n\times n\) identity matrix. Here we find that:
With the immunodominance hierarchy \(s_i\le s_{i+1}\), then \(y_i^{*}>0\) if \(s_1>\sum _{i>1}(s_n-s_i)\) and \(z_i^*>0\) if \(\mathcal R_i\left( n-1-\sum _i s_i\right) >n-1\). If these conditions are satisfied, then the equilibrium \({\mathcal {E}}^{1*}\) is saturated in the subsystem restricted to \({\mathcal {S}}_1\). In Browne and Smith (2018) we showed that in the larger network of viral strains, the equilibrium \({\mathcal {E}}^{1*}\) is always unstable in the case with equal reproduction numbers \({\mathcal {R}}_1=\mathcal R_2=\dots ={\mathcal {R}}_n\).
Now consider invasion by the wild strain \(y_0\), which can result in an \(n+1\) strain equilibrium \(\widetilde{{\mathcal {E}}}^{1*}\) consisting of the viral strain network \(\tilde{{\mathcal {S}}}_1\). By Proposition 1, the positive components \(x^*, {\widetilde{y}}^{1*},z^*\) of \(\widetilde{{\mathcal {E}}}^{1*}\) satisfies:
The above equations are difficult to analyze in general, but when \(x^*>0, {\widetilde{y}}^{1*}> {\textbf{0}}, z^*> {\textbf{0}}\), the \(n+1\) strain “at most one mutation” equilibrium will be positive. Furthermore, if the linear forms of invasion circuits (21) are positive, then by Proposition 4, \(\widetilde{{\mathcal {E}}}^{1*}\) will be stable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Browne, C.J., Yahia, F. Virus-immune dynamics determined by prey-predator interaction network and epistasis in viral fitness landscape. J. Math. Biol. 86, 9 (2023). https://doi.org/10.1007/s00285-022-01843-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-022-01843-y
Keywords
- Virus-immune response model
- Predator–prey network
- Fitness landscape
- HIV quasispecies
- Epistasis
- Eco-evolutionary dynamics