Abstract
This is the first study addressing validation of the early growth stages (including the first increment) in the beaks of juvenile cuttlefishes. The age validation in juveniles of Sepia officinalis was performed by comparison of the number of increments observed in the rostrum surface of lower jaws with their true age. A total of 159 individuals were reared at 18 ºC and 21 ºC, with ages up to 31 days from hatching. The number of growth increments in the beak was counted and contrasted with the days of life after hatching, validating the hypothesis of one increment of growth corresponding to one day of life. The mean coefficient of variation between readings (measuring precision) was 2.95 ± 5.98%. The growth of the reading area (rostrum surface) and the periodicity of increment deposition showed no difference between the two culture temperatures and therefore daily deposition was confirmed at these temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since Young (1960), hard structures have become a routine tool for age estimation of cephalopods (Arkhipkin et al. 2018). However, despite the fact that in 1965 Clarke observed the growth lines in Moroteuthis ingens beaks (Moroteuthopsis longimana, as it was confirmed later by Cherel 2020), statoliths have been the most frequently used hard structure for ageing cephalopods (Jereb et al. 1991; Morris 1988; Rodhouse and Hatfield 1990; Šifner 2008; Arkhipkin and Shcherbich 2012). Perales-Raya and Hernández-González (1998) suggested that beak sections of Octopus vulgaris are suitable for age estimation, and Hernández-López et al. (2001) improved Clarke’s method (1965) by counting increments on the inner surface of the lateral wall of the lower jaw, confirming daily periodicity in octopus paralarvae up to 30 days of age. Both methods were later compared and improved (Perales-Raya et al. 2010), although the daily deposition of beak increments across the entire age range of the species in both the lateral wall surfaces (LWS) and sections was not validated until several years later (Perales-Raya et al. 2014b), confirming later the first increment using embryos and new hatchlings (Armelloni et al. 2020). Moreover, Liu et al. (2015) concluded that beaks present greater advantages in age studies than statoliths, due to the relative simplicity of this processing method. After validation in O. vulgaris, the beaks have been used for age estimation in a number of cephalopod species, mainly squids and octopuses (e.g. Fang et al. 2016; Liu et al. 2017; Donlon et al. 2019; ** et al. 2019; Schwarz et al. 2019; Batista et al. 2021).
Daily growth increments in other structures such as stylets or statoliths have been validated for several benthic cephalopod species, including Octopus vulgaris (Hermosilla et al. 2010); O. pallidus (Leporati et al. 2008), and O. maya (Rodríguez-Domínguez et al. 2013). With regard to neritic/pelagic cephalopods as loliginids (Jackson 1990; Lipinski et al. 1998; Cordella de Aguiar et al. 2012; Hoving and Robison 2017) and Oegopsida squids (Hurley et al. 1985; Nakamura and Sakurai 1991), statoliths were the validated structures, although gladius and beaks were cross-verified by comparing their counts with those from statoliths (Arkhipkin and Bizikov 1991; Hu et al. 2016, among others).
The common cuttlefish, Sepia officinalis (Linnaeus 1758), is a target species in many industrial and artisanal fisheries in the Eastern Atlantic and the Mediterranean Sea (Boletzky 1983; Denis and Robin 2001), and frequently reported as a by-catch of bottom trawling (Rathjen and Voss 1987; Jereb et al. 2015). Age determination is essential for cuttlefish population dynamic modelling, and therefore for the assessment and management of its fisheries. However, according to Jackson and Moltschaniwskyj (1999), age estimation methods must be accurate and precise and not laborious or very time-consuming. Most of Sepia officinalis age estimation studies have been based on polymodal decomposition of length frequencies (Boucaud-Camou and Boismery 1991; Mion et al. 2014), the analysis of statolith microstructure (Perales-Raya et al. 1994; Bettencourt and Guerra 2001) or the cuttlebone (Nabhitabhata et al. 2022) although limited estimations have been obtained.
The beaks in sepiid species were tested in adults of Sepia apama (Hall et al. 2007), and more recently in S. officinalis by Lishchenko et al. (unpublished results), but validation is still necessary. The aim of the present study is to estimate, for the first time, the ages of the juveniles in sepiid species by counting the growth increments observed in the beaks, to validate daily deposition and the age of the first increment in known-age cuttlefishes reared in captivity.
Materials and methods
A total of 159 spring hatchlings of Sepia officinalis were obtained from fertilized eggs collected in shallow waters off Gran Canaria (Tufia; 27º57’N, 15º22’W). The eggs were attached to a rope at 7 m depth in a sandy bottom. They were collected in a single batch and the water temperature was 20.4 ºC. Eggs and newly hatched juveniles were reared at similar water temperature (21 ºC), and also at 18 ºC (as a small reduction within the “conventional” rearing temperature of 20 ± 2 °C described by Iglesias and Fuentes 2014) to evaluate the effect of small changes in temperature in the pattern of growth increments on beaks. The study was carried out in two phases. Phase I addressed two issues: (a) testing the hypothesis of 1 day = 1 increment, and (b) evaluating the impact of the temperature on the increment deposition rates. After Phase 1, Phase II consisted of replicating Phase I and standardizing the methodology used for the newly hatched Sepia officinalis. In this phase, all cultures were carried out at 21 ºC.
After sacrifice, the dorsal mantle length (DML) of each individual was measured to the nearest 0.1 mm. DML ranged from 5.08 mm to 8.89 mm, and their total weight varied from 0.1098 to 0.2712 g (Table 1). A total of 127 and 32 lower jaws of Sepia officinalis reared in the laboratory at 21 ºC and 18 ºC, respectively, were analysed for age determination following the methodology of Perales-Raya et al. (2018) in octopus paralarvae.
Rearing conditions
The eggs development occurred in the natural environment, collecting the eggs in the organogenesis period (Lemaire 1970). Then, the eggs were incubated in our lab under natural conditions (water temperature around 19–21 ºC, photoperiod of 14 h L / 10 h D, and a salinity of 36 PSU). All juveniles were reared with the aim of sacrificing them randomly to validate the daily deposition in beaks.
Newly hatched individuals were reared inside the laboratory in 25 transparent 10-L square glass tanks (20 × 20x25, densities not greater than three individuals per tank), with aeration, recirculating seawater at a natural photoperiod. No artificial shelters were provided to hatchlings, their feeding was performed ad libitum (3 times per day) with live Artemia salina on a daily basis. Thirty-two individuals were reared at 18 ºC, while 127 were kept at 21 ºC. In Phase I, 112 individuals were cultured, of which 32 were reared at 18 ºC and 80 at 21 ºC. There were differences in the number of specimens cultured at each temperature, but the total number of specimens cultured at each temperature condition was enough to check the significance of differences between temperatures. In Phase II, 47 individuals were cultured at 21 ºC. No individuals were cultivated at 18 ºC because in Phase I differences between those cultured at 18 ºC and 21 ºC were no significant. The specimens were euthanized by anaesthetic overdose (clove oil, following Ayala-Soldado 2014) and dissected fresh.
Animal rearing was performed in compliance with Spanish law 53/2013 within the framework of the European Union’s adopted directive 2010/63/EU regarding animal welfare for the protection of animals employed for scientific purposes, following the Guidelines for the Care and Welfare of Cephalopods in Research, as proposed by Fiorito et al. (2015). The present study was also approved (register document OEBA-ULPGC 04/2019R1) by the Ethics Committee for Animal Research (Comité Ético de Experimentación Animal of the University of Las Palmas de Gran Canaria, CEEA-ULPGC, Spain).
Beak extraction, preparation and analysis
The beaks were extracted, cleaned, labelled and stored in distilled water at 5 ºC, as recommended by Perales-Raya et al. (2014b, 2018) for Octopus vulgaris paralarvae. Previously, it had been observed that the conservation of the beaks in 70% ethanol resulted in a low visibility of the growth increments. Upper and lower jaws were tested for age estimation. Each jaw was placed whole, without cutting, in the ventral position (convex side up) on a drop of water. It was then covered with a coverslip left tilted until the jaw was positioned correctly. Once its position was secured, some pressure was exerted on the coverslip to ensure fixation of the jaw and obtain standardized images (see Armelloni et al. 2020) (Fig. 1a). Image acquisition was carried out according to Perales-Raya et al. (2014b, 2018) and Armelloni et al. (2020).
After the correct placement, two areas of the jaws—the LWS and the rostrum surface—were explored to select the one that showed higher viability for age estimation. The increments were finally counted on the surface of the pigmented area (rostrum) of the lower jaw under transmitted light with a Nikon Microscope Multizoom AZ100 (400 × magnification). The system is equipped with a differential interference contrast attachment (DIC-Nomarski) that creates a 3-dimensional image of the rostrum surface, where the sequence of micro-increments is revealed. The regions of the jaw were identified, as well as the first increment (hatching; Fig. 1b), to standardize the reading methodology.
The increments were counted twice by the same trained reader. The coefficient of variation (CV) was calculated for each specimen to determine the precision (reproducibility) of the counts:
where R1 and R2 were the mean number of increments from the first and the second reading, respectively; R was the mean number of increments for both readings (Perales-Raya et al. 2018). The sample was classified into 7 age groups (Table 1): Group 0: 0–2 days; Group 1: 3–7 days; Group 2: 8–12 days; Group 3: 13–17 days; Group 4: 18–22 days; Group 5: 23–27 days; Group 6: 28–31 days. To avoid any bias by age groups, the CVs were averaged for each age group, and CVs < 7.6% were taken as valid (Campana et al. 2001). The mean CV was then calculated for each temperature group to assess and compare the precision of their readings. The number of increments was compared with the true age. The width of the reading area (WRA) was measured (μm) in the widest region of the reading area, where the increments were counted, in accord with Perales-Raya et al. (2018) (Table 1).
Statistical analysis
All statistical analyses were carried out using R v-4.1.1 (R Core Team 2021). A general linear model (GLM) was used to test the effect of the temperature, the age, and the interaction of age with temperature on increment numbers following Perales-Raya et al. (2018). ANOVA of the GLM fits was carried out to analyse the possible significant differences in relation to the variables described (Blanca-Mena et al. 2017; Foster 1b), as described by Perales-Raya et al. (2018) for the Octopus vulgaris paralarvae. Increments in the rostrum of lower jaws were clear (Fig. 2). Nevertheless, from the culture at 21 ºC, only 121 of the 127 collected beaks (95.28%) were suitable for age estimation. In the case of cuttlefishes reared at 18 ºC, 29 beaks of the 32 collected (90.63%) were readable.
Mean reading precision (CV) of readings in the rostral surface of S. officinalis was 2.95 ± 5.98% for all individuals. Mean values of CV for each age group were lower than the usual adopted value of 7.6% for annual and daily structures (Campana 2001; Table 1), and therefore, no additional individuals were discarded based on the CV. The CV in individuals cultured at 21 ºC (n = 120) was 3.26 ± 6.75%, and 2.01 ± 2.47% for those cultured at 18 ºC (n = 27). The age assigned to each individual was the average of the two readings.
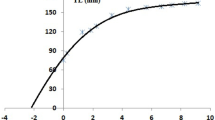
The relationship between the number of increments and the true age (Fig. 3) in newly hatched cuttlefish at both temperatures showed a linear trend (Table 2). The regressions between age and number of increments shown in Table 2 confirm daily deposition of increments in juveniles of S. officinalis at 18ºC and 21ºC. In Phase I, ANOVA of the GLM fit showed no significant differences in the increment deposition determined by the temperature alone or the factor Age x Temperature (Table 2 b). The same results were obtained for WRA growth, with no significant differences between cultures at different temperatures (ANOVA, p > 0.05). Phase II results are shown in Table 3.
Growth of cultured newly hatched Sepia officinalis: a Relationship between the mean number of increments counted in the lower jaw and the true age; b Dorsal Mantle Length and the true age; c Lower jaw growth (width of the reading area; see Fig. 1) with true age. Data grouped by temperature in the different phases
Discussion
This is the first time that the beak microstructure of Sepia officinalis has been shown in its early stages, where the visualization and the count of growth increments in the lower jaw’s rostrum was relatively easy. In contrast to the hatchlings of Octopus vulgaris, newly hatched Sepia officinalis showed no teeth as well as higher and wider pigmentation in the rostrum of lower jaws. This pigmentation in the reading area of the lower jaws probably allowed a better estimation of the true age, while for O. vulgaris the visibility of increments is much better in the upper jaws (Perales-Raya et al. 2014b; Armelloni et al. 2020). The lower jaws of O. vulgaris have a narrower and less pigmented rostrum, with deeper teeth and fewer increments visible compared with the upper jaws. It should also be noted that the upper jaws of S. officinalis juveniles are harder than those of O. vulgaris paralarvae, which complicates their manipulation and increase the risk of damaging them.
Regarding the LWS of S. officinalis beaks, they showed unclear growth marks that could be confused with roughness of the structure. Additionally, and according to Armelloni et al. (2020) for O. vulgaris paralarvae, the LWS in cuttlefish did not show highly developed pigmentation during these early stages and, consequently, some of the observed marks could be false increments. On the other hand, the rostral surface, which is already pigmented at hatching, showed a continuous sequence of growth increments in S. officinalis, making it possible to identify the age of the first increment. It is mandatory for correct validation of the methodology (Campana 2001).
The sizable agreement among readings indicates a high precision of readings in the rostral surface of Sepia officinalis. The reading precision (estimated using CV) was high because the readings differed with a maximum of ± 2.95%, although the variability of CV was high too (± 5.98), which is probably related to the complexity of the structure (Perales-Raya et al. 2018). The juveniles used in the present study were fed with soft diet but feeding may erode the beaks and careful should be taken to avoid age underestimations.
The GLM analysis showed that our results support the validation of this methodology for age estimation using beak microstructure as has been shown in other benthic species such as Octopus vulgaris (Hernández-López et al. 2001; Perales-Raya et al. 2014b, 2018; Armelloni et al. 2020) and O. maya (Rodriguez-Domínguez et al. 2013; Villegas-Bárcenas et al. 2014). The present study is the first validating the daily deposition of increments in early stages and the age of the first increment in the beaks of Sepia officinalis.
Ageing cuttlefish using the beak rostrum seems a suitable method for these species, in comparison with other commonly used methodologies, which are length-frequency analysis and direct ageing methods using statoliths and cuttlebones. Length-frequency distributions have some problems because the age of cohorts are not independently validated, the cephalopods show a high inter-individual variability, and modelling methods ignore variable growth rates which are influenced by environmental conditions (Semmens et al. 2004; Arkhipkin et al. 2021). Jackson et al. (1997) compared this methodology against the use of statoliths, since several authors used length-frequency analysis to analyse growth rate and growth forms (e.g. Jereb and Ragonese 1995; Mohamed 1996 among others). Jackson et al. (1997) concluded that length-frequency analysis should not be used for these age determinations, being the statoliths the promising technique at that time. As for statolith-based age determination, it is relatively laborious involving the extraction, handling, storage, mounting, both-side grinding and polishing, and counting under a microscope (Arkhipkin and Shcherbich 2012). In addition, its application is limited in cuttlefishes due to the complex crystalline structure of their statoliths (Natsukari and Tashiro 1991; Perales-Raya et al. 1994). Using the sequence of increments observed in the lateral dome (Perales-Raya et al. 1994), Bettencourt and Guerra (2001) validated increment deposition till 240 days of age in S. officinalis; but in older specimens, the age was underestimated due to the poor resolution of newer increments. Regarding the internal shell (cuttlebone), it has been used since the 1960s in several sepiid species, but formation of the shell stripes, or lamellae, is determined by physiological and environmental conditions (Yagi 1960; Choe 1963; Richard 1969; Ming-Tsung and Wang 2013, among others). It has also been used in S. officinalis (Richard 1969; Re and Narciso 1994, among others) and Sepia hierredda (Perales-Raya 2001). The absence of a daily deposition in the cuttlebone of S. officinalis was also described by Ré and Narciso (1994) and Le Goff et al. (1998) where they conclude that the cuttlebone lamellae should not be used for age estimation. The number of lamellae does not correspond to the true age and the temporal periodicity can be defined only if the temperature where the animal lives is considered (Bettencourt and Guerra 2001). The internal shell was recently analysed in several loliginid and sepiid species of known age by Nabhitabhata et al. (2022), reevaluating the accuracy in the neritic species living the tropical zone where environmental conditions are more stable. Moreover, some studies proposed to use the concentration of lipofuscin, a pigment that accumulates in tissues, as a proxy of age (Gras et al. 2016), but this method is considered complex and inaccurate (Doubleday and Semmens 2011). The use of these methodologies for age estimation were promising, although biological factors (length-frequency analysis) and morphological factors of the analysed structures (statolith and cuttlebone analysis) may influence the under- or over-estimation of age.
Using the rostrum surface of juveniles to determine age is equivalent to using rostrum sagittal sections in adults, as demonstrated in Octopus vulgaris (Perales-Raya et al. 2018, Lishchenko et al. unpublished results). Their similarities indicate that both structures are equivalent and suitable for S. officinalis. Cuttlefish beaks, once validated in adult stages, could be a promising method to determine the age of cuttlefishes.
The water temperature seems to be one of the most powerful factors influencing cuttlefish growth in cultured conditions. Domínguez et al. (2006) compared wild and reared Sepia officinalis observing that temperature and culture space play an important role in the growth of individuals. In the case of Octopus vulgaris, Perales-Raya et al. (2018) showed that the readings in octopuses cultured at 14 ºC and 21 ºC differed in width, whereas octopuses cultured at 21 ºC had a bigger reading area than octopuses reared at 14 ºC. Moreover, octopus paralarvae reared at 14 ºC showed apparent compaction of increments in the beaks and growth slowed or stopped at that temperature, thus affecting increment depositions (Perales-Raya et al. 2018). However, in our study, cuttlefishes reared at 18 ºC and 21 ºC did not show significant differences in the WRA. This could be due to the experimental temperatures, since they were both within the optimal range for S. officinalis, and this seems irrelevant for the increase in rostrum width during the first month of life. Nevertheless, temperature impact on the structure size was observed in other hard structures of cephalopods, such as statoliths. In particular, Villanueva (2000) reported that the size of the statoliths of Loligo vulgaris is determined by the temperatures at which the animal grows.
In conclusion, our results proved the validity of using the rostrum surface of beaks for age estimation in the early stages of S. officinalis and show that this methodology (using the microstructure of the beak rostrum) can be promising, once validated in adult stages, to determine the age in cuttlefishes since other structures such as statoliths or cuttlebone have limitations for a routine ageing method. The feeding factor should also be evaluated in future studies to estimate how much it might affect eroding the beak rostrum to avoid age underestimation. This study confirms the daily deposition in the first 31 days of life, fulfilling the initial hypotheses of one increment per day, but it is necessary to validate the age estimation of beaks in the full ontogenetic range of the species.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to are being processed for further analysis, but are available from the corresponding author on reasonable request.
References
Arkhipkin AI, Shcherbich ZN (2012) Thirty year’’ progress in age determination of squid using statoliths. Jour Mar Bio Assoc UK 92(6):1389–1398
Arkhipkin AI, Bizikov VA, Doubleday ZA, Laptikhovsky VV, Lishchenko FV, Perales-Raya C, Hollyman PR (2018) Techniques for estimating the age and growth of Molluscs: Cephalopoda. Jour Shell Res 37(4):783–793
Arkhipkin AI, Hendrickson LC, Payá I, Pierce GJ, Roa-Ureta RH, Robin JP, Winter A (2021) Stock assessment and management of cephalopods: advances and challenges for short-lived fishery resources. ICES Jour Mar Sci 78(2):714–730
Arkhipkin AI, Bizikov VA (1991) Comparative analysis of age and growth rates estimation using statoliths and gladius in squids. In: Squid Age Determination Using Statoliths. Proceedings of the International Workshop held in the Istituto di Technologia della Pesca e del Pescato (ITPP–CNR), Mazara del Vallo, Italy, 1989, Jereb P., Ragonese, S., Boletzky S. von (Eds.), 1991. Special Publications, pp. 19–37.
Armelloni EN, Lago-Rouco MJ, Bartolome A, Felipe BC, Almansa E, Perales-Raya C (2020) Exploring the embryonic development of upper beak in Octopus vulgaris Cuvier, 1797: new findings and implications for age estimation. Fish Res 221:105375
Ayala-Soldado N (2014) Estudio comparativo de los efectos de los anestésicos Metasulfonato de Tricaina (MS-222) y Eugenol para su uso en el pez cebra (Danio rerio) como modelo experimental. Doctoral Thesis
Batista BB, Matthews-Cascon H, Marinho RA, Kikuchi E, Haimovici M (2021) The growth and population dynamics of Octopus insularis targeted by a pot longline fishery in north-eastern Brazil. Jour Mar Biol Assoc UK 101(6):935–946
Bettencourt V, Guerra A (2001) Age studies based on daily growth increments in statoliths and growth lamellae in cuttlebone of cultured Sepia officinalis. Mar Biol 139(2):327–334
Blanca Mena MJ, Alarcón Postigo R, Arnau Gras J, Bono Cabré R, Bendayan R (2017) Non-normal data: Is ANOVA still a valid option?. Psico.
Boletzky SV (1983) Sepia officinalis. In: Boyle PR (ed) Cephalopod Life Cycles, vol 1. Species Account. Academic Press, London, pp 31–52
Boucaud-Camou E, Boismery J (1991) The migrations of the cuttlefish (Sepia officinalis L.) in the English Channel. In: Boucaud-Camou E (ed) La seiche/The cuttlefish. Centre de publication de l’Universite´ de Caen Basse-Normandie, Caen, pp 179–189
Campana SE (2001) Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Jour Fish Biol 59(2):197–242
Cherel Y (2020) A review of Southern ocean squids using nets and beaks. Mar Biod 50:98. https://doi.org/10.1007/s12526-020-01113-4
Choe D (1963) Daily age markings on the shell of cuttlefishes. Nat 197(4864):306–307
Clarke MR (1965) “Growth rings” in the beaks of the squid Moroteuthis ingens (Oegopsida, Onycoteuthidae). Malac 3(2):287–307
Cordella de Aguiar DC, Rossi-Wongtschow CLDB, Perez JAA (2012) Validation of daily growth increments of statoliths of Brazilian squid Doryteuthis plei and D. sanpaulensis (Cephalopoda: Loliginidae). Bio não-corrente 26(1):13–21
Denis V, Robin JP (2001) Present status of the French Atlantic fishery for cuttlefish (Sepia officinalis). Fish Res 52(1–2):11–22
Dominguez PM, Bettencourt V, Guerra A (2006) Growth of Sepia officinalis in captivity and in nature. Vie Mil 56(2):109–120
Donlon EM, Damsteegt EL, McKinnon J, Higgins FA, Lamare MD (2019) Growth and age of the midget octopus. Octopus Huttoni Aqua Ecol 53(4):689–706
Doubleday ZA, Semmens JM (2011) Quantification of the age-pigment lipofuscin in known-age octopus (Octopus pallidus): a potential tool for age determination. Jour Exp Mar Bio Eco 397(1):8–12
Fang Z, Li J, Thompson K, Hu F, Chen X, Liu B, Chen Y (2016) Age, growth, and population structure of the red flying squid (Ommastrephes bartramii) in the North Pacific Ocean, determined from beak microstructure. Fish Bull 114(1):34–44
Fiorito G, Affuso A, Basil J, Cole A, de Girolamo P, D’angelo L, Andrews PL (2015) Guidelines for the care and welfare of cephalopods in research–a consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab Ani 49:1–90
Foster R (2021) ANOVA for Some Non-Normal Data by Inverting Reliability Estimators, PsyAr**v, 25 pp.
Gras M, Safi G, Lebredonchel H, Quinquis J, Foucher E, Koueta N, Robin JP (2016) Stock structure of the English Channel common cuttlefish Sepia officinalis (Linnaeus 1758) during the reproduction period. Jour Biol Asso UK 96(1):167–176
Guerra A, Sánchez P (1985) Crecimiento relativo del estatolito de Sepia officinalis (Cephalopoda, Sepioidea) de la ría de Vigo. Inves Pesq 49(4):545–557
Hall KC, Fowler AJ, Geddes MC (2007) Evidence for multiple year classes of the giant Australian cuttlefish Sepia apama in northern Spencer Gulf, South Australia. Rev Fish Biol Fish 17:367–384
Hermosilla CA, Rocha F, Fiorito G, González AF, Guerra A (2010) Age validation in common octopus Octopus vulgaris using stylet increment analysis. ICES Jour Mar Sci 67(7):1458–1463
Hernández-López JL, Castro-Hernández JJ, Hernandez-Garcia V (2001) Age determined from the daily deposition of concentric rings on common octopus (Octopus vulgaris) beaks. Fish Bull 99(4):679–684
Hoving HJT, Robison BH (2017) The pace of life in deep-dwelling squids. Deep Sea Res Part i 126:40–49
Hu G, Fang Z, Liu B, Yang D, Chen X, Chen Y (2016) Age, growth and population structure of jumbo flying squid Dosidicus gigas off the Peruvian Exclusive Economic Zone based on beak microstructure. Fish Sci 82(4):597–604
Hurley GV, O’Dense P, O’Dor RK, Dawe EG (1985) Strontium labelling for verifying daily growth increments in the statoliths of the short-finned squid (Illex illecebrosus). Can Jour Fish Aqua Sci 42:380–383
Iglesias J, Fuentes L (2014) Octopus vulgaris. Paralarval culture. In: Iglesias J, Fuentes L, Villanueva R (eds) Cephalopod culture. Springer, Dordrecht
Jackson GD (1990) Age and growth of the tropical nearshore loliginid squid Sepioteuthis lessoniana determined from statolith growth-ring analysis. Fish Bull 88:113–118
Jackson GD, Moltschaniwskyj NA (1999) Analysis of precision in statolith derived age estimates of the tropical squid Photololigo (Cephalopoda: Loliginidae). ICES Jour Mar Sci 56(2):221–227
Jackson GD, Forsythe JW, Hixon RF, Hanlon RT (1997) Age, growth, and maturation of Lolliguncula brevis (Cephalopoda: Loliginidae) in the northwestern Gulf of Mexico with a comparison of length-frequency versus statolith age analysis. Can J Fish Aquat Sci 54(12):2907–2919
Jereb P, Ragonese S (1995) An outline of the biology of the squid Illex coindetii in the sicilian chanel (Central Mediterranean). J Mar Biol Assoc UK 75:373–390
Jereb P, Allcock LA, Lefkaditou E, Piatkowski U, Hastie LC, Pierce GJ (2015) Cephalopod biology and fisheries in Europe: II. Species Accounts, ICES
Jereb P, Ragonese S, Boltezky SV (1991) Squid age determination using statoliths, In: Proceedings of the International Workshop held in the Istituto di Tecnologia della Pesca e del Pescato, NRT-ITPP, Special publication,1, 127.
** Y, Lin F, Chen X, Liu B, Li J (2019) Microstructure comparison of hard tissues (statoliths, beaks, and eye lenses) of Uroteuthis chinensis in the South China Sea. Bull Mar Sci 95(1):13–26
Le Goff R, Gauvrit E, Pinczon du Sel G, Daguzan J (1998) Age group determination by analysis of the cuttlebone of the cuttlefish Sepia officinalis L in reproduction in the Bay of Biscay. J Molluscan Stud 64(2):183–193
Lemaire J (1970) Table de dévelopment embryonnaire de Sepia officinalis L.(Mollusque Cephalopode). Bull Soc Zool France 95:773–782
Leporati SC, Semmens JM, Pecl GT (2008) Determining the age and growth of wild octopus using stylet increment analysis. Mar Eco Prog Ser 367:213–221
Linnaeus CV (1758) Systema Naturae per regna tria naturae. Secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio 1(10):823
Lipinski MR, Durholtz MD, Underhill LG (1998) Field validation of age readings from the statoliths of chokka squid (Loligo vulgaris reynaudii d’Orbigny, 1845) and an assessment of associated errors. ICES Jour Mar Sci 55(2):240–257
Liu BL, Chen XJ, Chen Y, Hu GY (2015) Determination of squid age using upper beak rostrum sections: technique improvement and comparison with the statolith. Mar Biol 162(8):1685–1693
Liu BL, Chen XJ, Chen Y, Hu GY, Yu W, Wang JT, Lin JY (2017) Periodic increments in the jumbo squid (Dosidicus gigas) beak: a potential tool for determining age and investigating regional difference in growth rates. Hydro 790(1):83–92
Ming-Tsung C, Wang CT (2013) Age validation of the growth lamellae in the cuttlebone from cultured Sepia pharaonis at different stages. Jour Exp Mar Biol Eco 447:132–137
Mion M, Pias C, Giovanardi O (2014) Growth dynamics of Mullus barbatus L, 1758 and Sepia officinalis L, 1758 in relation to the summer trawling ban. Biol Mar Med 21(1):289–290
Mohamed KS (1996) Estimates of growth, mortality and stock of the Indian squid Loligo duvauceli Orbigny, exploited off Mangalore, Southwest coast of India. Bull Mar Sci 58:393–403
Morris GC (1988) Statolith growth lines and statocyst function in the Cephalopoda. Dissertation. University of Cambridge
Nabhitabhata J, Suriyawarakul J, Yamrungrueng A, Tongtherm K, Tuanapaya S (2022) Relationships of growth increments of internal shells and age through entire life cycles in three cultured neritic cephalopods (Mollusca: Cephalopoda) with re-evaluation as application for age determination. Swiss Jour Palae 141(1):1–8
Nakamura Y, Sakurai Y (1991) Validation of daily growth increments in statoliths of Japanese common squid Todarodes pacificus. Nip Sui Gakk 57:2007–2011
Natsukari Y, Tashiro M (1991) Neritic squid resources and cuttlefish resources in Japan. Mar Fresh Beha Phy 18(3):149–226
Perales-Raya C (2001) Determinación de la edad y estudio del crecimiento del choco (Sepia hierredda Rang, 1837), el calamar (Loligo vulgaris Lamarck, 1798) y el pulpo (Octopus vulgaris Cuvier, 1797) de la costa noroccidental africana. Dissertation, Universidad de La Laguna, 192 pp.
Perales-Raya C, Fernandez-Nuñez M, Balguerías E, Hernández-González CL (1994) Progress towards ageing cuttlefish Sepia hierredda from northwestern African coast using statoliths. Mar Ecol Prog Ser 144(1–2):139–147
Perales-Raya C, Hernández-González CL (1998) Growth lines within the beak microstructure of the octopus Octopus vulgaris Cuvier, 1797. Afr J Mar Sci 20:135–142
Perales-Raya C, Bartolomé A, García-Santamaría MT, Pascual-Alayón P, Almansa E (2010) Age estimation obtained from analysis of octopus (Octopus vulgaris Cuvier, 1797) beaks: improvements and comparisons. Fish Res 106(2):171–176
Perales-Raya C, Jurado-Ruzafa A, Bartolomé A, Duque V, Carrasco MN, Fraile-Nuez E (2014a) Age of spent Octopus vulgaris and stress mark analysis using beaks of wild individuals. Hydro 725(1):105–114
Perales-Raya C, Almansa E, Bartolomé A, Felipe BC, Iglesias J, Sánchez FJ, Carrasco JF, Rodríguez C (2014b) Age validation in Octopus vulgaris beaks across the full ontogenetic range: beaks as recorders of life events in octopuses. Jour Shell Res 33(2):481–493
Perales-Raya C, Nande M, Roura A, Bartolomé A, Gestal C, Otero JJ, García-Fernández P, Almansa E (2018) Comparative study of age estimation in wild and cultured Octopus vulgaris paralarvae: effect of temperature and diet. Mar Ecol Prog Ser 598:247–259
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
Rathjen WF, Voss GL (1987) The cephalopod fisheries: a review. In: Boyle PR (ed) Cephalopod Life Cycle, vol 2. Acad Press. London, pp 253–275
Ré P, Narciso L (1994) Growth and cuttlebone microstructure of juvenile cuttlefish, Sepia Qfficinalis L, under controlled conditions. J Exp Marine Biol Ecol 177(1):73–78
Richard A (1969) The part played by temperature in the rhythm of formation of markings on the shell of cuttlefish (Sepia officinalis) L. (Mollusca, Cephalopoda). Expe 25(10):1051–1052
Rodhouse PG, Hatfield EMC (1990) Age determination in squid using statolith growth increments. Fish Res 8:323–334
Rodríguez-Domínguez A, Rosas C, Méndez-Loeza I, Markaida U (2013) Validation of growth increments in stylets, beaks and lenses as ageing tools in Octopus maya. Jou Exp Mar Biol Eco 449:194–199
Schwarz R, Piatkowski U, Robison BH, Laptikhovsky VV, Hoving HJ (2019) Quantification of beak increments to study the pace of life in pelagic deep-sea Octopodiformes Japetella diaphana and Vampyroteuthis infernalis. Assessing the lifespans of coldwater octopods (Cephalopoda: Octopodiformes), 93.
Semmens JM, Pecl GT, Villanueva R, Jouffre D, Sobrino I, Wood JB, Rigby PR (2004) Understanding octopus growth: patterns, variability and physiology. Mar Fresh Res 55:367. https://doi.org/10.1071/MF03155
Šifner SK (2008) Methods for age and growth determination in cephalopods. Ribar 66(1):25–34
Villanueva R (2000) Effect of temperature on statolith growth of the European squid Loligo vulgaris during early life. Mar Bio 136(3):449–460
Villegas-Bárcenas G, Perales-Raya C, Bartolomé A, Almansa E, Rosas C (2014) Age validation in Octopus maya (Voss and Solís, 1966) by counting increments in the beak rostrum sagittal sections of known age individuals. Fish Res 152:93–97
Yagi T (1960) On the growth of the shell in Sepia esculenta Hoyle caught in Tokyo Bay. Bull Jap Soc Sci Fish 26(7):646–652
Young JZ (1960) The statocysts of Octopus vulgaris. Proc Roy Soc Lon. Ser b, Biol Sci 152:3–29
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Guerra-Marrero was supported by a PhD-fellowship (PIFULPGC-2017-CIENCIAS-2) from the University of Las Palmas de Gran Canaria.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AGM, CPR and JJC. The first draft of the manuscript was written by AGM and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in compliance with Spanish law 53/2013 within the framework of the European Union’s adopted directive 2010/63/EU regarding animal welfare for the protection of animals employed for scientific purposes, following the Guidelines for the Care and Welfare of Cephalopods in Research. The present study was also approved (register document OEBA-ULPGC 04/2019R1) by the Ethics Committee for Animal Research (Comité Ético de Experimentación Animal of the University of Las Palmas de Gran Canaria, CEEA-ULPGC, Spain).
Consent to participate
No applicable.
Consent to publish
Not applicable.
Additional information
Responsible Editor: Z. Doubleday .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guerra-Marrero, A., Perales-Raya, C., Lishchenko, F. et al. Age validation in early stages of Sepia officinalis from beak microstructure. Mar Biol 170, 24 (2023). https://doi.org/10.1007/s00227-022-04165-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04165-1