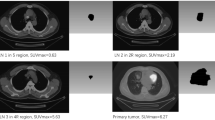
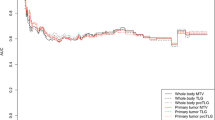
Abstract
Purpose
Standardized reporting of treatment response in oncology patients has traditionally relied on methods like RECIST, PERCIST and Deauville score. These endpoints assess only a few lesions, potentially overlooking the response heterogeneity of all disease. This study hypothesizes that comprehensive spatial-temporal evaluation of all individual lesions is necessary for superior prognostication of clinical outcome.
Methods
[18F]FDG PET/CT scans from 241 patients (127 diffuse large B-cell lymphoma (DLBCL) and 114 non-small cell lung cancer (NSCLC)) were retrospectively obtained at baseline and either during chemotherapy or post-chemoradiotherapy. An automated TRAQinform IQ software (AIQ Solutions) analyzed the images, performing quantification of change in regions of interest suspicious of cancer (lesion-ROI). Multivariable Cox proportional hazards (CoxPH) models were trained to predict overall survival (OS) with varied sets of quantitative features and lesion-ROI, compared by bootstrap** with C-index and t-tests. The best-fit model was compared to automated versions of previously established methods like RECIST, PERCIST and Deauville score.
Results
Multivariable CoxPH models demonstrated superior prognostic power when trained with features quantifying response heterogeneity in all individual lesion-ROI in DLBCL (C-index = 0.84, p < 0.001) and NSCLC (C-index = 0.71, p < 0.001). Prognostic power significantly deteriorated (p < 0.001) when using subsets of lesion-ROI (C-index = 0.78 and 0.67 for DLBCL and NSCLC, respectively) or excluding response heterogeneity (C-index = 0.67 and 0.70). RECIST, PERCIST, and Deauville score could not significantly associate with OS (C-index < 0.65 and p > 0.1), performing significantly worse than the multivariable models (p < 0.001).
Conclusions
Quantitative evaluation of response heterogeneity of all individual lesions is necessary for the superior prognostication of clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterogeneity of lesion response to treatment has been observed in many cancers including metastatic melanoma [1], lung [2], colorectal [3], lymphoma [4] and prostate cancers [5]. In cases where a heterogeneous response is present, it is currently difficult for clinicians to assess whether a patient is benefiting from their current treatment regimen and to decide how to proceed with treating the patient. When the overall disease burden may be improving, but there is still a significant number of lesions not responding to therapy, systemic treatment intensification may be required. On the other hand, in cases where the majority of lesions are favorably responding to treatment, targeting individual progressing lesions using localized ablation may extend benefit and prolong survival [6, 7]. Therefore, a better understanding of treatment response heterogeneity at the lesion level of analysis has significant implications for clinical decision making and strong potential for improving patient outcomes, reducing costs, and increasing drug development efficiency in clinical trials.
Due to the high prevalence of intra-patient response heterogeneity across many types of metastatic cancer [8], measurement of treatment response in all individual disease sites across the body is imperative. Positron emission tomography (PET) and computed tomography (CT) are commonly used to monitor response to therapy in metastatic cancer patients, as they provide a non-invasive method of measuring anatomic and functional changes across the whole body. Measuring response in all disease sites, particularly with whole body PET/CT images, is labor-intensive. Thus, a limited subset of lesions selected based on size or PET uptake is used for the sake of feasibility [9, 10]. As a result, treatment response heterogeneity is often difficult to appreciate as the treating provider only has access to semi-quantitative or descriptive reports interpreted from a subset of lesions present.
Several guidelines to standardize treatment response reporting, particularly in context of clinical trials, where improvements in the “mean” has been used to determine which treatment option was better, have been developed. These include the Response Evaluation Criteria In Solid Tumors (RECIST) [9], World Health Organization (WHO) [11] Criteria, Positron Emission tomography Response Criteria in Solid Tumors (PERCIST) [10] and Deauville Score [12]. The RECIST and WHO criteria were initially developed in the era of cytotoxic chemotherapy and therefore used changes in tumor size as an endpoint for clinical trials, assuming that a higher proportion of tumor shrinkage would reflect improvement in overall survival. RECIST and WHO guidelines stated that “mixed response” was uncommon, thus the assessment of a limited number of lesions was adequate to prioritize agents and choose dosing regimens for late-phase clinical trials. Based on that, RECIST 1.1 [9] later changed the number of assessed lesions from 10 to 5, and preserved the unidimensional measurement (WHO used 2 dimensional measurement) as a way to simplify yet provide “sufficient” standardization for clinical trials. PERCIST was developed for [18F]Fluoro-2-deoxy-2-D-glucose (FDG) PET with a similar goal of standardizing response and allowing comparison between trials. PERCIST is predominantly based on change in the lesions with the highest FDG uptake. Limitations include change in non-target and new lesions, which defers to clinical judgement or a subjective determination of whether it meets “unequivocal” progression. Nevertheless, all these criteria rely on the “sum of change” in target lesions, thus ignoring the impact of each individual lesion in the evaluation of treatment response heterogeneity.
Multiple studies have shown strong associations between FDG standardized uptake value (SUV) metrics and survival [13,14,15], but results across these studies are inconsistent. A study by Kurtipek et al. [16]. showed that average lesion uptake (SUVmean) and metabolic tumor volume (MTV) have a significant association with survival time, but maximum lesion uptake (SUVmax) showed no statistical significance. On the other hand, other studies showed SUVmax to have a strong prognostic value for survival [17,18,19]. Additionally, prognostic significance of measurement of tumor heterogeneity [20,21,22] has shown potential, but its exact impact on patient outcomes has not been determined. One of the main limitations was that for patients with multiple lesions, manual assessment is impractical and has poor reproducibility. Thus, automation is necessary to assess all lesions to improve ability and performance.
In this study, we explored a methodology aimed at providing clinicians with advanced automated TRAQinform IQ (AIQ Solutions, Madison, WI) software analysis. This analysis comprehensively characterizes lesion-level regions of interest (lesion-ROI), enabling early detection of both anatomical and functional changes and assessment of treatment response heterogeneity. Additionally, it evaluates how these changes impact the prognostic value of FDG PET/CT scans. This is the first study that looks at the impact of variation in lesion-ROI and heterogeneity on the prognostication of outcomes. Two cohorts of subjects with non-small cell lung cancer and diffuse large B-cell lymphoma were used in the study. Statistical modelling was used to simulate how a clinician would use quantitative features to prognosticate overall survival of patients. We hypothesized that quantitative evaluation of response heterogeneity of all individual lesions is necessary for superior prognostication of clinical outcome.
Methods
Patient population
Patients with metastatic non-small cell lung cancer (NSCLC) and diffuse large B-cell lymphoma (DLBCL), who received repeat FDG PET/CT imaging, were selected for our analysis. Selection of these cancer types, where FDG PET/CT is commonly used as a standard-of-care treatment response assessment tool [23,24,25,26,27,28,29], allowed for comparison of the applied methodology across different tumor types.
The DLBCL dataset from the randomized phase III CALGB50303 trial, which studied the efficacy of rituximab in combination with two different chemotherapy regimens, included 127 patients with multiple scans available for analysis [30, 31]. The NSCLC dataset from the multi-center ACRIN 6668 trial studying the use of early FDG PET/CT to predict long-term clinical outcome (survival) after definitive chemoradiotherapy included 114 patients with multiple scans available for analysis [13, 32]. The dataset included all data with at least the first two time points, which were the baseline and follow-up scans. Full information for each dataset, including publications of the primary objectives where relevant, is shown in Table 1.
Both studies had rigorous quality control of the FDG PET/CT imaging with centralized standardization based on phantoms as specified in their respective imaging protocols [33, 34]. PET/CT images were reviewed by an experienced research associate or technologist regarding technical specifications such as dosage, timing, acquisition, and reconstruction, and checked if they were compliant with the protocol. Images that were not compliant were either rectified or removed.
For the DLBCL patients, images were acquired at baseline and 2–3 weeks after cycle 2 of chemotherapy. For NSCLC, images were baseline and 12–16 weeks post radiation therapy (and at least 4 weeks post chemotherapy). Patients were required to fast for 4 h and have blood glucose levels less than 200 mg/dL before the FDG injection. The FDG dose was not mandated; the recommended dose was 0.14 to 0.21 MBq/kg (approximately 10 to 20 MBq). PET/CT scanning took place 50 to 70 min after FDG injection and included the body from mid cervical spine to proximal femurs. Reconstruction of PET images was performed in accordance with the imaging protocols for both studies [33, 34]. Scanner information is reported in Table 2.
Lesion-ROI level augmentative software analysis
For this study, analysis was performed by TRAQinform IQ software (AIQ Solutions, Madison, WI).The TRAQinform IQ software performs quantitative analysis of heterogeneity of change in volume and tracer-uptake using automated matching [35, 36] of lesion-ROI between FDG PET/CT images. TRAQinform IQ software also performs automatic organ segmentation, trained using the method described in Weisman et al., to provide locations of lesion-ROI [37]. The organ segmentation model is trained to include all malignancies within the segmented organ. Maximum intensity projections (MIPs) of the organ segmentation output of all patients were manually reviewed to ensure no major failures occurred. TRAQinform IQ is a software-only medical device intended for use by trained medical professionals.
From every PET/CT image, TRAQinform IQ software extracted single time-point image features from both baseline and follow-up scans in each individual lesion-ROI: SUVmax (the highest SUV within lesion-ROI), SUVmean (the average SUV in lesion-ROI), Volume (the total volume of lesion-ROI), SUVtotal (the total SUV in lesion-ROI), SUVhetero (standard deviations of all uptake in lesion-ROI), SUVpeak (defined as the average value in a 1 cm3 sphere centered around the highest uptake voxel in the lesion-ROI) and lesion-ROI count (the number of identified lesion-ROI). Additionally, response features, defined as the change in each feature, were calculated. TRAQinform IQ software tracked each individual lesion-ROI between the baseline and follow-up scans, and then it categorized as new, increasing, stable, decreasing, or disappeared based on the ± 30% change in SUVtotal approximating the repeatability coefficients of repeat FDG PET/CT scans [38].
This categorization allowed for the extraction of additional image heterogeneity features that quantify the heterogeneity of lesion-ROI changes which included: the count of lesion-ROI in each category (e.g., the count of new lesion-ROI), the fraction of lesion-ROI in each category (e.g., the fraction of lesion-ROI classified as new), and specific characteristics (such as SUVmax, SUVmean, SUVhetero, SUVtotal, and Volume) of lesion-ROI in each category (e.g., the highest SUV value of increasing lesion-ROI). Supplementary Table 1 contains a definition list of all the extracted features.
Comparator response criteria
Three standard methods for patient-level treatment response evaluation were implemented as comparators: RECIST [9], PERCIST [10] and Deauville score [39] for the DLBCL cohort and RECIST and PERCIST for the NSCLC cohort. The standard-of-care (SOC) treatment response assessment evaluations were automated using the outputs from TRAQinform IQ’s individual lesion-ROI assessment.
In the automated RECIST evaluation, CT measurements were obtained from each of the PET identified lesion-ROI, and the RECIST target lesions were selected based on the 5 largest volumes of all identified lesion-ROI (no more than 2 per auto segmented organ). The largest long-axis diameter (LAD) was selected by measuring the LAD across every axial slice of the selected lesion-ROI.
In the automated PERCIST evaluation, SUVpeak of the lesion-ROI with the highest SUVmax in each of the scans was used to select 1 target lesion.
In the automated Deauville score evaluation, the lesion-ROI were classified into five categories based on SUVpeak compared to the SUVmean of aorta or the SUVmean of the liver. Score 1–no uptake, Score 2- SUVpeak less than or equal to aorta SUVmean, Score 3- SUVpeak more than aorta SUVmean but less than or equal to liver SUVmean, Score 4- SUVpeak higher than the liver SUVmean but no higher than 3 times the liver SUVmean, and Score 5– SUVpeak more the 3 times the liver SUVmean.
A subset of 20 patients (10 DLBCL and 10 NSCLC) were manually assessed by two nuclear medicine physicians (SC − 13 years of experience, MC − 11 years of experience) to verify the automated treatment response evaluation for RECIST, PERCIST, and Deauville score.
Treatment outcome prediction
This study implemented multivariable Cox proportional hazards (CoxPH) regression models to predict overall survival (OS) utilizing features extracted from the TRAQinform IQ software produced lesion-ROI specific reports (Supplementary Table 1).
To mitigate the risk of overfitting, a feature selection process preceded the integration of features into the models. Initially, univariable p-values for all features were computed, and features with a univariable p-value below a threshold of 0.2 were considered for inclusion in the model, the threshold previously used to successfully correlate features with survival [40]. Subsequently, a bootstrapped stepwise backward selection model using the Bayesian Information Criterion (BIC) was applied [41, 42]. This approach systematically identified parsimonious features by selecting the most relevant ones across multiple bootstrap samples. From these iterations, percentages were generated, representing the frequency of feature selection across the samples. To arrive at a final set of features for integration into the model, only those features ranking within the top 40th percentile were chosen. This brought the number of features down to fewer than 14 features per model. Two analyses were performed to evaluate the need for prognostics models of OS account for all lesion-ROI rather than subgroups and for response of each lesion-ROI rather than whole patient trends.
Lesion-ROI subgroup analysis
First, to assess the value of using all lesion-ROI for analyzing a subject, multivariable CoxPH models were trained, including all features, for different number of lesion-ROI as input:
-
a)
All lesion-ROI: all lesion-ROI were assessed.
-
b)
5 biggest lesion-ROI: the five largest lesion-ROI by volume were assessed, with a limit of two lesion-ROI per organ, as prescribed by RECIST criteria.
-
c)
1 hottest lesion-ROI: the single lesion-ROI with the highest SUVpeak value was assessed, as prescribed by PERCIST criteria.
Feature subgroup analysis
Second, to assess the need to include change of all lesion-ROI, multivariable CoxPH models were trained with different feature sets, extracted for all lesion-ROI, given as input. (Supplementary Table 1)
-
a)
All features: Baseline (BL: single timepoint whole patient features on the baseline scans), Follow-up (FU: single timepoint whole patient features on the follow-up scans), Patient-level Response (Response: change in each single-timepoint whole patient feature from baseline to follow-up), and intra-patient heterogeneity features.
-
b)
Baseline + Follow-up + Patient-level Response (BL + FU + Response): Baseline and Follow-up along with percent change in each single-timepoint whole patient feature from baseline to follow-up.
-
c)
Follow-up (FU): Only single timepoint whole patient features on the follow-up scans.
-
d)
Baseline (BL): Only single timepoint whole patient features on the baseline scans.
Comparison to previously existing methods
The top-performing model from our previous analyses was compared against previously established methods.
RECIST, PERCIST, and Deauville score each output distinct response categories for every patient. These were standardized into a 1–5 numerical scale reflecting best to worst prognosis assigned by each criterion. For RECIST/PERCIST the scale was 1: complete response (CR/CMR), 2: partial response (PR/PMR), 3: stable disease (SD/ SMD), 4: progressive disease (PD/PMD). For Deauville the scale was 1: Score 1, 2: Score 2, 3: Score 3, 4: Score 4 and 5: Score 5.
Univariable CoxPH models were fit using RECIST, PERCIST or Deauville score as features for comparison with the selected model. Moreover, the selected model also underwent comparisons with models fitted using individual predictive features previously identified as predictive in these trial cohorts, post-treatment SUVpeak and pre-treatment molecular tumor volume (Volume) for NSCLC [13, 43, 44], and change of SUVmax for DLBCL [45, 46].Univariable CoxPH models were fit using RECIST, PERCIST or Deauville Score as features for comparison with the selected model. Moreover, the selected model also underwent comparisons with models fitted using individual predictive features previously identified as predictive in these trial cohorts, post-treatment SUVpeak and pre-treatment molecular tumor volume (Volume) for NSCLC [13, 43, 44], and percent change of SUVmax for DLBCL [45, 46].
Statistical analysis
Overall survival (OS) was defined as time from the baseline FDG PET/CT scan to the date of patient death. OS for surviving patients was censored at the date of the last survival assessment. Censoring was used for patients that did not die during the monitoring period of the trials [44, 46].
The performance of survival predictions of the CoxPH models was assessed using the concordance index (c-index), which is a generalization of the area under the receiver operating characteristic curve that accounts for the prediction of time to event such as overall survival with censored observations [47]. A c-index of 1 indicates perfect model performance, and indicates the model was able to order a set of patients correctly according to their risk.
The stability of each model was ensured by employing a robust bootstrap** method where the original data was resampled to generate 1000 unique bootstrap samples [48, 49]. For each combination of input feature and lesion-ROI, a CoxPH model was then trained on every bootstrap sample, producing a C-index for each sample outcome. The final C-index of the specific input combination was calculated as the median C-index across the 1000 bootstrap model outcomes. Comparisons of C-indices between models were conducted using a paired t-test based on the bootstrap estimated standard errors of paired differences.
The hazard ratio (HR), its 95% confidence interval (CI), and associated p-value were also derived for the final model.
The Proportional Hazard assumptions for the CoxPH models were verified prior to analysis by performing Scaled Schoenfeld residuals statistical testing [35]. On the other hand, TRAQinfrom IQ software quantifies this change for all lesion-ROI and allows for more complex analyses to better predict clinical outcomes of patients from their imaging.
The main finding of this work is not the ability of models to prognosticate outcomes, but rather that models had superior performance when factoring in response of all lesions rather than subsets of lesions. The results suggest that when treating patients with multiple lesions, physicians would have a better understanding of the prognosis of patients if quantitative information of response of all lesions was available to them. This could allow clinicians to make better decisions on how to treat patients with metastatic cancer.
Previous analyses of the ACRIN 6668 trial (source of the NSCLC data) identified post-treatment SUVpeak and pre-treatment molecular tumor volume (MTV) as a significant predictor of OS [13, 43, 44]. Our analysis supported these measures as significant univariate predictors. Previous analysis of the CALGB50303 trial (source of the DLBCL data) showed that percent change of SUVmax was a predictor of OS [45, 46]. In our analyses, this was not a significant predictor of survival. This difference could be due to differences in the assessment tools and that only a subset of the total patients was made available for analysis by TRAQinform IQ.
Several noteworthy features selected by the multivariable all-feature models were heterogeneity features focusing on the change of individual lesion-ROI, such as decreasing SUVtotal (SUVtotal of the decreasing lesion-ROI), decreasing volume (volume of the decreasing lesion-ROI), decreasing SUVmax (SUVmax of decreasing lesion-ROI), among others. Conversely, the majority of univariable predictors demonstrated minimal to negligible significance in terms of hazard ratios. This underscores the need to use multiple features in tandem to prognosticate patient outcomes.
While this work trained statistical models that could be used to predict outcomes of patients, this was not the aim of this study. These models were trained and evaluated with bootstrap** for the purpose of evaluating if stable and strong predictive models could be trained based on varied sets of information to determine which information created the highest performing model. Validation of models with an external dataset should be performed before using these models for decision making.
The retrospective nature of this study may raise concerns about the applicability of our findings to prospective settings. Given that the data is derived solely from independent singular studies, the transferability of our results to wider populations is potentially limited. Variations in the quality of acquisition, potential biases introduced by cohorts not representative of real-world populations, and changes in imaging technology over time further contribute to the limitations of this study.
To strengthen the robustness and generalizability of our findings, it is crucial to undertake further validation using additional patient datasets encompassing diverse populations. This validation process would serve to mitigate the limitations posed by the retrospective design and the exclusive reliance on singular study data. Additionally, future endeavors should include validation in prospective studies, incorporating different tracers to provide a more comprehensive understanding of the findings. Note that PET quantification can be dependent on scanner capabilities and reconstruction parameters. As the images acquired in this study were part of two prospective clinical trials with strict imaging protocols, further work is needed to ensure these prognostic trends remain on standard of care images across multiple imaging centers.
Furthermore, it is important to acknowledge the limitations of Cox proportional hazards regression and the statistical feature selection in effectively capturing non-linearities within the data. Machine learning models are emerging as alternatives showing superior performance to Cox regression [51, 52]. It is reasonable to expect that superior performance for outcome modeling will also apply when response of all lesions is used. Therefore, future work should investigate the development and implementation of state-of-the-art machine learning models using larger datasets to allow for training and external validation of these models. These advanced models will be instrumental in compensating for the complexities associated with non-linear relationships within the datasets and enhancing the accuracy and reliability of predictive models.
Conclusion
In this work, we investigated the impact of using TRAQinform IQ software to quantify response heterogeneity of all lesion-ROI on statistical models predicting overall survival patients receiving serial FDG PET/CT images. The best performance was observed when imaging features characterizing the response heterogeneity between scans of all lesion-ROI were considered. The models were able to prognosticate outcomes in two different patient populations with varying disease burden and varying incidence of heterogeneity, while standard of care response criteria (RECIST, PERCIST and Deauville score) were not. Use of automated methods like TRAQinform IQ software is necessary to provide the clinician with a more complex analysis of individual patients which allows for better understanding of patient status e.g. treatment response from their imaging exams as the input. The characterization of the treatment response may allow for earlier identification of patients who will fail and to what extent on specific drugs, enabling a more patient-specific approach to optimal treatment decision making.
Data availability
The datasets were obtained from The Cancer Imaging Archive (TCIA). The Diffuse Large B-Cell lymphoma data is from the dataset NCT00118209 from the NCTN/NCORP Data Archive of the National Cancer Institute’s (NCI’s) National Clinical Trials Network (NCTN). Data was originally collected from clinical trial NCT number NCT00118209, CALGB-50303. The non-small cell lung cancer data is from the clinical trial NCT number NCT00083083, ACRIN6668. All analyses and conclusions in this manuscript are the sole responsibility of the authors and do not necessarily reflect the opinions or views of the clinical trial investigators, the NCTN, the NCORP or the NCI.
References
Grzywa TM, Paskal W, Włodarski PK. Intratumor and Intertumor Heterogeneity in Melanoma. Transl Oncol. 2017;10.
Saito Y, Horiuchi S, Morooka H, Ibi T, Takahashi N, Ikeya T et al. Inter-tumor heterogeneity of PD-L1 expression in non-small cell lung cancer. J Thorac Dis [Internet]. 2019 [cited 2023 Jan 29];11. https://jtd.amegroups.com/article/view/34565.
Hendlisz A, Deleporte A, Delaunoit T, Maréchal R, Peeters M, Holbrechts S, et al. The Prognostic significance of metabolic response heterogeneity in metastatic colorectal Cancer. PLoS ONE. 2015;10:e0138341.
Roider T, Seufert J, Uvarovskii A, Frauhammer F, Bordas M, Abedpour N, et al. Dissecting intratumour heterogeneity of nodal B-cell lymphomas at the transcriptional, genetic and drug-response levels. Nat Cell Biol. 2020;22:896–906.
Kyriakopoulos CE, Heath EI, Ferrari A, Sperger JM, Singh A, Perlman SB, et al. Exploring spatial-temporal changes in 18F-Sodium fluoride PET/CT and circulating Tumor cells in metastatic castration-resistant prostate Cancer treated with Enzalutamide. J Clin Oncol. 2020;38:3662–71.
Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA, et al. Local ablative therapy of Oligoprogressive Disease Prolongs Disease control by tyrosine kinase inhibitors in Oncogene-Addicted non–small-cell Lung Cancer. J Thorac Oncol. 2012;7:1807–14.
Jairam V, Park HS, Decker RH. Local ablative therapies for Oligometastatic and Oligoprogressive non–small cell Lung Cancer. Cancer J. 2020;26:129.
Humbert O, Chardin D. Dissociated response in metastatic Cancer: an atypical pattern brought into the spotlight with immunotherapy. Front Oncol. 2020;10:566297.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med off Publ Soc Nucl Med. 2009;50:S122–50.
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14.
El-Galaly TC, Villa D, Gormsen LC, Baech J, Lo A, Cheah CY. FDG-PET/CT in the management of lymphomas: current status and future directions. J Intern Med. 2018;284:358–76.
Machtay M, Duan F, Siegel BA, Snyder BS, Gorelick JJ, Reddin JS, et al. Prediction of survival by [18 F]Fluorodeoxyglucose Positron Emission Tomography in patients with locally Advanced non–small-cell Lung Cancer undergoing definitive chemoradiation therapy: results of the ACRIN 6668/RTOG 0235 Trial. J Clin Oncol. 2013;31:3823–30.
Hack RI, Becker AS, Bode-Lesniewska B, Exner GU, Müller DA, Ferraro DA, et al. When SUV matters: FDG PET/CT at baseline correlates with survival in soft tissue and Ewing Sarcoma. Life. 2021;11:869.
Wrenn SM, Moore AL, Shah HJ, Barletta JA, Vaidya A, Kilbridge KL et al. Higher SUVmax on FDG-PET is associated with shorter survival in adrenocortical carcinoma. Am J Surg [Internet]. 2022 [cited 2023 Feb 23]; https://www.sciencedirect.com/science/article/pii/S0002961022005438.
Kurtipek E, Çayci M, Düzgün N, Esme H, Terzi Y, Bakdik S, et al. (18)F-FDG PET/CT mean SUV and metabolic tumor volume for mean survival time in non-small cell lung cancer. Clin Nucl Med. 2015;40:459–63.
Bailly C, Carlier T, Berriolo-Riedinger A, Casasnovas O, Gyan E, Meignan M, et al. Prognostic value of FDG-PET in patients with mantle cell lymphoma: results from the LyMa-PET project. Haematologica. 2020;105:e33–6.
Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized Uptake Value (SUVmax) measured on Fluorodeoxyglucose Positron Emission Tomography (FDG-PET) is of Prognostic Value for Survival in Non-small Cell Lung Cancer (NSCLC): a systematic review and Meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12.
Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, et al. Preoperative F-18 fluorodeoxyglucose-Positron Emission Tomography maximal standardized uptake value predicts Survival after Lung Cancer Resection. J Clin Oncol. 2004;22:3255–60.
Hughes NM, Mou T, O’Regan KN, Murphy P, O’Sullivan JN, Wolsztynski E, et al. Tumor heterogeneity measurement using [18F] FDG PET/CT shows prognostic value in patients with non-small cell lung cancer. Eur J Hybrid Imaging. 2018;2:25.
Yang Z, Shi Q, Zhang Y, Pan H, Yao Z, Hu S, et al. Pretreatment 18F-FDG uptake heterogeneity can predict survival in patients with locally advanced nasopharyngeal carcinoma——a retrospective study. Radiat Oncol. 2015;10:4.
Zhao Y, Liu C, Zhang Y, Gong C, Li Y, **e Y, et al. Prognostic value of Tumor Heterogeneity on 18F-FDG PET/CT in HR + HER2 – metastatic breast Cancer patients receiving 500 mg fulvestrant: a retrospective study. Sci Rep. 2018;8:14458.
AL-Jahdali H, Khan AN, Loutfi S, Al-Harbi AS. Guidelines for the role of FDG-PET/CT in lung cancer management. J Infect Public Health. 2012;5:S35–40.
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP et al. Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–58.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and Response Assessment of Hodgkin and Non-hodgkin Lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–67.
D’souza MM, Jaimini A, Bansal A, Tripathi M, Sharma R, Mondal A, et al. FDG-PET/CT in lymphoma. Indian J Radiol Imaging. 2013;23:354–65.
Voltin C-A, Mettler J, Grosse J, Dietlein M, Baues C, Schmitz C, et al. FDG-PET imaging for Hodgkin and diffuse large B-Cell Lymphoma—An updated overview. Cancers. 2020;12:601.
Ambrosini V, Nicolini S, Caroli P, Nanni C, Massaro A, Marzola MC, et al. PET/CT imaging in different types of lung cancer: an overview. Eur J Radiol. 2012;81:988–1001.
Volpi S, Ali JM, Tasker A, Peryt A, Aresu G, Coonar AS. The role of positron emission tomography in the diagnosis, staging and response assessment of non-small cell lung cancer. Ann Transl Med. 2018;6:95–95.
CALGB50303 [Internet]. Cancer Imaging Arch. TCIA. [cited 2024 Mar 31]. https://www.cancerimagingarchive.net/collection/calgb50303/.
Bartlett NL, Wilson WH, Jung S-H, Hsi ED, Maurer MJ, Pederson LD, et al. Dose-adjusted EPOCH-R compared with R-CHOP as Frontline therapy for diffuse large B-Cell lymphoma: clinical outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol. 2019;37:1790–9.
ACRIN-NSCLC-FDG-PET [Internet]. Cancer Imaging Arch. TCIA. [cited 2024 Mar 31]. https://www.cancerimagingarchive.net/collection/acrin-nsclc-fdg-pet/.
Alliance for Clinical Trials in Oncology. Phase III Randomized Study of R-CHOP V. Dose-Adjusted EPOCH-R With Molecular Profiling in Untreated De Novo Diffuse Large B-Cell Lymphomas [Internet]. clinicaltrials.gov; 2021 Nov. Report No.: NCT00118209. https://clinicaltrials.gov/study/NCT00118209.
ACRIN Legacy Trials [Internet]. [cited 2024 Mar 31]. https://www.acr.org/Research/Clinical-Research/ACRIN-Legacy-Trials.
Huff DT, Santoro-Fernandes V, Chen S, Chen M, Kashuk C, Weisman AJ, et al. Performance of an automated registration-based method for longitudinal lesion matching and comparison to inter-reader variability. Phys Med Biol. 2023;68:175031.
Santoro-Fernandes V, Huff DT, Scarpelli ML, Perk TG, Albertini MR, Perlman S et al. Development and validation of a longitudinal soft-tissue metastatic lesion matching algorithm. Phys Med Biol. 2021;0–13.
Weisman AJ, Huff DT, Govindan RM, Chen S, Perk TG. Multi-organ segmentation of CT via convolutional neural network: impact of training setting and scanner manufacturer. Biomed Phys Eng Express. 2023;9.
Lodge MA. Repeatability of SUV in oncologic 18F-FDG PET. J Nucl Med off Publ Soc Nucl Med. 2017;58:523–32.
Pfleger R. Deauville five-point scale | Radiology Reference Article | Radiopaedia.org [Internet]. Radiopaedia. [cited 2023 Jan 29]. https://radiopaedia.org/articles/deauville-five-point-scale?lang=us.
Harmon SA, Perk T, Lin C, Eickhoff J, Choyke PL, Dahut WL, et al. Quantitative Assessment of Early [18F]Sodium Fluoride Positron Emission Tomography/Computed tomography response to treatment in men with metastatic prostate Cancer to bone. J Clin Oncol. 2017;35:2829–37.
Haga T, Okuno T. Selection of variables in multiple regression analysis. In: Maruyama G, Prokhorov JV, editors. Proc Third Jpn — USSR Symp Probab Theory. Berlin, Heidelberg: Springer Berlin Heidelberg; 1976. pp. 713–22.
Wit E, Heuvel EVD, Romeijn J. All models are wrong… an introduction to model uncertainty. Stat Neerlandica. 2012;66:217–36.
Ohri N, Duan F, Machtay M, Gorelick JJ, Snyder BS, Alavi A, et al. Pretreatment FDG-PET metrics in stage III non-small cell lung cancer: ACRIN 6668/RTOG 0235. J Natl Cancer Inst. 2015;107:djv004.
Bazan JG, Duan F, Snyder BS, Horng D, Graves EE, Siegel BA, et al. Metabolic tumor volume predicts overall survival and local control in patients with stage III non-small cell lung cancer treated in ACRIN 6668/RTOG 0235. Eur J Nucl Med Mol Imaging. 2017;44:17–24.
Torka P, Pederson LD, Knopp MV, Poon D, Zhang J, Kahl BS et al. Is local review of positron emission tomography scans sufficient in diffuse large B-cell lymphoma clinical trials? A CALGB 50303 analysis. Cancer Med [Internet]. 2023 [cited 2023 Mar 22];n/a. https://doi.org/10.1002/cam4.5628.
Schöder H, Polley M-YC, Knopp MV, Hall N, Kostakoglu L, Zhang J, et al. Prognostic value of interim FDG-PET in diffuse large cell lymphoma: results from the CALGB 50303 clinical trial. Blood. 2020;135:2224–34.
Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6.
Chen CH, George SL. The bootstrap and identification of prognostic factors via Cox’s proportional hazards regression model. Stat Med. 1985;4:39–46.
Denne C, Maag S, Heussen N, Häusler M. A new method to analyse the pace of child development: Cox regression validated by a bootstrap resampling procedure. BMC Pediatr. 2010;10:12.
Xue X, **e X, Gunter M, Rohan TE, Wassertheil-Smoller S, Ho GY, et al. Testing the proportional hazards assumption in case-cohort analysis. BMC Med Res Methodol. 2013;13:88.
Spooner A, Chen E, Sowmya A, Sachdev P, Kochan NA, Trollor J, et al. A comparison of machine learning methods for survival analysis of high-dimensional clinical data for dementia prediction. Sci Rep. 2020;10:1–10.
Moncada-Torres A, Van Maaren MC, Hendriks MP, Siesling S, Geleijnse G. Explainable machine learning can outperform Cox regression predictions and provide insights in breast cancer survival. Sci Rep. 2021;11:6968.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Ojaswita Lokre, Timothy G. Perk, Amy J. Weisman, Rajkumar Munian Govindan, Song Chen, Meijie Chen. The first draft of the manuscript was written by Ojaswita Lokre and Timothy G. Perk and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The clinical and imaging data have been anonymized by The Cancer Imaging Archive (TCIA). These data are available on an open-access database. Therefore, the institutional review board approval was exempted. For this type of retrospective study, formal consent is not required.
Consent to publish
No individual participant data is included in the submitted manuscript.
Competing interests
The authors Ojaswita Lokre, Timothy G. Perk, Amy J. Weisman, Rajkumar Munian Govindan, Glenn Liu and Robert Jeraj are employees of AIQ Solutions.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lokre, O., Perk, T.G., Weisman, A.J. et al. Quantitative evaluation of lesion response heterogeneity for superior prognostication of clinical outcome. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06764-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06764-0