Abstract
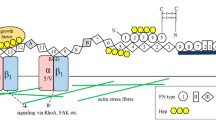
Constructing bionic extracellular matrix (ECM) is an attractive proposition for tissue engineering and clinical regeneration therapy involving the stemness of stem cells. Here, a novel recombinant protein fibronectin-collagen peptide (FCP) was designed to modulate the function of ECM expressed by Picha. pastoris strain X33. This FCP promotes cell migration and adhesion and maintains rBMSC stemness by binding integrin β3. Its effects were blocked by both integrin β3 siRNA and the integrin β3 inhibitor Cilengitide. A template-independent ab initio prediction modeling approach is the best approach to construct a stable FCP protein model, which predicts the binding sites between FCP and integrin β3. FCP may be used in the in vitro culture and clinical regeneration of stem cells that highly express integrin β3, such as hematopoietic stem cells. The study provides information on the molecular structure of FCP and its bioactivity, which can be used to design new compounds.
Key points
• Design a novel recombinant fibronectin-collagen peptide biomimetic ECM.
• FCP promotes cell adhesion, migration, and proliferation.
• Predicted and verified FCP structure and affinity with integrin β3.
• FCP binds integrin β3 to maintain rBMSC stemness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem cells play an important role in tissue engineering and clinical regeneration therapy due to their ability to self-replicate and potential to differentiate into multiple cell lines, called stemness (Keating 2012). The clinical demand for stem cells from various sources far exceeds their supply (Van Zant and Liang 2012). In this regard, an effective solution may be to obtain stem cells through in vitro culture. However, multiple passages of in vitro cultivation gradually decrease the therapeutic properties of stem cells by weakening their proliferation and multipotent differentiation potential, eventually leading to treatment failure (Truong et al. 2019; Zaim et al. 2012). Therefore, develo** more substances that can maintain or even enhance the stemness of stem cells during large-scale in vitro culture is important for their clinical applications.
Extracellular matrix (ECM), a noncellular three-dimensional macromolecular network composed of collagens, fibronectin, elastin, and several other glycoproteins (Ishihara et al. 2014; Isomursu et al. 2019), exerts essential functions on the proliferation and differentiation of stem cells (Du et al. 2011). Extensive research and testing have shown that collagen promotes stem cell migration and adhesion (Sorushanova et al. 2019). Fibronectin promotes cell adhesion similar to collagen, and it also fixes collagen to maintain the ECM stability. In addition, the ECM communicates with cells through integrins located on the surface of the cell membrane, such as αvβ3, αvβ1, and αvβ6, via Arg-Gly-Asp (RGD) sequences (Pierschbacher and Ruoslahti 1984). FN10, a part of the fibronectin type III module, contains RGD sequences that specifically bind αvβ1 to guide the fate of the cells (Bharadwaj et al. 2017; Hocking et al. 1996). Moreover, collagen and fibronectin support the growth of human embryonic stem cells (hESC) without the use of a feeder layer under certain conditions (Lu et al. 2006) and play a role in maintaining the stemness of stem cells (Akhir and Teoh 2020; Thaweekitphathanaphakdee et al. 2019). Notably, as the main strain matrix element, collagen relies on fibronectin, fibronectin-bound, and collagen-bound integrins to complete its corresponding functions (Kadler et al. 2008; Kubow et al. 2015). Meanwhile, natural collagen and fibronectin from animal sources have some problems such as unstable quality and difficult dissolution (Davison-Kotler et al. 2019). In contrast, the recombinant proteins based on collagen and fibronectin and prepared by synthetic biology possess the excellent characteristics of high yield, easy amplification, low cost, and not easily contaminated by mammalian pathogens. These characteristics are slowly being recognized as contributors leading to the future application of recombinant proteins based on collagen and fibronectin in regenerative medicine (Ferrer-Miralles and Villaverde 2013). Therefore, it is necessary to use genetic engineering technology to synthesize a novel functional protein that acts as a biomimetic ECM for stem-cell therapy.
Integrin plays an important role in the long-term ex vivo culture of stem cells. As a member of the integrin family, integrin β3 is a transmembrane receptor that mediates interactions between cells and the ECM and which widely exists on the surface of hematopoietic stem cells and bone marrow mesenchymal stem cells (Pei et al. 2011; Umemoto et al. 2012). The integrin-αvβ3 found on hematopoietic stem cells (HSCs) plays an important role in maintaining stem cell activity by regulating stem cell function and affecting stemness through signal transduction (Umemoto et al. 2012; de Graaf and Metcalf 2011; Ishihara et al. 2014). These integrin functions are dependent on specific ECM ligand–receptor interactions and the specific molecular interactions that lead to cytoskeletal changes that result in different migratory behavior or changes in growth and differentiation (Hynes 2002; Isomursu et al. 2019). It is interesting to design innovative proteins based on the functional characteristics of the principal ECM structural proteins that contain specific ECM ligands, such as RGD, and which can bind with integrin β3 to de-anchor and adsorb cells.
Here, we reported a recombinant protein FCP improved according to the early exploration of our laboratory. FCP structure and functions, including binding to integrin β3, were predicted and recombinant fusion expression assays were conducted. Experiments verified that FCP promoted cell migration and adhesion and maintained cell stemness by binding integrin β3. Its effects could be blocked both by integrin β3 silencing and employing the integrin β3 inhibitor Cilengitide. Therefore, FCP may be used in the in vitro culture of stem cells, such as hematopoietic stem cells and other stem cells with high expression of integrin β3, or even in the field of tissue regeneration engineering.
Materials and methods
Materials
Vectors pPICZαA (Invitrogen, Guangzhou, China) and P. pastoris strain X33 (Invitrogen, Guangzhou, China, Invitrogen™ C18000, ATCC® 28,485™) were used for cloning and heterologous expression. PCR purification kits, gel extraction kits, and micro preparation kits were purchased from Tiangen (Bei**g, China). CC (a collagen-like protein, designed from COLA1, ID: BC036531.2, 2120-2164nt) was artificially synthesized by GL Biochem (Shanghai, China). FN10(ID: U42594.1, 633-770nt) was recombinantly expressed in our Lab (Fig. S2).
ECV304 (Human umbilical vein endothelial cells, ATCC® CRL-1998) and ECV304-eGFP (Human umbilical vein endothelial cells-enhanced green fluorescent protein, ATCC® PCS-100–010) cells were purchased from the Chinese Academy of Sciences (Shanghai, China). They were cultured in Roswell Park Memorial Institute 1640 medium (RPMI 1640, Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum, penicillin (100 I.U./mL), and streptomycin (100 μg/mL) (Sangon Biotech, Shanghai, China). Rat bone marrow mesenchymal stem cells (rBMSCs) were extracted in our laboratory approved by the Animal Care Committee of **an University (JNU20200826-11) and were performed in accordance with animal ethics guidelines of Agricultural Animals for Research and Teaching at **an University. rBMSCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% fetal bovine serum, penicillin (100 I.U./mL), and streptomycin (100 μg/mL).
Construction and identification of FCP
The cDNA encoding FCP was inserted into the pPICZαΑ vector to give a recombinant plasmid named pPICZαΑ-FCP; then, the pPICZαΑ-FCP was transformed into P. pastoris strain X33. Zeocin-resistant clones were selected on YPD plates containing 0.1 mg/mL of Zeocin to obtain different copies of the integrated DNA of FCP. All strains were cultivated overnight at 30 °C in 5 mL of YPD medium, and 1% of the culture was transferred to 25 mL of YPG in a 250 mL baffled flask. The cells were grown at 30 °C in a shaking incubator until the OD600 reached 1.5. The cell pellets were then induced in YPM. At the same time, methanol (0.5, 1.0, 1.5, 2.0%) was added to the culture, and 1-mL samples were collected at 0, 24, 48, and 72 h after induction. The expressions of proteins were monitored by SDS-PAGE. FCP proteins were purified using the Ni Sepharose 6 Fast Flow column combined with gel filtration Sephadex G-25. Polymerase chain reaction (F: 5′-CATGGTGCTCCAGGTGCCCCTGGATC-3′, R: 5′-TGGCTTATCAATTTCAGTTCTGTAG-3′), agarose gel electrophoresis, and western blot (His-Tag Antibody, Affinity, Biosciences, Changzhou, China), etc., were used to verify the authenticity of FCP. Expanded culture of highly expressing P. pastoris strain X33 in 2L YPD media was performed. Additionally, the circular dichroism (CD) spectrum of FCP was measured with a Chirascan plus Circular Dichroism Spectrophotometer (Applied Photophysics Ltd., Leatherhead, Surrey, UK).
Cell migration assay: scratch wound assay
An in vitro scratch wound healing model was used to assess the cell migration induced by FCP. Briefly, ECV304 cells were plated in 12‐well plates and cultured until approximately 95% confluence before the scratch study was undertaken. A scratch was created with a 1 ml sterile pipette tip. After scratching, cells were washed using PBS to remove cell debris caused by the scratch. Another 1 mL treatment solution (250 nmol/L FCP, or FN10, or CC in 1640 medium containing 1% FBS, respectively) was added to cells. Three images of the scratch area were photographed using an inverted microscope (Olympus IX70, Tokyo, Japan) at 0, 18, and 36 h. The ImageJ software was used to determine percentage closure (%).
In another separate experiment, ECV304 cells were treated with or without FCP, integrin β3 siRNA, and NC group (negative control: cells transfected with scrambled siRNA), respectively. All operations are performed as above.
Cell adhesion assay: crystal violet staining
Crystal violet staining was performed to assess the adhesion of FCP. Briefly, culture plates were coated with 250 nmol/L of FCP or Cilengitide overnight at 4 °C. Next, the plates were washed three times with PBS. Subsequently, ECV304 (1.0 × 105 cells/mL) were seeded on tissue culture plates (96-well) and allowed to attach for 4 h at 37 °C. After incubation, non-attached cells were removed by three rinses with PBS. The remaining cells were fixed with 2% paraformaldehyde for 20 min and stained with 1% crystal violet (Solarbio, Bei**g, China) for 20 min.
Immunofluorescence staining assay
After adhesion, cell spread areas were characterized by vinculin and TRITC Phalloidin and detected by immunofluorescence staining assays via a confocal microscope. Briefly, ECV304 (2.0 × 105 cells/mL) were seeded on plates (24-well) and calculated approximately 95% confluence. Then, after being treated with different test substances for 24 h, we discarded culture supernates and washed ECV304 cells using PBS 3 times, following fixed them in 4% paraformaldehyde. In the next moment, washed with PBS and then permeabilized with ice-cold 0.5% Triton X-100. The cells were blocked with PBS containing 2% bovine serum albumin, then incubated with primary antibody of vinculin (Proteintech, Wuhan, China, Cat#26,520–1-AP, 1:100) overnight at 4 °C; after that, Alexa Fluor 488 goat anti-rabbit IgG (Sigma, UK, Ca#SLBX2002, 1:80) were co-incubated at room temperature for 30 min. Next, TRITC Phalloidin (Solarbio, Bei**g, China, Cat#CA1610, 1:200) was incubated at room temperature for 10 min. Finally, the sections were stained with 4′,6-diamidino-2- phenylindole (DAPI) (Cell Biolabs INC, San Diego, CA, Cat#112,002, 1:1000) and imaged with a confocal laser scanning fluorescence microscope (LSM700, Zeiss, Wetzlar, Germany).
There were three separate immunofluorescence staining assays. The test substances of the first immunofluorescence staining assays were FCP, CC, and FN10 (250 nmol/L). ECV304 cells were treated with FCP, integrin β3 siRNA, or scramble siRNA for 24 h separately in the second separated assays. In the last separate experiment, ECV304 cells were treated with FCP, Cilengitide, and FCP combined with Cilengitide (250 nmol/L) for 24 h. All test corresponding control groups were given.
Tube formation assay
The tube formation assay was performed with ECV304-eGFP. Briefly, 96-well plates were coated with 50 μL/well matrigel, then polymerized at 37 °C for 30 min. Then, ECV304-eGFP (3 × 104) were added to each well and incubated at 37 °C and 5% CO2 for 12 h. Cells were respectively treated with or without FCP, FN10, and CC (250 nmol/L) for 4 h. Then, tube formation was observed and captured with an inverted microscope (Olympus IX70, Tokyo, Japan).
Protein modeling and molecular docking
The structural modeling of FCP was performed using bioinformatic webservers such as SWISS-MODEL (https://swissmodel.expasy.org/) (Waterhouse et al. 2018), I-TASSER (https://zhanggroup.org/I-TASSER/) (Yang and Zhang 2015), RoseTTAFold (https://robetta.bakerlab.org/) (Baek et al. 2021) and Alphafold2 (https: // colab.research.google.com / github / deepmind / alphafold / blob / main / notebooks / AlphaFold.ipynb) (Jumper et al. 2021). These webservers cover different modeling methods including homology modeling, threading modeling, deep learning-based ab initio modeling. FCP structure model was evaluated and verified using Ramachandran Plot (Carugo and D**ovic-Carugo 2013), Errat (Colovos and Yeates 1993), Prove (Pontius et al. 1996), Whatcheck (Hooft et al. 1996) and Verify3D (Bowie et al. 1991) evaluation procedures by SAVES6.0 (https://saves.mbi.ucla.edu/).
To explore the molecular interaction, the PatchDock server (http://bioinfo3d.cs.tau.ac.il/PatchDock/php.php) (Schneidman-Duhovny et al. 2005) was used to simulate the interaction between FCP (the highest quality model) and integrin β3 (PDB ID: IJV2).
Utilizing the FireDOCK server (http://bioinfo3d.cs.tau.ac.il/FireDock/) to screen the docking results from PatchDock. Finally, 3D and 2D plot analyses of the protein interaction surface through PyMoL v.2.5.1 and Ligplot+v.2.2 respectively.
Surface plasmon resonance (SPR)
The interactions between FCP and integrin β3 were examined by SPR (Nicoya Life Science, Waterloo, Canada) (** biomaterials is to design biomaterials that contain specific domains which can interact with biological systems to meet various medical and health needs. Protein-functional materials have a distinct advantage in medical and green manufacturing due to their good biocompatibility, biodegradability, multifunctionality, and environmental friendliness.
This study designed a novel recombinant human fusion protein, FCP, with specific protein domains that bind the integrin β3 receptor to communicate with specific stem cells. FCP is composed of FN10 and CC. FN10 is a functional domain of FN that contains the RGD sequence. CC is a recombinant collagen-like protein, developed by our laboratory, that promotes the migration and adhesion of NIH/3T3 cells (Cheng et al. 2020). In a full-thickness animal wound model, CC dressing significantly promotes wound healing and angiogenesis. However, CC exhibits thermal instability, a characteristic that leaves great room for improvement. Thus, in the present study, the CC fragment was combined with FN10 to construct and express FCP protein, improving the performance of the recombinant protein to meet the needs of tissue engineering. Compared with the individual CC and FN10 components, FCP promotes improved cellular proliferation, adhesion, and angiogenesis. The comparative analysis of three proteins shows a satisfying result. All aspects of the performance of FCP improved significantly.
To better understand the structure and function of FCP, the FCP protein structure was predicted using various protein modeling methods. Model quality assessment showed that the FCP model based on ab initio prediction without a homologous template, which was optimized by deep learning, reflected the FCP structure most realistically. Various modeling approaches were tested in optimally constructing a stable FCP structure. The homology and threading methods accurately and efficiently construct the spatial conformations of target proteins with homologous sequences and similar topologies based on the vast amount of known protein structure information obtained from the database. However, these methods are not applicable to unknown proteins that lack template information (Waterhouse et al. 2018; Yang and Zhang 2015). In contrast, for newly designed proteins, such as FCP, a template-independent ab initio prediction modeling approach is best for constructing stable protein models. However, this modeling method is computationally resource intensive and time-consuming. The Alphafold2 server based on a deep learning algorithm was also tested for protein structure modeling; however, the model quality was not satisfactory (Supplement Fig. 3). We speculate that this may be due to the largely irregular curl structure of FCP leading to poor construction.
After obtaining a high-quality structural model of FCP, molecular docking studies of FCP and integrin β3 were performed. Computer simulations showed that the FN10 C-terminus within the FCP protein could form a stable complex with the extracellular segment of integrin β3, consistent with our original design goal. According to these results, modification of the residues on the docking interaction surface of the FCP protein and integrin β3 may potentially enhance the affinity of FCP for integrin β3.
The high affinity of FCP for integrin β3 was verified by SPR assay. Integrin β3 protein, captured on an NTA chip, bound FCP with an affinity constant of 5.37 × 10–8 M, as determined by the SPR assay. Thus, the integrin β3 protein strongly binds the FCP protein (10–8 ~ 10–10 M). The SPR technique is a powerful tool in the study of target molecule interaction and has the advantages of low cost and direct and quantifiable results. However, because SPR is sensitive to interfering factors within the sample, it represents an indirect interaction between proteins.
Collectively, these data suggest that integrin β3, an important receptor on the surface of ECV304 cells, may be a key target for FCP to exert its biological effects. By knocking down integrin β3 expression in ECV304 cells and competitively blocking integrin β3 binding sites with Cilengitide, FCP-induced angiogenesis, migration, and adhesion of ECV304 cells were reduced. rBMSCs were chosen as the model system in the present studies due to ethical constraints and other difficulties in obtaining hHSCs. Considering that rBMSCs are one of the most widely used stem cells in the field of tissue engineering (Chen et al. 2018; Li et al. 2019) and that integrin β3 is highly expressed on the surface of the BMSC cytomembrane (Pei et al. 2011), rBMSCs were utilized in the cloning formation assays. FCP enhanced the colony-forming ability of rBMSCs, and this effect was significantly decreased by Cilengitide pretreatment. After treatment with both FCP and Cilengitide, the related genes were upregulated compared with those in the Cilengitide only group. Likewise, the stemness-related genes NANOG and REX1 expression was increased following FCP administration when compared to control, and this increase remained 14 days after treatment. The gene expression levels of both SOX and ALP, markers of chondrogenic and osteogenic differentiation abilities of rBMSCs, were both higher than that observed in other genes. However, the gene expression of PPAR-γ2, an adipogenic gene, was similar to that of the control (P > 0.05). This finding is difficult to explain, but we speculate that it is related to the function of integrin β3. Further study of these results was outside the scope of the current study due to laboratory constraints. For a sample, we are unable to test karyotypic stability after transfer generation.
Functional recombinant proteins that are used as replacements for native proteins have many unique characteristics. Their high molecular weight, high-frequency amino acids, and special posttranslational modifications serve as bottlenecks in the artificial cell synthesis field and lead to low expression rates and poor adaptation between functional elements and host cells. Functional recombinant protein structures may be unstable or ineffective, limiting efficient production and application of protein-functional materials. Therefore, it is particularly important to ab initio design protein molecules and construct a cell factory to realize functional directional enhancement and industrial production of these functional recombinant proteins. Fibronectin and collagen are principal structural proteins of the ECM. By combining their ECM functions allows us to more comprehensively understand the molecular structure of ECM and its bioactivity in order to design new therapeutic compounds in the future. FCP combines the FN10 (one of fibronectin functional domain-containing RGD sequence) and CC (a kind of human-like collagen) proteins, which have an ideal domain to provide mechanical support for cells. The FCP bionic ECM may provide a microenvironment to support stem cell localization, proliferation, differentiation, and stemness maintenance. In the future, FCP may be used in the in vitro culture of stem cells, such as hematopoietic stem cells and other stem cells with high expression of integrin β3, or even in the field of tissue regeneration engineering.
Data availability
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Code availability
Not applicable.
References
Akhir HM, Teoh PL (2020) Collagen type I promotes osteogenic differentiation of amniotic membrane-derived mesenchymal stromal cells in basal and induction media. Biosci Rep 40:5. https://doi.org/10.1042/bsr20201325
Baek M, DiMaio F, Anishchenko I, Dauparas J, Ovchinnikov S, Lee GR, Wang J, Cong Q, Kinch LN, Schaeffer RD, Millán C, Park H, Adams C, Glassman CR, DeGiovanni A, Pereira JH, Rodrigues AV, van Dijk AA, Ebrecht AC, Opperman DJ, Sagmeister T, Buhlheller C, Pavkov-Keller T, Rathinaswamy MK, Dalwadi U, Yip CK, Burke JE, Garcia KC, Grishin NV, Adams PD, Read RJ, Baker D (2021) Accurate prediction of protein structures and interactions using a three-track neural network. Science 373:871–876. https://doi.org/10.1126/science.abj8754
Bharadwaj M, Strohmeyer N, Colo GP, Helenius J, Beerenwinkel N, Schiller HB, Fässler R, Müller DJ (2017) αV-class integrins exert dual roles on α5β1 integrins to strengthen adhesion to fibronectin. Nat Commun 8:14348. https://doi.org/10.1038/ncomms14348
Bowie JU, Lüthy R, Eisenberg D (1991) A method to identify protein sequences that fold into a known three-dimensional structure. Science 253:164–170. https://doi.org/10.1126/science.1853201
Carugo O, D**ovic-Carugo K (2013) Half a century of Ramachandran plots. Acta Crystallogr D Biol Crystallogr 69:1333–1341. https://doi.org/10.1107/S090744491301158X
Chen HY, Pan L, Yang HL, **a P, Yu WC, Tang WQ, Zhang YX, Chen SF, Xue YZ, Wang LX (2018) Integrin alpha5beta1 suppresses rBMSCs anoikis and promotes nitric oxide production. Biomed Pharmacother 99:1–8. https://doi.org/10.1016/j.biopha.2018.01.038
Cheng Y, Li Y, Huang S, Yu F, Bei Y, Zhang Y, Tang J, Huang Y, **ang Q (2020) Hybrid freeze-dried dressings composed of epidermal growth factor and recombinant human-like collagen enhance cutaneous wound healing in rats. Front Bioeng Biotechnol 8:742. https://doi.org/10.3389/fbioe.2020.00742
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2:1511–1519. https://doi.org/10.1002/pro.5560020916
Correcirc DHA, Ramos CHI (2009) The use of circular dichroism spectroscopy to study protein folding, form, and function. Afr J Biochem Res 3:164–173. https://doi.org/10.5897/AJBR.9000245
Davison-Kotler E, Marshall WS, García-Gareta E (2019) Sources of collagen for biomaterials in skin wound healing. Bioengineering (Basel) 6:56. https://doi.org/10.3390/bioengineering6030056
Graaf CA, Metcalf D (2011) Thrombopoietin and hematopoietic stem cells. Cell Cycle 10:1582–1589. https://doi.org/10.1196/annals.1349.018
Du J, Chen X, Liang X, Zhang G, Xu J, He L, Zhan Q, Feng X-Q, Chien S, Yang C (2011) Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci USA 108:9466–9471. https://doi.org/10.1073/pnas.1106467108
Ferrer-Miralles N, Villaverde A (2013) Bacterial cell factories for recombinant protein production; expanding the catalogue. Microb Cell Fact 12:113. https://doi.org/10.1186/1475-2859-12-113
Hariharan S, Gustafson D, Holden S, McConkey D, Davis D, Morrow M, Basche M, Gore L, Zang C, O’Bryant CL, Baron A, Gallemann D, Colevas D, Eckhardt SG (2007) Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann Oncol 18:1400–1407. https://doi.org/10.1093/annonc/mdm140
Hocking DC, Smith RK, McKeown-Longo PJ (1996) A novel role for the integrin-binding III-10 module in fibronectin matrix assembly. J Cell Biol 133:431–444. https://doi.org/10.1083/jcb.133.2.431
Hooft RW, Vriend G, Sander C, Abola EE (1996) Errors in protein structures. Nature 381:272. https://doi.org/10.1038/381272a0
Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110:673–687. https://doi.org/10.1016/s0092-8674(02)00971-6
Ishihara J, Umemoto T, Yamato M, Shiratsuchi Y, Takaki S, Petrich BG, Nakauchi H, Eto K, Kitamura T, Okano T (2014) Nov/CCN3 regulates long-term repopulating activity of murine hematopoietic stem cells via integrin alpha v beta 3. Int J Hematol 99:393–406. https://doi.org/10.1007/s12185-014-1534-x
Isomursu A, Lerche M, Taskinen ME, Ivaska J, Peuhu E (2019) Integrin signaling and mechanotransduction in regulation of somatic stem cells. Exp Cell Res 378:217–225. https://doi.org/10.1016/j.yexcr.2019.01.027
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589. https://doi.org/10.1038/s41586-021-03819-2
Kadler KE, Hill A, Canty-Laird EG (2008) Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol 20:495–501. https://doi.org/10.1016/j.ceb.2008.06.008
Keating A (2012) Mesenchymal Stromal Cells: New Directions. Cell Stem Cell 10:709–716. https://doi.org/10.1016/j.stem.2012.05.015
Kubow KE, Vukmirovic R, Zhe L, Klotzsch E, Smith ML, Gourdon D, Luna S, Vogel V (2015) Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat Commun 6:8026. https://doi.org/10.1038/ncomms9026
Li X, Peng B, Zhu X, Wang P, Sun K, Lei X, He H, Tian Y, Mo S, Zhang R, Yang L (2019) MiR-210-3p inhibits osteogenic differentiation and promotes adipogenic differentiation correlated with Wnt signaling in ERα-deficient rBMSCs. J Cell Physiol 234:23. https://doi.org/10.1002/jcp.28916
Lu J, Hou RH, Booth CJ, Yang SH, Snyder M (2006) Defined culture conditions of human embryonic stem cells. Proc Natl Acad Sci U S A 103:5688–5693. https://doi.org/10.1073/pnas.0601383103
Pei M, He F, Kish VL (2011) Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A 17:3067–3076. https://doi.org/10.1089/ten.tea.2011.0158
Pierschbacher MD, Ruoslahti E (1984) Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309:30–33. https://doi.org/10.1038/309030a0
Pontius J, Richelle J, Wodak SJ (1996) Deviations from standard atomic volumes as a quality measure for protein crystal structures. J Mol Biol 264:121–136. https://doi.org/10.1006/jmbi.1996.0628
Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ (2005) PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res 33:W363–W367. https://doi.org/10.1093/nar/gki481
Sorushanova A, Delgado LM, Wu Z, Shologu N, Kshirsagar A, Raghunath R, Mullen AM, Bayon Y, Pandit A, Raghunath M, Zeugolis DI (2019) The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv Mater 31:e1801651. https://doi.org/10.1002/adma.201801651
Thaweekitphathanaphakdee S, Chanvorachote P, Prateepchinda S, Khongkow M, Sucontphunt A (2019) Abalone collagen extracts potentiate stem cell properties of human epidermal keratinocytes. Mar Drugs 17:424. https://doi.org/10.3390/md17070424
Truong NC, Bui KH-T, Van Pham P (2019) Characterization of senescence of human adipose-derived stem cells after long-term expansion. Adv Exp Med Biol 1084:109–128. https://doi.org/10.1007/5584_2018_235
Umemoto T, Yamato M, Ishihara J, Shiratsuchi Y, Utsumi M, Morita Y, Tsukui H, Terasawa M, Shibata T, Nishida K, Kobayashi Y, Petrich BG, Nakauchi H, Eto K, Okano T (2012) Integrin-αvβ3 regulates thrombopoietin-mediated maintenance of hematopoietic stem cells. Blood 119:83–94. https://doi.org/10.1182/blood-2011-02-335430
Van Zant G, Liang Y (2012) Concise review: hematopoietic stem cell aging, life span, and transplantation. Stem Cells Transl Med 1:651–657. https://doi.org/10.5966/sctm.2012-0033
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:W296–W303. https://doi.org/10.1093/nar/gky427
Wei Y, Thyparambil AA, Latour RA (2014) Protein helical structure determination using CD spectroscopy for solutions with strong background absorbance from 190 to 230 nm. Biochim Biophys Acta 1844(12):2331–2337. https://doi.org/10.1016/j.bbapap.2014.10.001
**e J, Shen Q, Huang K, Zheng T, Cheng L, Zhang Z, Yu Y, Liao G, Wang X, Li C (2019) Oriented assembly of cell-mimicking nanoparticles via a molecular affinity strategy for targeted drug delivery. ACS Nano 13:5268–5277. https://doi.org/10.1021/acsnano.8b09681
Yang J, Zhang Y (2015) I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res 43:W174–W181. https://doi.org/10.1093/nar/gkv342
Zaim M, Karaman S, Cetin G, Isik S (2012) Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol 91:1175–1186. https://doi.org/10.1007/s00277-012-1438-x
Acknowledgements
The authors would like to acknowledge the faculty and staff at the Biopharmaceutical R&D Center of **an University.
Funding
This work was supported by grants from the Natural Science Fund of Guangdong Province (Grant No. 2021A1515012480), and the Special Innovation Projects of Universities in Guangdong Province (Grant No. 2019KTSCX011). The Key Areas Research and Development Program of Guangzhou (Grant No.202103030003). Special Fund for Marine Economic Development of Department of Natural Resources of Guangdong Province. (YUEZIRANZIHE [2021]50#).
Author information
Authors and Affiliations
Contributions
QX and YH contributed to the study conception and design. XL and DG contributed to the acquisition of data and study conduct. XL, DG, and ZL contributed to the analysis of data. ZL, YZ, TY, and QZ contributed to the materials. XL and DG wrote the manuscript. QX modified the manuscript. XL and DG contributed equally to this work. All the authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval
The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of **an University (Approval number: 20200826–11). All experiments were conducted according to the guidelines for animal care and use of China, and they were approved by the animal ethics committee of the Chinese Academy of Medical Sciences.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, X., Geng, D., Zhang, Q. et al. Recombinant expression a novel fibronectin—collage fusion peptide modulating stem cell stemness via integrin β3. Appl Microbiol Biotechnol 106, 3765–3776 (2022). https://doi.org/10.1007/s00253-022-11965-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11965-4