Abstract
Fungi can colonize organic matter present in subterranean sites and have a significant role as dwellers in different microniches of cave habitats. In order to analyze the content of airborne fungal propagules in different parts of “Stopića Cave,” a touristic site in Serbia, air sampling was carried out in three seasons during 2020, prior to and during the onset of COVID-19 pandemic. Culturable mycobiota was identified using both microscopic techniques and ITS region/BenA gene barcoding, while multivariate analyses were employed to establish the link between fungal taxa and different environmental factors. The maximal measured fungal propagule concentrations were recorded during spring sampling which were based on fungal propagule concentration categories; the cave environment matches the category V. A total of 29 fungal isolates were identified, while Aspergillus, Cladosporium, Fusarium, Lecanicillium, Mucor, and Penicillium were the most diverse genera. According to the trophic mode, most of the isolated fungal species were pathotrophs (75.86%), but when regarding ecological guilds, the most dominant were undefined saprobes and animal pathogens (41.38% for each). Show caves are especially vulnerable to human impacts, and the fungal propagules’ concentration within the caves could be good indices for the level of ecological disturbance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caves are defined as subterranean sites, characterized by constant temperatures and relative humidity all year long and with a moderate to low organic carbon input due to the absence of primary production [1, 2]. However, a variety of substrata within the caves are susceptible to microbial colonization, mostly by oligotrophic microorganisms [1]. In general, fungi can colonize any organic matter present in subterranean sites, and among the cave microbiota, they have a significant role as dwellers in different microniches, such as sediments, vermiculations on cave walls, carcasses of troglobites or troglophiles, and bat guano [3, 4]. Fungal cave dwellers play a significant ecological role as decomposers and parasites [5]. According to Wasti et al. [6], fungi are among the most dominant cave organisms due to their high rate of spores’ dissemination, capability for colonization of various substrata, and tolerance to a wide range of pH values. Unfortunately, some fungal cave inhabitants may be hazardous for mammal health, such as Pseudogymnoascus destructans, a causative agent of white-nose syndrome in Chiroptera [7], and Histoplasma capsulatum, which triggers systemic histoplasmosis (“Darling’s disease”) in humans and others [8]. Furthermore, in caves with Paleolithic art, as well as in caves repurposed as sacral objects during the history of mankind, fungi can cause biodeterioration of both prehistoric wall paintings as well as murals and other artifacts deposited within [9,10,11]. In that sense, due to the significance of fungal presence in the caves, Polish scientists introduced a novel term — speleomycology, which encompasses all kinds of research with main focus on cave mycobiota [12].
Fungal propagules of underground sites are suspended as airborne particles, which form air fractions called bioaerosols [13]. Furthermore, fungi are also present as propagules carried by water, bats, arthropods, and humans, although airborne ones are the most abundant [14]. In the caves of touristic significance (show caves), novel amounts of fungal propagules are frequently introduced by lint, hair, and dander of human visitors [15]. Viable propagules settled on different surfaces within the caves, along with favorable growth conditions (humidity, temperature, nutrients), lead to successful colonization of available substrata, enabling fungi to complete their life cycle and to form another generation of spores within the caves’ interior [16]. In that sense, aerobiological analyses have been proved as a suitable approach for investigations of airborne fungal dispersion in subterranean environments in the last decade. These investigations are therefore essential for the detection of the potential hazardous effect of fungi on the visitors’ health, potential detrimental effect on the cave art, and as agents of geological alterations of cave walls and sediments [17, 18].
The aim of this research was to analyze the content of airborne fungal propagules in different parts of “Stopića Cave”, a show cave in Serbia, and to link the fungal presence with different environmental factors and to discuss their features and relation to cave habitat.
Material and Methods
Study Area and Sampling Sites
Stopića Cave is located on the left bank of the river Prištavica, at an altitude of 711 m, on the eastern edge of the Zlatibor mountain in western parts of Serbia. The entrance to the cave is 30–40 m wide and is located at the bottom of a 50-m high vertical limestone cliff [19]. Stopića Cave consists of five morphological units: Light Hall, Dark Hall, Hall with Tubs, Channel with Tubs, and River Channel [19]. Light Hall — the very beginning of the cave (the entrance zone), Dark Hall (transitional zone), and Hall with Tubs are accessible and adapted for tourists. The most famous tourist attraction is the hall with Tufa tubs (rimstone dams), where many of Tufa tubs are recognized as the biggest and the deepest (over 7 m) of all other caves in Serbia [20]. The cave has been protected by the Serbian government as a natural good of the first category since 2005. Sampling was carried out in three seasons during 2020: Winter (mid-January 2020, prior to the COVID-19 pandemic lockdown), Spring (early June, at the beginning of the tourist season peak, after COVID-19 pandemic lockdown), and Summer (early September, at the end of the tourist season peak), and in four selected sites within the cave: entrance, crossroads, Tufa bathtubs, and waterfall (Fig. 1). The opening hours in January are shorter (7 h) than in June and September (8–9 h).
Measurement of Microclimate Parameters
Environmental parameters, temperature (T, °C), and relative humidity (RH, %) were measured in situ using temperature/humidity meter (VELLEMAN DEM105).
Air Sampling and Estimation of Propagule Concentrations
Airborne fungi were sampled by the volumetric air sampling method using the SAS Super DUO 360 Air Sampler. Cave indoor air (100 L) was vacuumed and subsequently inoculated on three different mycological media: potato dextrose agar (PDA) and Sabouraud dextrose agar (SDA) for mesophilic fungi and malt yeast 40% sucrose agar (M40Y) for xerophiles and xerotolerants. Before each sampling, the sampler was sterilized with ethanol (70 %) to prevent cross-contamination.
Petri dishes with inoculated nutrient media were transported to the laboratory and incubated in a thermostat (UE 500, Memmert) at 25 ± 2 °C for a week. After the incubation period, the Petri dishes were examined, and all visible colonies were counted and labeled. The obtained numbers of the colonies were then converted to probable statistical total values and calculated by formula given by Feller [21] and expressed as CFU m−3 of air. All morphologically different colonies were inoculated in order to obtain pure cultures.
Identification of Fungi
Isolated fungi were inspected using stereomicroscope (Stemi DV4, Carl Zeiss) and light microscope Zeiss Axio Imager M.1) and identified according to morphological criteria (colony morphology and microscopic characteristics of reproductive structures) using identification keys: Watanabe [22] and Samson et al. [23]. Molecular methods are used to confirm preliminary identification, as well to identify non-sporulating isolates. In that sense primary fungal isolates were reinoculated on PDA, SDA, and M40Y media and incubated at 25 ± 2 °C for a week. For DNA extraction, dry peripheral mycelia (approx. 40 mg) were harvested according to the manufacturer’s instructions of a DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA). PCR amplification of the ITS region and BenA gene was carried out using selected ITS1/ITS4 [24] and Bt2a/Bt2b primers [25], respectively, as described previously [26]. The amplified DNA fragments were fractionated in agarose gels (1%) in 0.5 × TBE buffer. Midori Green stain was used for DNA visualization by UV illumination [26]. The obtained PCR products were then shipped for purification and sequencing to Macrogene (the Netherlands). The resulting sequences were then compared with other related sequences deposited to the National Center for Biotechnology Information (NCBI) using the BLAST program (BLAST+ 2.7.1 of the NCBI). Finally, obtained fungal DNA sequences were then deposited in the relevant GenBank database of NCBI.
Phylogenetic Analysis of Airborne Fungal Communities
Sequence alignment was carried out using the CLUSTALW algorithm in MEGAX software [27]. The phylogenetic tree was built on the basis of the alignment and DNA sequences comparison by employing maximum likelihood phylogeny (1000 bootstrap replicas). Kimura 2 parameter model was determined as the best for estimating genetic distances between tested sequences — measured in the terms of nucleotide substitutions per site. Rhizophydium brooksianum JEL 136 (NR_119550.1) was used as the outgroup.
Ecological Indicators
In order to estimate indoor air quality in the “Stopića pećina” cave, obtained results of the concentration of fungal propagules in the different cave rooms were expressed in CFU m−3 and compared with ecological indicators proposed by Porca et al. [1]. According to these authors, the true cave atmosphere has been classified into five categories based on fungal propagule concentration: (1) caves with fungal concentration less than 50 CFU m−3 (category I — indicating no problem with fungi); (2) caves with fungal concentration between 50 and 150 CFU m−3 (category II — requires some periodic controls and studies to eliminate fungal problem); (3) caves with fungal concentration between 150 and 500 CFU m−3 (category III — threatened by fungi and requires different cave management and controls); (4) caves with fungal concentration between 500 and 1000 CFU m−3 (Category IV – already affected by fungi as a result of massive visits or spillage); and (5) caves with fungal concentration above 1000 CFU m−3 (category V — have irreversible ecological disturbance).
Fungal Communities by Trophic Mode and Ecological Guild
All fungal isolates were sorted using FUNGuild v1.0 tool [28] (Guilds_ v1.1.py script, database: http://stbates.org/funguild_db.php) and literature data [23, 29,30,31,32,33]. Species were classified on the basis of their trophic mode into ecological categories: pathotroph (P), saprotroph (S), and symbiotroph (Sy), and to corresponding ecological guilds: animal pathogen (ap), endophyte (en), epiphyte (ep), fungal parasite (fp), lichen parasite (lp), litter saprotroph (ls), soil saprotroph (ss) plant pathogen (pp), undefined saprotroph (us), and wood saprotroph (ws).
Statistical Analyses
Statistical analyses were performed using software Canoco for Windows [34], Microsoft Excel, and the statistical package XLSTAT [35].
Canonical correspondence analysis (CCA) was done to see the relationship between documented fungal genera (presence/absence) and sampling season that is used as explanatory variable, while temperature, relative air humidity, and CFU m−3 were used as supplementary variables. Documented fungal taxa were observed also against variables referring to cultivation medium and part of the cave where sampling was done, but those analyses showed no significance.
Categories (CI–CV) of cave atmosphere based on fungal propagule concentration proposed by Porca et al. [1] were also observed in relation to season, part of the cave and medium using constrained analysis (CCA). Only analysis that shows categories (CI–CV) of cave atmosphere in relation to sampling season was significant.
Results
Fungal Concentration
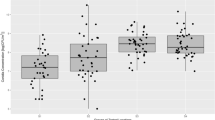
The maximal measured fungal propagule concentrations were recorded during spring sampling on all nutrient media used: entrance (3400 CFU m−3, 2680 CFU m−3 and 2040 CFU m−3 on PDA, SAB and M40Y respectively) and crossroads (2490 CFU m−3 on PDA). Furthermore, only three sampling points during spring showed fungal propagule concentrations lower than 1000 CFU m−3: Tufa bathtubs (980 CFU m−3 and 930 CFU m−3 on M40Y and SAB respectively) and waterfall (510 CFU m−3 on M40Y). During all other sampling periods, obtained fungal concentrations were lower than 1000 CFU m−3 and the lowest were detected on crossroads sampling point in the summer on SAB (50 CFU m−3). Categories (CI–CV) of cave atmosphere based on fungal propagule concentration were observed in relation to season, growth media and part of the cave where the sampling was done (Fig. 2). Category CV was exclusively documented in spring, CIV predominantly in summer, while CIII and CII in winter. Finally, CI was documented only once in the sampling point “crossroads” during the summer on SAB (Figs. 2 and 3a). CCA analysis showing relationship of categories and seasons confirmed the mentioned distribution and was found significant (F = 9.7, P = 0.002).
Fungal propagule concentrations in the air of investigated “Stopića Cave” parts with the reference to the ecological indicators proposed by Porca et al. [1]: (a) entrance, (b) Tufa bathtubs, (c) crossroads, (d) waterfall
Considering part of the cave where the sampling was done, according to performed multivariate analyses, CI was connected only to crossroads, CII to entrance, crossroads and Tufa bathtubs, CIII to Tufa bathtubs and waterfall, CIV and CV to all sections. All were detected on all three media, except CI that was documented only on SAB.
Identified Fungi
In the study presented here, a total of 29 fungal isolates were identified to species level, apart from one Aspergillus isolate which is identified to the section (A. sect. Nigri) and a Stereum isolate which was identified to the genus level. The most diverse genus was Aspergillus with 4 identified species, followed by Cladosporium, Fusarium, Lecanicillium, Mucor, and Penicillium genera with 3 identified species (Table 1). A majority of identified fungi were members of the division Ascomycota (79.31%), followed by Mucoromycota (13.79%) and Basidiomycota (6.9%). Aspergilli and Penicillia formed a well-supported Eurotiales clade (bootstrap value, bpv = 96). Likewise, members of genera Lecanicillium, Cordyceps, Trichoderma, and Fusarium formed well-supported Hypocreales clade (bpv = 95), while Alternaria spp. and Ascochyta phacae grouped together as members of Pleosporales clade (bpv = 95). Botrytis cynerea grouped as a member of Leotiales clade (bpv = 98), while Cladosporium spp. were grouped as members of Capnodiales clade (bpv = 96). Within the division Mucoromycota two clades were present: Mucorales (bpv = 99) and Mortieralles (bpv = 100), while two well-supported clades Polyporales (bpv = 100) and Russulales (bpv = 100) were within division Basidiomycota (Fig. 4).
CCA (F = 2.2, P = 0.002) representing documented fungal genera in relation to sampling season is shown in Fig. 5a. Taxa characteristics for only one season were Ascochyta, Bjerkandera, and Stereum (winter), Mortierella and Mucor (spring), and Lecanicillium and Botrytis (summer). Alternaria was recorded in spring and summer and Epicoccum and Trichoderma in winter and summer. The rest of documented taxa were found in all three seasons. T and RH had higher values during spring and summer and had positive correlation with Alternaria, Botrytis, Lecanicillium, Mortierella, Mucor, and Fusarium (Fig. 5b). The rest were negatively correlated with these two parameters. It is also observed that CFU m−3 was highest in spring and lowest in winter.
When part of the cave where sampling was performed and growth medium was used as explanatory variables in relation to fungal taxa (in separate CCA analyses), the analyses were not significant. However, certain taxa were found in certain sections only: entrance, Bjerkandera; crossroads, Stereum; Tufa bathtubs, Botrytis; and waterfall, Ascochyta and Trichoderma. Considering medium, Ascochyta and Trichoderma grew only on PDA medium, Stereum and Bjerkandera on SAB, while M40Y did not have specific taxon growing only on this medium (Botrytis appeared on M40Y and PDA).
Ecological Categories of Cave Mycobiota
Most of isolated fungal species were pathotrophs (75.86%) which were followed by saprotrophs (58.62%), while symbiotrophs were the least frequent (27.59%). On the other hand, when regarding ecological guilds, the most dominant were undefined saprobes and animal pathogens (41.38% for each) followed by plant pathogens (34.48%) and endophytes (27.59%). Wood saprobes represented 13.79% of species while all other groups were present with only 3.45%. Pathotrophs were dominated by animal pathogens (54.55% of all pathotrophs) but closely followed by plant pathogens (45.45%). The only fungal pathotroph was Trichoderma harzianum, and the only lichen pathotroph was Epicoccum nigrum. Likewise, saprotrophes were mostly represented by undefined saprotrophes (70.59% of all saprotrophes) which were followed by wood saprotrophes (23.52%). Finally, all symbionts were endophytes, while only Cladosporium cladosporioides also belonged to epiphyte guild.
Discussion
Aerobiological studies conducted in cave environments could be used for the monitoring of fungal presence and for the prevention of potential fungal outbreaks in the caves of touristic importance. The results of quantitative and qualitative aeromycological analyses obtained in this study correspond to the work of other authors in the terms of fungal propagule concentration and species composition in subterranean sites [14, 17, 36]. The measurement of the fungal propagule concentration in the summer period showed that the majority of tested sampling points belong to categories IV or V. According to Porca et al. [1], these categories are due to fungal outbreaks caused by frequent visitors. Bearing in mind that sampling of air-borne fungi in “Stopića Cave” has been carried out during COVID-19 pandemic, when due to restrictions to travel abroad, many Serbian citizens were encouraged to travel within Serbian borders, and in that particular time, “Stopića Cave” emerged as novel visiting hotspot for many tourists; high fungal propagule concentration during spring and summer could be connected to more frequent visits. Highest fungal loads in the air were documented during the spring and summer, i.e., after COVID-19 lockdown, which can be attributed to the higher influx of visitors during that time than during winter periods prior to lockdown. Other factors within the “Stopića pećina” cave that could lead to high concentrations of fungal propagules are vegetation period, and big entrance which make “Stopića Cave” more susceptible to climate factors in surroundings (wind, temperature, humidity), in addition to the narrow and short paths for visitors, which could be vectoric carriers of fungal propagules into the deeper parts of the cave. It should be emphasized that there are no official reference standards and limits regarding fungal propagule concentration in indoor air regarding the human health [16]. However, severe impacts on human health are reported only upon exposure to concentrations above 50,000 CFU m−3 [37, 38]. Having in mind that visitors usually do not spend more than few hours during the tour within the cave, the high fungal propagule concentrations could not be regarded as a potential threat for human health.
According to the official site https://www.zlatibor.org.rs/sr/sta-videti/atrakcije/stopica-pecina/, 90,600 tourists visit cave during 2019, but in 2020, that number reached 100,000 which is an absolute record since the opening of the cave to the public (and it was achieved having in mind that the cave was closed for several months due to pandemic). Fernandez-Cortes et al. [39] reported that in Galeria del Calvario room, famous for their paleolithic paintings and engravings in the cave Cueva de Ardales (Spain); fungal propagule concentration (expressed in CFU m−3) increased by 100 times after a visit of only 32 people. Similar trend was observed in our study since observed CFU counts were 10 to 30 times higher after the lockdown (in June) compared to the winter (prior to the lockdown). Therefore, most of the rooms during the summer period were classified into the category IV. On the other hand, Martin-Sanchez et al. [40] reported high fungal contamination levels during winter sampling of aeromycobiota in all investigated rooms from famous Lascaux Cave (France) which contains valuable Paleolithic art. All investigated Lascaux rooms belonged to categories III or IV, and this contamination level was explained by convection currents created by the climate system established for preventing condensation of water vapor on the walls which evacuated the airborne bacteria and fungi. Dominguez-Moñino et al. [18] investigated the aerobiology of caves in Southern Spain and reported a high level of fungal contamination for the Cueva del Tesoro cave. All tested rooms in this cave during spring sampling belonged to categories IV and V, as was documented in our study. Relatively small dimensions of the rooms and galleries, which could contribute to the concentration of spores, in addition to the abundance of phototrophic biofilms all over the cave walls are considered to be main factors contributing to the fungal propagule abundance in the air of Cueva del Tesoro Cave. Jurado et al. [41] investigated aeromycobiota of Cueva de Nerja (Spain) and during sampling in winter found two rooms with extremely high fungal propagules concentration: Kitchen Hall and Heracles Hall with 2170 and 1330 CFU m−3, respectively. However, and contrary to our findings, both mentioned that Cueva de Nerja rooms during previous sampling in summer had low fungal propagules concentration and belonged to category I. Kokurewicz et al. [14] conducted the aeromycological study in Nietoperek Bat Reserve (Western Poland) and detected the highest level of fungal spores reaching the highest number in November and January which placed investigated rooms to category V, but the authors also noticed the number of fungal spores in the air significantly declining in March. According to Kokurewicz et al. [14], the number of bats in that hibernation site was the primary factor determining the fungal propagule concentration in the indoor air. Duan et al. [11] conducted an aeromycological survey in cave temples Maijishan Grottoes in China and reported the highest fungal concentration of 1389 CFU m−3 (category V) in Upper Seven Buddha Pavilion during summer, which was positively correlated with relative humidity and higher visitor density. According to Bercea et al. [42], the high YM (yeast and molds) concentration in the cave Meziad (Western Romania) is correlated with the number of tourists and CO2 level.
The structures of airborne fungal communities documented in research presented here showed that no unique fungal composition could be distinguished on any sampling site. The majority of isolated fungi were members of genera: Alternaria, Aspergillus, Cladosporium, Fusarium, Lecanicilium, Mucor, and Penicillium. The results presented in this paper correspond with similar findings presented by other authors, but mostly of those reported by Rafael Ogórek, researcher who investigates air mycobiota from different caves in Slovakia. Ogórek et al. [5] reported the presence of Alternaria alternata, Aspergillus fumigatus, A. niger, Botrytis cinerea, Cladosporium cladosporioides, and Epicoccum nigrum in the indoor air of Harmanecká Cave, the most important subterranean site of bat occurrence in Slovakia. In the air of Driny Cave, which is also open for public in Slovakia, A. alternata, A. fumigatus, C. cladosporioides, C. herbarum, E. nigrum, Fusarium equiseti, Mucor hiemalis, and Trichoderma harzianum were found. Also, the presence of A. niger, B. cinerea, C. cladosporioides, C. herbarum, E. nigrum, M. hiemalis was reported by Ogórek et al. [13] for Demänovska´ Ice Cave, a very popular attraction in Slovakia. Apart from Slovakian caves, Popkova et al. [43] reported the presence of A. alternata, A. fumigatus, A. niger, B. cinerea, C. cladosporioides, C. herbarum, M. hiemalis, Penicillium expansum, and T. harzianum in the indoor air of two caves: Novoafonskaya (Abkhazia, Georgia) and Ali-Sadr (Iran). The similarities in fungal diversity and mycobiota composition between “Stopića Cave” and investigated caves in Slovakia, Georgia, and Iran could be explained by several factors: (1) fungal species in common (members of genera Alternaria, Aspergillus, Cladosporium, Fusarium, Mucor, Penicillium, and Trichoderma) are according to Vanderwolf et al. [15] recognized as the most characteristic for the subterranean sites; (2) all fungal species in common produce small-sized conidia or sporangiospores which are easily suspended in the air as bioaerosols; and (3) all caves are open for visitors. Apart from ubiquitous, some other fungal isolates are documented less frequently and could be regarded as uncommon due to their narrow ecological valence. The presence of documented entomopathogenic fungi Cordyceps farinosa, and Lecanicilium psaliotae, members of animal pathogens’ guild [29, 44], could be explained by troglobiotic entomofauna within the Stopića Cave, namely, “Stopića Cave” is a habitat of the stenoendemic subspecies of troglobiotic ground beetle (Carabidae) Rascioduvalius stopicensis [45]. Also, trogloxenic ground beetle Trechus obtusus is also documented in Stopića Cave [19]. It should be noted that Bjerkandera adusta and Stereum sp. are the only two basidiomycetes documented in this research, both of them members of wood saprotrophs’ guild, namely, the members of the division Basidiomycota are seldom reported as constituents of cave aeromycobiota. However, B. adusta and Trametes hirsuta were documented in the air of Demänovska´cave (Slovakia) by Ogórek et al. [13]. Bercea et al. [42] reported the presence of basidiomycetous yeast, Trichosporon sp., an opportunistic pathogen, in the cave “Ursilor” (Western Romania). Potential source of Cladosporia, Fusaria, Penicillia, Trichoderma spp., and Basidiomycota spores could be surrounding vegetation since they are potential plant pathogens, soil and wood decaying fungi. Furthermore, Stopića pećina is located on the slopes of Zlatibor mountain and is surrounded by beech forest and has large entrance, which enables air communication between exterior and interior of the cave and consequently spore flow via air currents. It should be emphasized that animal pathogens were documented with high frequency in the indoor air of Stopića pećina (41.38%), and hence, their presence in indoor environments could be regarded as a potential threat for visitors’ health. Among them, Aspergillus fumigatus is a known animal and human pathogen and a causative agent of pulmonary aspergillosis [23]. However, it should be emphasized that A. fumigatus is in research presented here, documented only once (entrance, winter sampling), so it could be assumed the spores of this pathogen are not frequent inside the cave. Our work presents a risk assessment study which lays foundations for the further ecological investigations which will be carried out in the future. Health risk assessment is very important in the understanding of fungal diversity and is especially significant in assessing a potential impact for the visitors.
Conclusion
Caves of touristic importance are especially vulnerable to human impacts, and the fungal propagules’ concentration within the caves could be good indices for the level of ecological disturbance. In order to prevent fungal outbreaks in such caves, which could affect the health of visitors and cave workers, as well as the biodeterioration of cave art, speleomycological research, including aeromycological studies, is essential and therefore should be introduced to cave management as a routine monitoring.
Data availability
The data sets generated during the current study are available from the corresponding author on a reasonable request.
References
Porca E, Jurado V, Martin-Sanchez PM, Hermosin B, Bastian F, Alabouvette C, Saiz-Jimenez C (2011) Aerobiology: an ecological indicator for early detection and control of fungal outbreaks in caves. Ecol Indic 11:1594–1598. https://doi.org/10.1016/j.ecolind.2011.04.003
Wang W, Ma X, Ma Y, Mao L, Wu F, Ma **aojun An L, Feng H (2011) Molecular characterization of airborne fungi in caves of the Mogao Grottoes, Dunhuang, China. Int Biodeter Biodegr 65:726–731. https://doi.org/10.1016/j.ibiod.2011.04.006
Kozlova EV, Mazina SE (2020) Biodiversity of fungi in the photic and aphotic zones of Montenegro caves. Aerobiologia 36:589–604. https://doi.org/10.1007/s10453-020-09654-8
Dimkić I, Fira D, Janakiev T, Kabić J, Stupar M, Nenadić M, Unković N, Ljaljević Grbić M (2021) The microbiome of bat guano: for what is this knowledge important? Appl Microbiol Biotechnol 105:1407–1419. https://doi.org/10.1007/s00253-021-11143-y
Ogórek R, Višňovská Z, Tančinová D (2016) Mycobiota of underground habitats: case study of Harmanecká Cave in Slovakia. Microb Ecol 71:87–99. https://doi.org/10.1007/s00248-015-0686-4
Wasti IG, Khan FAA, Bernard H, Hassan NH, Fayle T, Sathiya Seelan JS (2021) Fungal communities in bat guano, speleothem surfaces, and cavern water in Madai cave, Northern Borneo (Malaysia). Mycology 12:188–202. https://doi.org/10.1080/21501203.2021.1877204
Pavlinić I, Đaković M, Lojkić I (2015) Pseudogymnoascus destructans in Croatia confirmed. Eur J Wildl Res 61:325–328. https://doi.org/10.1007/s10344-014-0885-1
Rodrigues AM, Beale MA, Hagen F, Fisher MC, Terra PPD, de Hoog S, Brilhante RSN, de Aguiar CR, de Souza CMCB, MFG R, JJC S, de Camargo ZP (2020) The global epidemiology of emerging Histoplasma species in recent years. Stud Mycol 97:100095. https://doi.org/10.1016/j.simyco.2020.02.001
Bastian F, Jurado V, Nováková A, Alabouvette C, Saiz-Jimenez C (2010) The microbiology of Lascaux Cave. Microbiology 156:644–652. https://doi.org/10.1099/mic.0.036160-0
Leplat J, François A, Touron S, Galant P, Bousta F (2019) Aerobiological behavior of Paleolithic decorated caves: a comparative study of five caves in the Gard department (France). Aerobiologia 35:105–124. https://doi.org/10.1007/s10453-018-9546-2
Duan Y, Wu F, He D, Gu J-D, Feng H, Chen T, Liu G, Wang W (2021) Diversity and spatial–temporal distribution of airborne fungi at the world culture heritage site Maijishan Grottoes in China. Aerobiologia 37:681–694. https://doi.org/10.1007/s10453-021-09713-8
Pusz W, Ogórek R, Uklańska-Pusz C, Zagożdżon P (2014) Speleomycological research in underground Osówka complex in Sowie Mountains (Lower Silesia, Poland). IJS 43:27–34. https://doi.org/10.5038/1827-806X.43.1.3
Ogórek R, Kozak B, Višňovská Z, Tančinová D (2018) Phenotypic and genotypic diversity of airborne fungal spores in Demänovská Ice Cave (Low Tatras, Slovakia). Aerobiologia 34:13–28. https://doi.org/10.1007/s10453-017-9491-5
Kokurewicz T, Ogórek R, Pusz W, Matkowski K (2016) Bats increase the number of cultivable airborne fungi in the “Nietoperek” bat reserve in Western Poland. Microb Ecol 72:36–48. https://doi.org/10.1007/s00248-016-0763-3
Vanderwolf K, Malloch D, McAlpine D, Forbes G (2013) A world review of fungi, yeasts, and slime molds in caves. IJS 42:77–96. https://doi.org/10.5038/1827-806X.42.1.9
Savković Ž, Stupar M, Unković N, Ivanović Ž, Blagojević J, Popović S, Vukojević J, Ljaljević Grbić M (2021) Diversity and seasonal dynamics of culturable airborne fungi in a cultural heritage conservation facility. Int Biodeter Biodegr 157:105163. https://doi.org/10.1016/j.ibiod.2020.105163
Docampo S, Trigo MM, Recio M, Melgar M, García-Sánchez J, Cabezudo B (2011) Fungal spore content of the atmosphere of the Cave of Nerja (southern Spain): diversity and origin. Sci Total Environ 409:835–843. https://doi.org/10.1016/j.scitotenv.2010.10.048
Dominguez-Moñino I, Jurado V, Rogerio-Candelera MA, Hermosin B, Saiz-Jimenez C (2021) Airborne fungi in show caves from Southern Spain. Appl Sci 11:5027. https://doi.org/10.3390/app11115027
Kličković M (2009) Naš krš. Centar za krš i speleologiju 13:65–76
Lazarević R (2012) Stopića pećina – Zlatibor. Drugo dopunjeno izdanje, Ženid, Beograd
Feller W (2009) An introduction to probability theory and its applications. Vol. 1,3. ed., rev. print., [Nachdr.]. (ed) Wiley series in probability and mathematical statistics. Wiley, S.l
Watanabe T (2010) Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species3rd edn. CRC Press/Taylor & Francis, Boca Raton
Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B (2019) Food and indoor fungi. Westerdijk laboratory manual series2nd edn. Westerdijk Fungal Biodiversity Institute, Utrecht
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
Savković Ž, Stupar M, Unković N, Ivanović Ž, Blagojević J, Vukojević J, Ljlaljević Grbić M (2019) In vitro biodegradation potential of airborne Aspergilli and Penicillia. Sci Nat 106(3–4):8. https://doi.org/10.1007/s00114-019-1603-3
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Nguyen NH, Song Z, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG (2016) FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248. https://doi.org/10.1016/j.funeco.2015.06.006
Zimmermann G (2008) The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): biology, ecology and use in biological control. Biocontrol Sci Tech 18:865–901. https://doi.org/10.1080/09583150802471812
Bensch K, Braun U, Groenewald JZ, Crous PW (2012) The genus Cladosporium. Stud Mycol 72:1–401. https://doi.org/10.3114/sim0003
Lu X-L, Najafzadeh MJ, Dolatabadi S, Ran Y-P, Gerrits van den Ende AHG, Shen Y-N, Li C-Y, ** L-Y, Hao F, Zhang Q-Q, Li R-Y, Hu Z-M, Lu G-X, Wang J-J, Drogari-Apiranthitou M, Klaassen C, Meis JF, Hagen F, Liu W-D, de Hoog GS (2013) Taxonomy and epidemiology of Mucor irregularis, agent of chronic cutaneous mucormycosis. Pers - Int Mycol J 30:48–56. https://doi.org/10.3767/003158513X665539
Woudenberg JHC, Groenewald JZ, Binder M, Crous PW (2013) Alternaria redefined. Stud Mycol 75:171–212. https://doi.org/10.3114/sim0015
Tseng Y-H, Rouina H, Groten K, Rajani P, Furch ACU, Reichelt M, Baldwin IT, Nataraja KN, Uma Shaanker R, Oelmüller R (2020) An endophytic Trichoderma strain promotes growth of its hosts and defends against pathogen attack. Front Plant Sci 11:573670. https://doi.org/10.3389/fpls.2020.573670
Šmilauer P, LepŠ J (2014) Multivariate analysis of ecological data using Canoco 52nd edn. Cambridge University Press, Cambridge, United Kingdom; New York
Addinsoft (2020) XLSTAT statistical and data analysis solution, New York, USA. https://www.xlstat.com. Accessed 15 Aug 2022
Pusz W, Król M, Zwijacz-Kozica T (2018) Airborne fungi as indicators of ecosystem disturbance: an example from selected Tatra Mountains caves (Poland). Aerobiologia 34:111–118. https://doi.org/10.1007/s10453-017-9498-y
Hedenstierna G, Alexandersson R, Belin L, Wimander K, Rosen G (1986) Lung function and Rhizopus antibodies in wood trimmers: A cross-sectional and longitudinal study. Int Arch Occup Environ Heath 58:167–177. https://doi.org/10.1007/BF00432098
Oppliger A, Rusca S, Charriere N, Vu Duc T, Droz PO (2005) Assessment of bioaerosols and inhalable dust exposure in Swiss sawmills. Ann Occup Hyg. https://doi.org/10.1093/annhyg/meh105
Fernandez-Cortes A, Cuezva S, Sanchez-Moral S, Cañaveras JC, Porca E, Jurado V, Martin-Sanchez PM, Saiz-Jimenez C (2011) Detection of human-induced environmental disturbances in a show cave. Environ Sci Pollut Res 18:1037–1045. https://doi.org/10.1007/s11356-011-0513-5
Martin-Sanchez P, Jurado V, Porca E, Bastian F, Lacanette D, Alabouvette C, Saiz-Jimenez C (2014) Airborne microorganisms in Lascaux Cave (France). IJS 43:295–303. https://doi.org/10.5038/1827-806X.43.3.6
Jurado V, Del Rosal Y, Liñan C, Martin-Pozas T, Gonzalez-Pimentel JL, Saiz-Jimenez C (2021) Diversity and seasonal dynamics of airborne fungi in Nerja Cave Spain. Appl Sci 11:6236. https://doi.org/10.3390/app11136236
Bercea S, Năstase-Bucur R, Mirea IC, Măntoiu DŞ, Kenesz M, Petculescu A, Baricz A, Andrei A-Ş, Banciu HL, Papp B, Constantin S, Moldovan OT (2018) Novel approach to microbiological air monitoring in show caves. Aerobiologia 34:445–468. https://doi.org/10.1007/s10453-018-9523-9
Popkova A, Kozlova E, Khazaei S, Kochetkov S, Fedorov A, Benitsiafantoka EU, Soanjara RB, Mazina S (2020) An comparative analysis of airborne and terrestrial fungi in show caves Novoafonskaya (Caucasus) and Ali-Sadr (Iran). Ecol Monten 37:11–18. https://doi.org/10.37828/em.2020.37.2
Senthil Kumar CM, Jacob TK, Devasahayam S, D’Silva S, Kumar NKK (2015) Isolation and characterization of a Lecanicillium psalliotae isolate infecting cardamom thrips (Sciothrips cardamomi) in India. BioControl 60:363–373. https://doi.org/10.1007/s10526-015-9649
Ćurčić SB, Ćurčić BPM, Vrbica M (2013) Remarks on some trechine ground beetle taxa from the Balkan peninsula (Coleoptera: Carabidae: Trechini). Arch biol sci (Beogr) 65:1675–1686. https://doi.org/10.2298/ABS1304675C
Funding
The authors received financial support from the Ministry of Education, Science and Technological Development of the Republic of Serbia for their research (Contract No. 451-03-9/2021-14/200178).
Author information
Authors and Affiliations
Contributions
SP contributed to the study conception and design. Field sampling was performed by SP, MS, and ŽS. MS and ŽS conducted experiments. The first draft of the manuscript was written by MS, ŽS, and SP. All other authors commented on the previous versions of the manuscript. Funding acquisition: GSS and MLjG. Supervision: MLjG and GSS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to participate
All authors agreed to participate.
Consent for publication
All authors are informed and agreed to publish.
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stupar, M., Savković, Ž., Popović, S. et al. Speleomycology of Air in Stopića Cave (Serbia). Microb Ecol 86, 2021–2031 (2023). https://doi.org/10.1007/s00248-023-02214-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-023-02214-w