Abstract
Moyamoya disease is characterized by progressive internal carotid artery (ICA) occlusion. Extracranial-intracranial bypass surgery is effective, particularly in pediatric patients; imaging plays a crucial role in evaluating intracranial perfusion pre- and post-surgery. Arterial spin labeling (ASL) is a magnetic resonance technique employed for noninvasive, whole-brain perfusion assessment by magnetically labeling inflowing blood. However, ASL cannot evaluate the territories and development of each vessel perfusion compared with digital subtraction angiography (DSA). Recently, super-selective ASL (SS-ASL) has been developed, performing pinpoint labeling on a specific artery at a time, and offering a tomographic view that distinctly displays blood supply areas for each vessel. Unlike DSA, SS-ASL is noninvasive and can be repeatedly performed in pediatric patients. In conclusion, SS-ASL is useful for evaluating bypass development over time and understanding the pathophysiology of pediatric moyamoya disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Moyamoya disease (MMD) is characterized by steno-occlusive lesions in the terminal portion of the bilateral internal carotid arteries (ICAs) and the development of compensatory fine and fragile arteries [1, 2]. This disease is predominantly found in East Asian countries, with an incidence of 0.54/100,000/year [3]. Diagnosis often relies on ischemia in children. Vascular reconstruction surgery such as extracranial to intracranial (EC-IC) bypass is recommended in symptomatic patients. Furthermore, 6.8% of pediatric patients with MMD exhibit progressive posterior cerebral artery (PCA) stenosis and require bypass surgery [4], necessitating, postoperative assessment of cerebral hemodynamics. Although digital subtraction angiography (DSA) and/or magnetic resonance angiography (MRA) are commonly employed for this purpose, invasiveness and radiation exposure make DSA less suitable for pediatric patients. Though MRA is noninvasive, it does not show the perfusion territory in details. Recently, sonography has been reported to be useful for assessing bypass function easily and noninvasively; however, the details are difficult to assess [5]. Arterial spin labeling (ASL) is a magnetic resonance (MR) imaging technique used for evaluating cerebral perfusion noninvasively by magnetically labeling the inflowing blood. However, standard ASL can only visualize perfusion in the whole brain. Recently, super-selective ASL (SS-ASL) has been developed and can label separately into the external carotid artery (ECA), ICA, and vertebral artery (VA). This technique uniquely enables rapid and non-invasive visualization of each vascular territory on tomographic images [6, 7]. Herein, we report the usefulness of SS-ASL imaging for evaluating cerebral perfusion post-vascular reconstruction surgery in pediatric patients with MMD.
Methods
MR imaging
MR imaging was performed using a 3 T whole-body system (Ingenia Elition; Philips Healthcare, Best, Netherlands) with a whole-body radiofrequency coil for transmission and a 32-channel phased array head coil for signal reception. Scanning and tagging parameters of the SS-ASL adhered to the technical guidelines in principle [8, 9]. These parameters at our facility were: FOV 240 × 240 mm, voxel size of 2.5 × 2.6 × 10 mm3, 3D fast field echo-planar imaging readout, TR/TE/FA = 4220 ms/18 ms/90-degree, labeling duration of 1.80 s, post labeling delay of 2.20 s, with background suppression consisting of 4 pulses after a saturation pulse that preceded the labeling. Fourteen slices of labeled and control images were acquired at a scan time of approximately 2 min. The scan plane was angulated in the same manner as the labeling plane. In the planning steps of SS-ASL, the labeling stack was initially positioned on a 3D time-of-flight (TOF) MRA image of the cervical vessels. The circular labeling spot was adjusted in the graphic user interface to cover the target vessel in its craniocaudal extension of at least 20 mm (Fig. 1a). Regarding the posterior circulatory perfusion specifically, labeling of the bilateral VAs was simultaneously performed (Fig. 1b). The diameter of the circular label focus was set to 8 mm. Second, the readout slab was positioned to cover the entire brain perfusion territory [10]. If patient movement was detected during examination, TOF scan was repeatedly performed, the labeling locations were updated directly before performing the SS-ASL sequence to minimize the loss of labeling efficiency due to motion.
Planning steps of superselective arterial spin labeling (SS-ASL) sequence. The labeling stack is positioned on a Three-Dimensional Time-of-Flight Magnetic Resonance Angiography (3D TOF MRA) of the cervical vessels (e.g. Right external carotid artery (ECA) (a) and bilateral vertebral arteries (VAs) (b)). Labeling locations are shown in yellow boxes
Case presentation
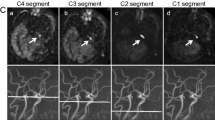
A 9-year-old girl presented with stage IV MMD (Fig. 2a). Preoperative standard ASL showing reduced cerebral blood flow in bilateral cerebral hemispheres (Fig. 2b). First, bypass surgery was performed bilaterally for anterior circulation. Two years later, progression of the right PCA stenosis caused the appearance of ivy signs indicating ischemia in the right occipital lobe, and bypass surgery was performed for the right PCA territory (Fig. 2c, d). The patient’s postoperative course was uneventful (Fig. 2e, f). Three years post-initial surgery, MRA showed progression of bilateral ICA stenosis and right posterior cerebral artery occlusion, as well as excellent development of the bypasses from the bilateral ECA (Fig. 2g). Standard ASL showed improved blood flow in the whole brain (Fig. 2h). SS-ASL showed areas of blood supply from each vessel separately on the tomographic view (Fig. 2i-m).
(a) Preoperative magnetic resonance angiography (MRA) demonstrating multiple changes in arterial vascular calibers and (b) Preoperative arterial spin labeling (ASL) showing reduced cerebral blood flow. (c) Preoperative left (Lt.) vertebral angiography (VAG) showing right posterior cerebral artery (PCA) stenosis (arrow) and (d) Preoperative Fluid-attenuated inversion recovery (FLAIR) showing ivy sign (arrow). (e) Postoperative ASL showing improved blood flow in the whole brain and (f) Postoperative FLAIR showing disappearance of ivy sign and no abnormal findings. (g) MRA showing excellent development of the bypasses from the bilateral external carotid artery (ECA). (h) Standard ASL showing improved blood flow in the whole brain (in ml/100 g/min). (i, j) superselective-ASL (SS-ASL) showing that each internal carotid artery (ICA) supplies the ipsilateral basal ganglia, (k) bilateral vertebral arteries (VAs) supply the left occipital lobe, (l) the right ECA supplies cortical areas of the anterior and posterior circulation in the right cerebral hemisphere and (m) the left ECA supplies cortical areas of the left anterior circulation including the right posterior circulation
Discussion
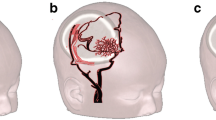
Postoperative cerebral perfusion is evaluated with single photon emission computed tomography (SPECT), positron emission tomography (PET), computed tomography (CT) perfusion, DSA, and ASL [1, 11]. Although SPECT, PET, CT perfusion, and DSA involve radiation exposure and SPECT, PET, and CT perfusion could only evaluate perfusion in the entire brain, DSA is an ideal modality for assessing the territory and development of bypass perfusion. However, it is not suitable for evaluating changes over time because it cannot be performed frequently due to the risks associated with the procedure, such as cerebral infarction [12]. Furthermore, pediatric patients sometimes require general anesthesia to maintain their posture during DSA. ASL is a safe and universal examination method that can evaluate cerebral perfusion without radiation exposure or contrast medium because it uses protons in arterial blood as endogenous tracers using radio waves to label them magnetically and is also suitable for follow-up. However, ASL can only evaluate cerebral perfusion in the whole brain because it labels the entire axial section of the cervical vessels in the neck and cannot evaluate cerebral perfusion in individual arteries, as DSA can. By contrast, SS-ASL labels a specific artery and shows the cerebral circulation supplied by the selected artery (Fig. 3). Hwang et al. reported that SS-ASL findings were consistent with those of DSA [13]. In the case presented here, SS-ASL clearly evaluated perfusion and showed that the cortical areas of the bilateral anterior and right posterior circulation received perfusion from their associated ECAs. Using DSA, taking images of each vessel to evaluate the area of blood supply is necessary, which can also be evaluated by comprehensively assessing all image findings obtained by taking images from several angles. This requires advanced anatomical knowledge and abundant experience. By contrast, SS-ASL can evaluate the perfusion profiles of each vessel in the whole brain on tomographic images. Therefore, visually evaluating the perfusion territory is easy not only in the cortex but also in the medulla, such as the basal ganglia and corona radiata. SS-ASL takes only approximately 2 min per vessel using our method and can be performed during an outpatient visit, making evaluating cerebral hemodynamic changes over time possible and being useful for understanding the pathophysiology of pediatric MMD.
Schematic diagram showing the difference between standard arterial spin labeling (ASL) and superselective-ASL (SS-ASL). The red area in the diagram shows the labeling area. (a) standard ASL can only evaluate cerebral perfusion in the whole brain because it labels the entire axial section of the cervical vessels in the neck. (b) SS-ASL labels a specific artery and shows the cerebral circulation supplied by the selected artery
There are some limitations in the present study. First, movement was a significant limitation in SS-ASL acquisition due to the need for precise placement of the labeling volumes. Although this might be problematic in general patient populations, it could be much more problematic in pediatric cases. However, although mild sedation was required to adequately perform MR imaging in most of pediatric patients, none of the cases required general anesthesia. Furthermore, SS-ASL takes only approximately 2 min per vessel using our method, the rate of failure in our institutions is as low as 1 in 125 cases (data not shown). Second, turbulent flows may have decreased labeling efficiency or caused signal inhomogeneities during readout. Additionally, the lumen size of the labeled structure could have influenced perfusion related signal changes in the readout volume. Lastly, although diagnostic worth was not excluded, ASL could be dependent on heart rate, blood pressure, hematocrit, blood CO2 levels, and blood flow velocity at the time of the imaging. Therefore, ASL could not quantitatively assess cerebral blood flow. As a result, a correction method should be developed and optimized to evaluate with high reproducibility.
Conclusions
SS-ASL is a unique modality that could show the blood supply areas from each vessel separately on tomographic view. In addition, its noninvasive nature could allow for repeated use in children, making it useful as a postoperative evaluation method for cerebral hemodynamics in pediatric patients with MMD.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ASL:
-
Arterial spin labeling
- Bil:
-
Bilateral
- CT:
-
Computed tomography
- DSA:
-
Digital subtraction angiography
- ECA:
-
External carotid artery
- FLAIR:
-
Fluid-attenuated inversion recovery
- ICA:
-
Internal carotid artery
- PCA:
-
Posterior cerebral artery
- PET:
-
Positron emission tomography
- SPECT:
-
Single photon emission computed tomography
- VA:
-
Vertebral artery
- VAG:
-
Vertebral angiography
References
Scott RM, Smith ER (2009) Moyamoya disease and Moyamoya syndrome. N Engl J Med 360:1226–1237. https://doi.org/10.1056/NEJMra0804622
Suzuki J, Kodama N (1983) Moyamoya disease–a review. Stroke 14:104–109. https://doi.org/10.1161/01.str.14.1.104
Kuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S et al (2008) Prevalence and clinicoepidemiological features of Moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke 39:42–47. https://doi.org/10.1161/STROKEAHA.107.490714
Hayashi T, Shirane R, Tominaga T (2009) Additional surgery for postoperative ischemic symptoms in patients with Moyamoya disease: the effectiveness of occipital artery-posterior cerebral artery bypass with an indirect procedure: technical case report. Neurosurgery 64:E195–E196; discussion E196. https://doi.org/10.1227/01.NEU.0000336311.60660.26
Connolly F, Alsolivany J, Czabanka M, Vajkoczy P, Valdueza JM, Röhl JE et al (2021) Blood volume flow in the superficial temporal artery assessed by duplex sonography: predicting extracranial-intracranial bypass patency in moyamoya disease. J Neurosurg 135:1666–1673. https://doi.org/10.3171/2020.9.JNS202709
The Japan Stroke Society (2021) Japanese guidelines for the management of stroke 2021. Kyowa Kikaku Ltd, Tokyo, Japan
Helle M, Norris DG, Rüfer S, Alfke K, Jansen O, van Osch MJ (2010) Superselective pseudocontinuous arterial spin labeling. Magn Reson Med 64:777–786. https://doi.org/10.1002/mrm.22451
Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116. https://doi.org/10.1002/mrm.25197
Lindner T, Bolar DS, Achten E, Barkhof F, Bastos-Leite AJ, Detre JA et al (2023) Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging. Magn Reson Med 89:2024–2047. https://doi.org/10.1002/mrm.29572
Helle M, Rüfer S, van Osch MJ, Nabavi A, Alfke K, Norris DG, Jansen O (2013) Superselective arterial spin labeling applied for flow territory map** in various cerebrovascular diseases. J Magn Reson Imaging 38:496–503. https://doi.org/10.1002/jmri.24041
Richter V, Helle M, van Osch MJ, Lindner T, Gersing AS, Tsantilas P et al (2017) MR imaging of individual perfusion reorganization using superselective pseudocontinuous arterial spin-labeling in patients with complex extracranial steno-occlusive disease. AJNR Am J Neuroradiol 38:703–711. https://doi.org/10.3174/ajnr.A5090
Piao J, Wu W, Yang Z, Yu J (2015) Research progress of Moyamoya disease in children. Int J Med Sci 12:566–575. https://doi.org/10.7150/ijms.11719
Hwang I, Cho WS, Yoo RE, Kang KM, Yoo DH, Yun TJ et al (2020) Revascularization evaluation in adult-onset Moyamoya disease after bypass surgery: superselective arterial spin labeling perfusion MRI compared with digital subtraction angiography. Radiology 297:630–637. https://doi.org/10.1148/radiol.2020201448
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
Open Access funding provided by Shimane University. No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Shimane University (No.20190927–1).
Informed consent
Informed consent was obtained from legal guardians.
Conflict of interest
We declare that we have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshikane, T., Hayashi, K., Obara, M. et al. The usefulness of super-selective arterial spin labeling for postoperative evaluation of pediatric moyamoya disease: technical note. Neuroradiology (2024). https://doi.org/10.1007/s00234-024-03402-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00234-024-03402-2