Abstract
Animal proteins from meat and its stuffs have recently been one of main concerns in the drive for sustainable food production. This viewpoint suggests that there are exciting prospects to reformulate meat products that are produced more sustainably and may also have health benefits by substituting high-protein nonmeat ingredients for some of the meat. Considering these pre-existing conditions, this review critically reviews recent data on extenders from several sources, including pulses, plant-based components, plant byproducts, and unconventional sources. We used the related keywords from Scopus-database without limiting the publishing date. With an emphasis on how these findings may impact the sustainability of meat products, it sees them as a great chance to enhance the functional quality and technological profile of meat. Therefore, to promote sustainability, meat alternatives such as plant-based meat equivalents are being made available. To boost consumer acceptability of these goods, further initiatives should also be developed to enhance the functioning of these innovative food items and increase public knowledge of plant-based meat analogues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
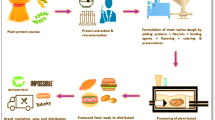
Meat is recognized as a very popular food item worldwide and it is well known as an excellent quality protein source with other nutritional characteristics along with its appealing taste. With the growing rate of the planet's population, the need for food security is rising as well, and to feed this growing population a greater amount of good quality food having proper protein, fat, and other nutrition is required. Meanwhile, increased environmental footprint awareness plays a significant role in meat analogues supply for the sustainable and transparent food security of the planet. Animal is the solitary bioresource of meat protein and with rapid population growth, the need for meat protein is also increasing. Various data show that the demand will be magnified near to twice by 2050 [1]. To cater to this high meat protein demand, more animal husbandry is required, but the scarcity of land, water, and other environmental factors constrain the growth of animal husbandry due to which meat price indices are continuously increasing, which makes it difficult in the adequate meat accessibility to the people of lower and lower-middle class group countries (Fig. 1). One of the major environmental concerns is also emission of greenhouse gases (carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O)) from livestock farming (Fig. 2). In the search for an alternative to conventional animal-derived meat protein, nowadays search for alternative protein has become very common, where plant-based meat analogues (PBMA) emerge as the most popular alternative [2]. Meat analogues are generally mock meat substitutes or imitation meat having all the structural and nutritional characteristics of meat along with the aesthetic qualities of specific types of meats [3]. The PBMA seems to be a wholesome solution to cater to all the following issues raised by animal meat such as high pricing of meat protein, religious concerns (halal/jhatka), greenhouse gas emissions from animal husbandry farms, and loss of biodiversity due to higher animal husbandry [4]. As people are becoming more aware of the importance of their health and environment, they are inclined to the PBMA [5]. Over a long period, scientists and researchers investigated different protein texturizing techniques to convert different plant-based proteins into meat analogues. The first attempt at texturing plant protein into meat analogues was made with soy protein isolate in 1970 where isolated soy protein was added to increase the protein level and enhance the texture of meatball, wiener, and hamburger compositions [3]. Nowadays to develop PBMA several protein sources like—soy protein, gluten, different legumes, vegetables, seeds, lentils, beans, peas, etc. are used as the main raw material. Plant-based proteins are converted to meat analogues mainly with the extrusion technology process and with time advancements in processing techniques resulting in mimic meats comprising enhanced nutritional qualities and functional meat-like characteristics [6, 7]. Based on moisture content PBMA is mainly two types—low moisture meat analogues that contain moisture content of less than 30% and high moisture content meat analogues that contain moisture content between 50 and 70% [8]. Low moisture content meat analogues are also known as texturized vegetable protein, and it is used with the combination of real meat to get a meat-like texture and qualities. High moisture content meat analogues are gaining rapid attention in the industries for their applicability as a whole meat substitute. In animal meat, one of the major concerns is high saturated fat content and high cholesterol content, which causes severe cardiovascular diseases and also other major problems such as hypertension, weight gain, etc. [9]. Different processed animal meat products like curing meat and smoking meat raised concerns about their possible carcinogenic phenomena [10]. PBMA—as a natural plant-based protein is a primary ingredient that imparts several vitamins, minerals, proteins, fibres, anti-oxidants, and polyphenols into our body system that keeps our body healthy [11]. In animal meat, there is a chance to transfer various animal-borne diseases into the body system through meat intake and this might cause serious illness in the human body, but the chances of similar issues from PBMA are low [2]. Nowadays from a healthy life point of view and different religious concerns, people are moving towards a vegan diet and for them, PBMA gives an excellent option to get meat-like taste, mouthfeel, and nutritional functionalities without meat intake.
This paper collects and synthetizes the available research on plant-based meat analogues. The Scopus database was used to retrieve the data. The Scopus database was searched for data in January 2023 using the following keywords: (plant proteins) AND (meat analogues OR meat alternatives OR meat formulation OR ingredients OR functionalities). The PRISMA guidelines (www.prisma-statement.org) were used for data refinement where the total number of primary searches was 806; after filtering the documents, the final number of relevant articles was 403. For this review, all titles, and abstracts of identified articles were screened by the authors and the full text was evaluated if appropriate. Additional inclusion criteria were that the article should deal specifically with meat analogues using plant proteins, not other sources. No temporal restrictions were applied to the literature search. Figure 3 reported the bibliometric analysis of plant protein-based meat analogues, data were captured from Scopus.
Meat analogues
Potential ingredients for meat analogues
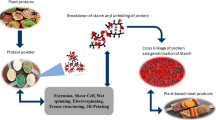
Figures 4 and 5 portray the necessary ingredients needed for develo** PBMA. The specific amount of all the ingredients with accurate formulation will result in the desired mock meat characteristics of the developed meat analogues.
Plant proteins as promising ingredients
Proteins are made up of amino acids that are critical for human health and are mostly used in food processing to create a variety of nutritious diets [12]. Dietary proteins are the basic source of nitrogen, and amino acids serve as construction blocks for human tissue while also requiring physiological enzymes to regulate chemical and biological reactions for the body to function properly [13]. Plant-based proteins (PBP) have gained popularity recently because of the change in particular dietary habits that most people are adopting. There are several PBP, for instance, soy protein, legume-based protein, wheat gluten, and various seed proteins are used in PBMA-develo** (Fig. 6). Soy protein is extensively used in PBMA-develo** due to its low cost, easily available, and superior functionalities. The reason for the increased focus on PBP is a recently discovered link between the intake of animal protein products and an enlarged hazard of chronic diseases [14]. Another reason for the increased prominence of PBP is the environmental issues caused by livestock agriculture. Recently, PBP was used as meat analogues in local and international markets. The global market for meat substitutes was $4,532.6 M in 2019 and is expected to reach $7,106.7 M in 2025. Between 2020 and 2025, this is with an estimated CAGR of 7.7%. Market trends and category development are influenced by regional variations; with 70% each, Europe and North America dominated the global market in 2019. Additionally, Asia Pacific held a significant share and is anticipated to grow at the fastest CAGR. The Middle East and Africa have a small share of the market, so they could be underexplored.
Usage of soy protein for meat analogues
Soybeans are rich in protein and contain water-soluble and insoluble proteins. Based on the dry weight of mature raw seeds, soybeans have ~ 8.5–13% moisture, ~ 33–40% protein, ~ 3–4% ash, ~ 18–20% fats, and ~ 9–12% fibers [15]. The variation in protein content may be due to cultivation environments, variety, and genetic modification. Soy protein has been utilized to create fabricated soy products, including soy protein isolates, soy protein concentrates, and soy-deflated flour [135]. Soluble proteins or aggregates migrate to the interface of the air–water, which reduces surface tension and keeps air bubbles in suspension, thus slowing the rate of coalescence. Recently, Zhu et al. [156] found that wet-separated pea protein had better foaming properties than that of wet-separated one, and the resulting dough of meat analogues was more solid-like with higher hardness. FCI of proteins from bell pepper seed, quinoa seed mung bean, hyacinth bean seed was reported to be 152.67–354.33, 58.37–78.62, 62.50, and 30.33–123.33, respectively, and the corresponding FSI was 113.33 to 119.00, 54.54–83.55 95.20%, and 27.32–84.44%, respectively [135, 136, 140]. The foam foaming property generally depends on protein solubility, as proteins with high solubility can diffuse easily and rapidly into the air–water interface for air bubble encapsulation, leading to improvements in FCI and FSI [136]. At present, the effect of the foaming property of plant protein on the quality of meat analogues is still lacking.
Gel forming ability of plant proteins
Gel-forming ability is the aggregation of unfolded proteins to form a 3D microstructure network through intermolecular interactions [143]. It is usually measured by the least gelation concentration (LGC) and gel strength (GS). Gel property is the foremost indicator for evaluating the usage of plant proteins in meat analogues. The gel-forming ability of plant proteins from diverse sources is significantly different. For example, when the concentration of mung bean protein is above 13%, it can form a gel [157]. The LGC of hyacinth bean seed and soy protein isolate is 16% and 15%, respectively [140, 143]. However, the LGC of rice protein isolate must reach more than 35% to make a gel [143]. The gel strength of plant proteins is significantly lesser as compared to proteins of meat flesh [155]. The nature and concentration of protein, environmental factors, and processing conditions are important influencing factors in the creation of gel [143]. Liu et al. [136] found that the neutral and alkaline environments in mung bean proteins were favoured to denature and unfold the proteins and expose the more embedded functional groups under heating, which improved the molecular interactions and gel texture.
Film forming property of plant proteins
Water vapour permeability (WVP) and tensile strength (TS) are the two critical parameters of film. The WVP of the film made from quinoa seeds, sesame seeds, and mung bean seeds were reported to be 1.98–4.76, 10.87–13.57, and 10.76 10−7g Pa−1 h−1 m−1, respectively and their TS were 1.88–4.28, 8.29, and 3.33 MPa, respectively [144,145,146]. The film-forming property can be influenced by the properties of proteins used for preparing the film such as solubility, the hydrophobic/hydrophilic nature, purity, free volume, molecular mobility, and flexibility [144,145,146]. Yang et al. [158] used proteins from distiller-dried grains to prepare film incorporating green tea extracts and reported that the film can be utilized as an anti-oxidative packaging material for pork meat. Plant protein film has the potential to be used for packaging meat analogues. However, there are few relevant reports at present.
Binding property of plant proteins
In meat analogues processing, adding flavor (such as vanillin) and nutrients (such as flavonoids) is easy to achieve. Protein contains a variety of functional groups, which can interact with flavonoids, vanillin, quercetin, rutin, and other compounds through covalent (Schiff bases) and non-covalent (hydrogen bond, hydrophobic interaction, electrostatic force, etc.). Therefore, on one side, proteins can bond flavonoids and anthocyanins reversibly and irreversibly to form complexes and improve their stability. Jia et al. [147] stated that 7S/11S soybean protein can bind quercetin and rutin. The binding constant was 0.03–97.43 105 L M−1, and the binding site was 0.77–1.65. Soybean protein isolate was reported to be able to bind to flavonoids, with a binding constant of 0.23–143.88 105 L M−1 and a binding site of 0.98–1.75 [147]. On the other hand, proteins can interact with vanillin, aldehydes, and ketones to cause the loss of flavor intensity or so-called flavor fade. Temthawee et al. [148] found that the binding of vanillin to coconut protein was enthalpy driven, the binding constant was 40.1–122.2 105 L M−1, and the binding site was 0.81–1.48 [148]. The interactions between the compounds and proteins can be influenced by several factors relevant to the functional groups of these compounds and the changes in the protein conformation [147, 148].
Extrusion ability of plant proteins
Extrusion is the most widely used technology to process plant proteins to meat analogues with rich fibrous structures and good springiness like real animal meat. Specific mechanical energy (SME) and fibrous degree (FD) are the most important indicators of extruded meat analogues. SME refers to the mechanical energy absorbed per unit mass of extrudates, which translates into the extent of molecular collapse or disintegration of materials during extrusion. The FD was computed by dividing the crosswise shear force lengthways, which can be used for characterizing the anisotropic structure in the extrudate. Soy protein concentrate, pea protein isolate, and peanut protein powder have been used for extruding into meat analogues [149, 150, 159]. Their SMEs were 1029.03, 985.07660.56–1135.67 kJ, respectively, while TD is 1.03, 1.30, and 1.33, respectively. SME is majorly connected with the contents of moisture and fat, and energy input intensity [151, 160]. Fatty acids, especially those with low unsaturation degrees, have a lubrication effect, which reduces die pressure and contributes to the smooth flow of protein melt in the extruder barrel, consequently reducing the mechanical energy consumption during extrusion processing. TD is related to the protein nature, functional additive, and operation conditions. Dou et al. [149] reported that adding Gums promoted the fibre formation.
Influence of processing on functionalities of proteins
The functional qualities of plant protein depend on processing procedures intrinsic to the chosen extraction processes, modification, heat treatment, etc. in addition to elements inherent to the protein and environmental conditions [161]. These treatments will cause desired and unwanted changes in the functional characteristics of proteins (Table 6). In this way, it is very necessary to explain the changes in protein functional properties during processing operations and the corresponding results from the perspective of chemistry and physics.
Effect of physical operations on proteins
Effect of high-pressure homogenization, micro-fluidization, and high hydrostatic pressure treatments
In recent years, high-pressure processing has received attention from both industry and academia and has smeared extensively to amend the functional characteristics of plant proteins. During the high-pressure homogenization process, a continuous flowing fluid or suspension is pushed through a narrow gap that is usually controlled by a valve, which leads to high turbulence and shear stress [85]. Micro-streams with high velocity are generated in the micro-fluidization process, as fluid is accelerated into a Y-type interaction chamber by a high-pressure pump, resulting in high shear and impact forces [152]. In high-pressure homogenization, the fluid circulates in the equipment for a period, whereas the fluid passes through the micro-fluidization device in a fleeting time. As for the high hydrostatic pressure treatment, protein isolate or concentrate is placed in a closed ultra-high-pressure container statically, and water is used as the medium to apply a pressure of 200 ~ 600 MPa usually, which is higher than that (typically in a range of 50–200 MPa) in the micro-fluidization and high hydrostatic pressure treatment [165].
Those pressure effects can induce significant modifications of the functional characteristics of plant proteins (Table 6). Regardless of the method and protein source, moderate pressure (50 ~ 200 MPa) treatment can significantly increase the solubility, emulsification property, foaming property, and gel-forming ability. The improvement of solubility can be attributed to the fact that moderate pressure treatment causes the proper dissociation of the quaternary structure, the diminution of protein particle size, and the swelling of the molecule, leading to an increase in solvent-protein interaction [166]. At the same time, moderate pressure treatment unfolds the protein conformational structure, which led to the disclosure of internal hydrophobic groups and the increase of molecular flexibility, thereby enhancing the intermolecular interactions and protein adsorption at the air–water and oil–water interfaces. Therefore, the foaming property, gel-forming ability, emulsification property, and film-forming property are improved. However, excessive pressure (> 300 MPa) treatment induces the creation of bigger protein aggregates, causing declined solubility and other functional characteristics [162,163,133, 143]. The exposure of hydrophobic groups induced by sonication facilitates the diffusion of protein molecules toward air–water or oil–water interfaces and their adsorption on them. Consequently, the foaming property and emulsification properties are remarkably improved on the condition that the structural changes have been balanced in terms of hydrophilicity and hydrophobicity after sonication [143, 153]. Although the hydrophobicity increases, the foaming property, and emulsification properties may decrease due to the decline in the solubility under the excessive sonication [133, 143]. Under moderate sonication conditions, gel-forming ability film-forming property, and extrudability increase, as indicated in the increase of TS while the decrease of WVP, a decrease of LGC, and an increase of TD, correspondingly [143, 144, 168]. The reason might be assigned to the partial unfolding of their three-dimensional molecular structures, resulting in the exposing of hydrophobic and/or sulfhydryl groups, thereby promoting the formation of more interconnected gel networks due to the presence of more junction zone per particle [143, 168].
Effect of water bath incubation, autoclaving, ohmic heating, microwave heating, and extrusion cooking
Heat treatment is a traditional processing method, including water bath incubation, autoclaving, ohmic heating, microwave heating, extrusion cooking, etc. These heating methods have distinct characteristics. For example, water bath incubation is easy to apply in industrial production. Autoclaving can make the material temperature reach over 100 ℃. Ohmic and microwave heating can quickly generate heat by utilizing the friction movement of polar molecules and the conductivity of ions, respectively, thus shortening the heating time. Extrusion cooking combines the functions of mixing, sterilization, heating, drying, and forming. Heat treatment affects the functional characteristics of plant proteins.
Generally, the solubility of protein decreased after thermal treatment, which is mainly due to the creation of insoluble aggregates [139, 165]. However, Hu et al. [166] reported that extrusion increased the solubility of Baijiu vinasse proteins and suggested that the changes in solubility during extrusion are primarily due to an interplay of shearing, temperature, and pressure, which led to the formation of the small particle size, porous structure, and air voids of proteins. Water bath incubation, ohmic heating, and extrusion cooking affect the foaming property and emulsification properties of proteins, which rely on the operation conditions. The changes in the foaming property and emulsification properties can be explained by the changes in solubility and conformational structure [139, 166]. After water bath incubation (90 °C), the gel-forming ability of cowpea protein isolates decreased. It could be due to the formation of aggregates that had a lower ability to realign and form the protein network [165]. Guo et al. [169] examined that the binding capacities of soy protein isolate for HxAc and HpAc decreased as the temperature increased in the protein aqueous solution. It was related to the formation of an additional hydrophobic surface because of the thermal denaturation (unfolding) of protein, which declined the affinity of primary binding sites. Whereas the binding capacities of soy protein isolate for LiFo, LiAc, linalool, and geraniol increased, the reason was that heat treatment increased the number of lower affinity secondary binding sites on the hydrophobic surface of soy protein isolate. TD showed a tendency to significantly decrease after autoclaving [170]. The result was thought to be related to the movement and redistribution of moisture caused by the vacuum-heat treatment process.
Effect of atmospheric cold plasma, ultraviolet radiation
In recent years, non-thermal technology, a potential alternative to traditional thermal processing for microbial inactivation to extend the shelf life, including atmospheric cold plasma and ultraviolet irradiation, etc., were pertained to improve the functional characteristics of plant proteins regarding solubility, gel-forming ability, film-forming properties, and foaming property [145, 172, There is no data available for this article. Kyriakopoulou K, Dekkers B, van der Goot AJ (2019) Plant-based meat analogues. Sustain Meat Prod Process. https://doi.org/10.1016/B978-0-12-814874-7.00006-7 Singh M, Trivedi N, Enamala MK, Kuppam C, Parikh P, Nikolova MP, Chavali M (2021) Plant-based meat analogue (PBMA) as a sustainable food: a concise review. Eur Food Res Technol 247(10):2499–2526. https://doi.org/10.1007/S00217-021-03810-1 Zahari I, Östbring K, Purhagen JK, Rayner M (2022) Plant-based meat analogues from alternative protein: a systematic literature review. Foods 11(18):2870. https://doi.org/10.3390/FOODS11182870 Boukid F (2020) Plant-based meat analogues: from niche to mainstream. Eur Food Res Technol 247(2):297–308. https://doi.org/10.1007/S00217-020-03630- Siddiqui SA, Bahmid NA, Karim I, Mehany T, Gvozdenko AA, Blinov AV, Lorenzo JM (2022) Cultured meat: processing, packaging, shelf life, and consumer acceptance. LWT 172:114192. https://doi.org/10.1016/j.lwt.2022.114192 Siddiqui SA, Alvi T, Sameen A, Khan S, Blinov AV, Nagdalian AA, Onwezen M (2022) Consumer acceptance of alternative proteins: a systematic review of current alternative protein sources and interventions adapted to increase their acceptability. Sustainability 14(22):15370. https://doi.org/10.3390/su142215370 Siddiqui SA, Khan S, Murid M, Asif Z, Oboturova NP, Nagdalian AA, Jafari SM (2022) Marketing strategies for cultured meat: a review. Appl Sci 12(17):8795. https://doi.org/10.3390/app12178795 Joshi V, Kumar S (2015) Meat analogues: plant based alternatives to meat products–a review. Int J Food Ferment 5(2):107. https://doi.org/10.5958/2277-9396.2016.00001.5 van Vliet S, Kronberg SL, Provenza FD (2020) Plant-based meats, human health, and climate change. Front Sustain Food Syst 4:128. https://doi.org/10.3389/FSUFS.2020.00128/BIBTEX Ishaq A, Irfan S, Sameen A, Khalid N (2022) Plant-based meat analogs: a review with reference to formulation and gastrointestinal fate. Curr Res Nutr Food Sci 5:973–983. https://doi.org/10.1016/J.CRFS.2022.06.001 Bryngelsson S, Moshtaghian H, Bianchi M, Hallström E (2022) Nutritional assessment of plant-based meat analogues on the swedish market. Int J Food Sci Nutri 73(7):889–901. https://doi.org/10.1080/09637486.2022.2078286 Sá AGA, Moreno YMF, Carciofi BAM (2019) Food processing for the improvement of plant proteins digestibility. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2019.1688249 Hertzler SR, Lieblein-Boff JC, Weiler M, Allgeier C (2020) Plant proteins: assessing their nutritional quality and effects on health and physical function. Nutrients 12(12):1–27. https://doi.org/10.3390/NU12123704 Päivärinta E, Itkonen ST, Pellinen T, Lehtovirta M, Erkkola M, Pajari AM (2020) Replacing animal-based proteins with plant-based proteins changes the composition of a whole nordic diet-a randomised clinical trial in healthy finnish adults. Nutrients. https://doi.org/10.3390/NU12040943 Qin P, Wang T, Luo Y (2022) A review on plant-based proteins from soybean: Health benefits and soy product development. J Agric Food Res 7:100265. https://doi.org/10.1016/J.JAFR.2021.100265 Sha L, **ong YL (2020) Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci Technol 102:51–61. https://doi.org/10.1016/J.TIFS.2020.05.022 Nishinari K, Fang Y, Guo S, Phillips GO (2014) Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocolloids 39:301–318. https://doi.org/10.1016/j.foodhyd.2014.01.013 Tarone AG, Fasolin LH, Perrechil FDA, Hubinger MD, Da Cunha RL (2013) Influence of drying conditions on the gelling properties of the 7S and 11S soy protein fractions. Food Bioprod Process 91(2):111–120. https://doi.org/10.1016/j.fbp.2012.11.010 Liu Y, Huang ZH, Hu ZX, Yu Z, An HZ (2023) Texture and rehydration properties of texturized soy protein: Analysis based on soybean 7S and 11S proteins. Int J Food Sci Technol 58(1):323–333 Chatterjee C, Gleddie S, **ao CW (2018) Soybean bioactive peptides and their functional properties. Nutrients. https://doi.org/10.3390/NU10091211 Kumar S (2016) Meat Analogs “Plant based alternatives to meat products: Their production technology and applications.” Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2016.1196162 Bakhsh A, Lee S-J, Lee E-Y, Hwang Y-H, Joo S-T (2021) Traditional plant-based meat alternatives, current, and future perspective: a review. J Agric Life Sci 55(1):1–11. https://doi.org/10.14397/JALS.2021.55.1.1 Mäkinen OE, Sozer N, Ercili-Cura D, Poutanen K (2016) Protein from oat: structure, processes, functionality, and nutrition. Sustain Protein Sources. https://doi.org/10.1016/B978-0-12-802778-3.00006-8 He J, Evans NM, Liu H, Shao S (2020) A review of research on plant-based meat alternatives: Driving forces, history, manufacturing, and consumer attitudes. ComprehRev Food Sci Food Safety 19(5):2639–2656. https://doi.org/10.1111/1541-4337.12610 Chiang JH, Loveday SM, Hardacre AK, Parker ME (2019) Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct 19:100102. https://doi.org/10.1016/J.FOOSTR.2018.11.002 Accoroni C, Godoy E, Reinheimer MA (2020) Performance evaluation of protein recovery from Argentinian soybean extruded-expelled meals under different operating conditions. J Food Eng 274:109849. https://doi.org/10.1016/J.JFOODENG.2019.109849 Jan A, Sood M, Sofi SA, Norzom T (2017) Non-thermal processing in food applications: a review. Int J Food Sci Nutr 2(6):171–180 Geerts MEJ, Dekkers BL, van der Padt A, van der Goot AJ (2018) Aqueous fractionation processes of soy protein for fibrous structure formation. Innov Food Sci Emerg Technol 45:313–319. https://doi.org/10.1016/J.IFSET.2017.12.002 Lin D, Lu W, Kelly AL, Zhang L, Zheng B, Miao S (2017) Interactions of vegetable proteins with other polymers: Structure-function relationships and applications in the food industry. Trends Food Sci Technol 68:130–144. https://doi.org/10.1016/j.tifs.2017.08.006 Peng Y, Kyriakopoulou K, Rahmani A, Venema P, van der Goot AJ (2021) Isochoric moisture heating as a tool to control the functionality of soy protein. LWT. https://doi.org/10.1016/j.lwt.2021.111979 Baune MC, Völler M, Schroeder S, Witte F, Heinz V (2019) Additive-free vegan emulsion-type sausages based on meat and fat substitutes. Int Congress Meat Sci Technol 23:392–394 Krintiras GA, Göbel J, Van Der Goot AJ, Stefanidis GD (2015) Production of structured soy-based meat analogues using simple shear and heat in a Couette Cell. J Food Eng 160:34–41. https://doi.org/10.1016/j.jfoodeng.2015.02.015 Wild F, Czerny M, Janssen AM, Kole APW, Zunabovic M, Domig KJ (2014) The evolution of a plant-based alternative to meat. From niche markets to widely accepted meat alternatives. Agro Food Ind Hi Tech 25(1):45–49 Palanisamy M, Töpfl S, Aganovic K, Berger RG (2018) Influence of iota carrageenan addition on the properties of soya protein meat analogues. Lwt 87:546–552 Tessari P, Lante A, Mosca G (2016) Essential amino acids: master regulators of nutrition and environmental footprint? Sci Rep 6(1):1–13. https://doi.org/10.1038/srep26074 USDA (2020) FoodData Central. USDA, Hutchinson Gorissen SHM, Crombag JJR, Senden JMG, Waterval WAH, Bierau J, Verdijk LB, van Loon LJC (2018) Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 50(12):1685–1695. https://doi.org/10.1007/S00726-018-2640-5 Gasparre N, Rosell CM (2023) Wheat gluten: A functional protein still challenging to replace in gluten-free cereal-based foods. Cereal Chem 100(2):243–255. https://doi.org/10.1002/CCHE.10624 Barak S, Mudgil D, Khatkar BS (2014) Influence of gliadin and glutenin fractions on rheological, pasting, and textural properties of dough. Int J Food Prop 17(7):1428–1438. https://doi.org/10.1080/10942912.2012.717154 Schmiele M, Nucci Mascarenhas MCC, da Silva Barretto AC, Rodrigues Pollonio MA (2015) Dietary fiber as fat substitute in emulsified and cooked meat model system. LWT Food Sci Technol 61(1):105–111. https://doi.org/10.1016/J.LWT.2014.11.037 Ooms N, Jansens KJA, Pareyt B, Reyniers S, Brijs K, Delcour JA (2018) The impact of disulfide bond dynamics in wheat gluten protein on the development of fermented pastry crumb. Food Chem 242:68–74. https://doi.org/10.1016/J.FOODCHEM.2017.09.007 Pietsch VL, Werner R, Karbstein HP, Emin MA (2019) High moisture extrusion of wheat gluten: Relationship between process parameters, protein polymerization, and final product characteristics. J Food Eng 259:3–11. https://doi.org/10.1016/j.jfoodeng.2019.04.006 Urade R, Sato N, Sugiyama M (2018) Gliadins from wheat grain: an overview, from primary structure to nanostructures of aggregates. Biophys Rev 10(2):435–443. https://doi.org/10.1007/S12551-017-0367-2 Samard S, Gu BY, Ryu GH (2019) Effects of extrusion types, screw speed and addition of wheat gluten on physicochemical characteristics and cooking stability of meat analogues. J Sci Food Agric 99(11):4922–4931. https://doi.org/10.1002/JSFA.9722 Guzmán C, Posadas-Romano G, Hernández-Espinosa N, Morales-Dorantes A, Peña RJ (2015) A new standard water absorption criteria based on solvent retention capacity (SRC) to determine dough mixing properties, viscoelasticity, and bread-making quality. J Cereal Sci 66:59–65. https://doi.org/10.1016/j.jcs.2015.10.009 Malav OP, Talukder S, Gokulakrishnan P, Chand S (2015) Meat analog: a review. Crit Rev Food Sci Nutr 55(9):1241–1245. https://doi.org/10.1080/10408398.2012.689381 Bessada SMF, Barreira JCM, Oliveira MBPP (2019) Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci Technol 93:53–68. https://doi.org/10.1016/J.TIFS.2019.08.022 **ao S, Li Z, Zhou K, Fu Y (2023) Chemical composition of kabuli and desi chickpea (Cicer arietinum L.) cultivars grown in **njiang, China. Food Sci Nutr 11(1):236–248. https://doi.org/10.1002/fsn3.3056 Gu J, Bk A, Wu H, Lu P, Nawaz MA, Barrow CJ, Dunshea FR, Suleria HAR (2023) Impact of processing and storage on protein digestibility and bioavailability of legumes. Food Rev Intl 39(7):4697–4724. https://doi.org/10.1080/87559129.2022.2039690 Anzani C, Boukid F, Drummond L, Mullen AM, Álvarez C (2020) Optimising the use of proteins from rich meat co-products and non-meat alternatives: Nutritional, technological and allergenicity challenges. Food Res Int 137:109575. https://doi.org/10.1016/J.FOODRES.2020.109575 Vogelsang-O’Dwyer M, Zannini E, Arendt EK (2021) Production of pulse protein ingredients and their application in plant-based milk alternatives. Trends Food Sci Technol 110:364–374. https://doi.org/10.1016/J.TIFS.2021.01.090 Brishti FH, Chay SY, Muhammad K, Ismail-Fitry MR, Zarei M, Karthikeyan S, Saari N (2020) Effects of drying techniques on the physicochemical, functional, thermal, structural and rheological properties of mung bean (Vigna radiata) protein isolate powder. Food Res Int 138:109783. https://doi.org/10.1016/J.FOODRES.2020.109783 Ayari S, Shankar S, Follett P, Hossain F, Lacroix M (2020) Potential synergistic antimicrobial efficiency of binary combinations of essential oils against Bacillus cereus and Paenibacillus amylolyticus-Part A. Microb Pathog 141:104008. https://doi.org/10.1016/J.MICPATH.2020.104008 Gharibzahedi SMT, Smith B (2020) The functional modification of legume proteins by ultrasonication: A review. Trends Food Sci Technol 98:107–116. https://doi.org/10.1016/j.tifs.2020.02.002 Vogelsang-O’Dwyer M, Petersen IL, Joehnke MS, Sørensen JC, Bez J, Detzel A, Busch M, Krueger M, O’Mahony JA, Arendt EK, Zannini E (2020) Comparison of Faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: techno-functional. Nutr Environ Perform Foods 9(3):322 Wang Y, Guldiken B, Tulbek M, House JD, Nickerson M (2020) Impact of alcohol washing on the flavour profiles, functionality and protein quality of air classified pea protein enriched flour. Food Res Int. https://doi.org/10.1016/j.foodres.2020.109085 Fetzer A, Herfellner T, Stäbler A, Menner M, Eisner P (2018) Influence of process conditions during aqueous protein extraction upon yield from pre-pressed and cold-pressed rapeseed press cake. Ind Crops Prod 112:236–246. https://doi.org/10.1016/J.INDCROP.2017.12.011 Náthia-Neves G, Alonso E (2021) Valorization of sunflower by-product using microwave-assisted extraction to obtain a rich protein flour: Recovery of chlorogenic acid, phenolic content and antioxidant capacity. Food Bioprod Process 125:57–67. https://doi.org/10.1016/j.fbp.2020.10.008 Wang M, Liu Y, Pan RL, Wang RY, Ding SL, Dong WR, Sun GB, Ye JX, Sun XB (2019) Protective effects of Myrica rubra flavonoids against hypoxia/reoxygenation-induced cardiomyocyte injury via the regulation of the PI3K/Akt/GSK3β pathway. Int J Mol Med. https://doi.org/10.3892/ijmm.2019.4131 Salgado PR, Ortiz SEM, Petruccelli S, Mauri AN (2012) Functional food ingredients based on sunflower protein concentrates naturally enriched with antioxidant phenolic compounds. JAOCS 89(5):825–836. https://doi.org/10.1007/s11746-011-1982-x Malik MA, Saini CS (2018) Rheological and structural properties of protein isolates extracted from dephenolized sunflower meal: Effect of high intensity ultrasound. Food Hydrocolloids 81:229–241. https://doi.org/10.1016/j.foodhyd.2018.02.052 Jia W, Rodriguez-Alonso E, Bianeis M, Keppler JK, van der Goot AJ (2021) Assessing functional properties of rapeseed protein concentrate versus isolate for food applications. Innov Food Sci Emerg Technol. https://doi.org/10.1016/j.ifset.2021.102636 Tan SH, Mailer RJ, Blanchard CL, Agboola SO, Day L (2014) Gelling properties of protein fractions and protein isolate extracted from Australian canola meal. Food Res Int 62:819–828. https://doi.org/10.1016/J.FOODRES.2014.04.055 Ainis WN, Ersch C, Ipsen R (2018) Partial replacement of whey proteins by rapeseed proteins in heat-induced gelled systems: Effect of pH. Food Hydrocolloids 77:397–406. https://doi.org/10.1016/J.FOODHYD.2017.10.016 Kim JH, Varankovich NV, Stone AK, Nickerson MT (2016) Nature of protein-protein interactions during the gelation of canola protein isolate networks. Food Res Int 89:408–414. https://doi.org/10.1016/j.foodres.2016.08.018 Baioumy AA, Bobreneva IV, Tvorogova AA, Shobanova TV (2018) Possibility of using quinoa seeds (Chenopodium quinoa) in meat products and its impact on nutritional and organoleptic characteristics. Biosci Res 15(4):3307–3315 Verma AK, Rajkumar V, Kumar S (2019) Effect of amaranth and quinoa seed flour on rheological and physicochemical properties of goat meat nuggets. J Food Sci Technol 56(11):5027–5035. https://doi.org/10.1007/S13197-019-03975-4 Zambrano V, González R, Viera C (2019) Quinoa as gelling agent in a mortadella formulation. Int Food Res J 26(3):1069–1077 Elsohaimy SA, Refaay TM, Zaytoun MAM (2015) Physicochemical and functional properties of quinoa protein isolate. Ann Agric Sci 60(2):297–305. https://doi.org/10.1016/J.AOAS.2015.10.007 Bučko S, Katona J, Popović L, Vaštag Ž, Petrović L, Vučiniće-Vasić M (2015) Investigation on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. LWT Food Sci Technol 64(2):609–615. https://doi.org/10.1016/J.LWT.2015.06.054 López DN, Ingrassia R, Busti P, Wagner J, Boeris V, Spelzini D (2018) Effects of extraction pH of chia protein isolates on functional properties. LWT 97:523–529. https://doi.org/10.1016/j.lwt.2018.07.036 Coelho MS, de Salas-Mellado M (2018) How extraction method affects the physicochemical and functional properties of chia proteins. LWT 96:26–33. https://doi.org/10.1016/J.LWT.2018.05.010 Rezig L, Chibani F, Chouaibi M, Dalgalarrondo M, Hessini K, Guéguen J, Hamdi S (2013) Pumpkin (cucurbita maxima) seed proteins: Sequential extraction processing and fraction characterization. J Agric Food Chem 61(32):7715–7721 Kleekayai T, Khalesi M, Amigo-Benavent M, Cermeño M, Harnedy-Rothwell P, FitzGerald RJ (2023) Enzyme-Assisted Extraction of Plant Proteins. Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development. Springer International Publishing, Cham, pp 131–178 Sari YW, Bruins ME, Sanders JPM (2013) Enzyme assisted protein extraction from rapeseed, soybean, and microalgae meals. Ind Crops Prod 43(1):78–83. https://doi.org/10.1016/j.indcrop.2012.07.014 Yao F, Chen F, Du Y, Zhang Q, Zhu T (2021) Functional and structural properties of soy 11S globulin: Influence of reverse micelle extraction. J Food Sci 86(8):3403–3412. https://doi.org/10.1111/1750-3841.15820 Varghese T, Pare A (2019) Effect of microwave assisted extraction on yield and protein characteristics of soymilk. J Food Eng 262:92–99. https://doi.org/10.1016/j.jfoodeng.2019.05.020 Grossmann L, McClements DJ (2023) Current insights into protein solubility: A review of its importance for alternative proteins. Food Hydrocolloids 137:108416. https://doi.org/10.1016/J.FOODHYD.2022.108416 Lu ZX, He JF, Zhang YC, Bing DJ (2020) Composition, physicochemical properties of pea protein and its application in functional foods. Crit Rev Food Sci Nutr 60(15):2593–2605. https://doi.org/10.1080/10408398.2019.1651248 Pickardt C, Eisner P, Kammerer DR, Carle R (2015) Pilot plant preparation of light-coloured protein isolates from de-oiled sunflower (Helianthus annuus L.) press cake by mild-acidic protein extraction and polyphenol adsorption. Food Hydrocolloids 44:208–219. https://doi.org/10.1016/j.foodhyd.2014.09.020 Dabbour M, He R, Ma H, Musa A (2018) Optimization of ultrasound assisted extraction of protein from sunflower meal and its physicochemical and functional properties. J Food Process Eng 41(5):e12799. https://doi.org/10.1111/JFPE.12799 Tirgar M, Silcock P, Carne A, Birch EJ (2017) Effect of extraction method on functional properties of flaxseed protein concentrates. Food Chem 215:417–424. https://doi.org/10.1016/j.foodchem.2016.08.002 Luo L, Zhang R, Palmer J, Hemar Y, Yang Z (2021) Impact of high hydrostatic pressure on the gelation behavior and microstructure of quinoa protein isolate dispersions. ACS Food Sci Technol 1(11):2144–2151. https://doi.org/10.1021/ACSFOODSCITECH.1C00332 Du L, Arauzo PJ, Meza Zavala MF, Cao Z, Olszewski MP, Kruse A (2020) Towards the properties of different biomass-derived proteins via various extraction methods. Molecules. https://doi.org/10.3390/molecules25030488 Luo L, Cheng L, Zhang R, Yang Z (2022) Impact of high-pressure homogenization on physico-chemical, structural, and rheological properties of quinoa protein isolates. Food Struct. https://doi.org/10.1016/j.foostr.2022.100265 Mota C, Santos M, Mauro R, Samman N, Matos AS, Torres D, Castanheira I (2016) Protein content and amino acids profile of pseudocereals. Food Chem 193:55–61. https://doi.org/10.1016/J.FOODCHEM.2014.11.043 Wouters AGB, Rombouts I, Fierens E, Brijs K, Delcour JA (2016) Relevance of the functional properties of enzymatic plant protein hydrolysates in food systems. Compreh Rev Food Sci Food Saf 15(4):786–800. https://doi.org/10.1111/1541-4337.12209 Kyriakopoulou K, Keppler JK, van der Goot AJ (2021) Functionality of ingredients and additives in plant-based meat analogues. Foods. https://doi.org/10.3390/FOODS10030600 Martins AJ, Lorenzo JM, Franco D, Vicente AA, Cunha RL, Pastrana LM, Quiñones J, Cerqueira MA (2019) Omega-3 and polyunsaturated fatty acids-enriched hamburgers using sterol-based oleogels. Eur J Lipid Sci Technol 121(11):1900111. https://doi.org/10.1002/EJLT.201900111 Li X, Li J (2020) The flavor of plant-based meat analogues. Cereal Foods World. https://doi.org/10.1094/CFW-65-4-0040 Chiang JH, Hardacre AK, Parker ME (2020) Effects of Maillard-reacted beef bone hydrolysate on the physicochemical properties of extruded meat alternatives. J Food Sci 85(3):567–575. https://doi.org/10.1111/1750-3841.14960 Duque-Estrada P, Kyriakopoulou K, de Groot W, van der Goot AJ, Berton-Carabin CC (2020) Oxidative stability of soy proteins: From ground soybeans to structured products. Food Chem 318:126499. https://doi.org/10.1016/J.FOODCHEM.2020.126499 He J, Liu H, Balamurugan S, Shao S (2021) Fatty acids and volatile flavor compounds in commercial plant-based burgers. J Food Sci 86(2):293–305. https://doi.org/10.1111/1750-3841.15594 Guo Z, Teng F, Huang Z, Lv B, Lv X, Babich O, Yu W, Li Y, Wang Z, Jiang L (2020) Effects of material characteristics on the structural characteristics and flavor substances retention of meat analogs. Food Hydrocolloids 105:105752. https://doi.org/10.1016/J.FOODHYD.2020.105752 Botella-Martínez C, Viuda-Martos M, Fernández-López JA, Pérez-Alvarez JA, Fernández-López J (2022) Development of plant-based burgers using gelled emulsions as fat source and beetroot juice as colorant: Effects on chemical, physicochemical, appearance and sensory characteristics. LWT 172:114193. https://doi.org/10.1016/J.LWT.2022.114193 Schreuders FKG, Dekkers BL, Bodnár I, Erni P, Boom RM, van der Goot AJ (2019) Comparing structuring potential of pea and soy protein with gluten for meat analogue preparation. J Food Eng 261:32–39. https://doi.org/10.1016/J.JFOODENG.2019.04.022 Akramzadeh N, Hosseini H, Pilevar Z, Karimian Khosroshahi N, Khosravi-Darani K, Komeyli R, Barba FJ, Pugliese A, Poojary MM, Khaneghah AM (2018) Physicochemical properties of novel non-meat sausages containing natural colorants and preservatives. J Food Process Preserv 42(9):e13660. https://doi.org/10.1111/JFPP.13660 Bolognesi VJ, Garcia CER (2018) Annatto carotenoids as additives replacers in meat products. Altern Replace Foods 17:355–384. https://doi.org/10.1016/B978-0-12-811446-9.00012-5 Arora B, Kamal S, Sharma VP (2017) Effect of binding agents on quality characteristics of mushroom based sausage analogue. J Food Process Preserv 41(5):e13134. https://doi.org/10.1111/JFPP.13134 Glorieux S, Goemaere O, Steen L, Fraeye I (2017) Phosphate reduction in emulsified meat products: impact of phosphate type and dosage on quality characteristics. Food Technol Biotechnol 55(3):390. https://doi.org/10.17113/FTB.55.03.17.5089 Nawrocka A, Szymańska-Chargot M, Miś A, Wilczewska AZ, Markiewicz KH (2017) Aggregation of gluten proteins in model dough after fibre polysaccharide addition. Food Chem 231:51–60. https://doi.org/10.1016/J.FOODCHEM.2017.03.117 Warnakulasuriya SN, Nickerson MT (2018) Review on plant protein–polysaccharide complex coacervation, and the functionality and applicability of formed complexes. J Sci Food Agric 98(15):5559–5571. https://doi.org/10.1002/JSFA.9228 Peters JPCM, Vergeldt FJ, Boom RM, van der Goot AJ (2017) Water-binding capacity of protein-rich particles and their pellets. Food Hydrocolloids 65:144–156. https://doi.org/10.1016/J.FOODHYD.2016.11.015 Zhang J, Liu L, Liu H, Yoon A, Rizvi SSH, Wang Q (2019) Changes in conformation and quality of vegetable protein during texturization process by extrusion. Crit Rev Food Sci Nutr 59(20):3267–3280. https://doi.org/10.1080/10408398.2018.1487383 Krintiras GA, Gadea Diaz J, Van Der Goot AJ, Stankiewicz AI, Stefanidis GD (2016) On the use of the Couette Cell technology for large scale production of textured soy-based meat replacers. J Food Eng 169:205–213. https://doi.org/10.1016/J.JFOODENG.2015.08.021 Dekkers BL, Nikiforidis CV, van der Goot AJ (2016) Shear-induced fibrous structure formation from a pectin/SPI blend. Innov Food Sci Emerg Technol 36:193–200. https://doi.org/10.1016/J.IFSET.2016.07.003 Dekkers BL, Emin MA, Boom RM, van der Goot AJ (2018) The phase properties of soy protein and wheat gluten in a blend for fibrous structure formation. Food Hydrocolloids 79:273–281. https://doi.org/10.1016/J.FOODHYD.2017.12.033 Chiang JH, Tay W, Ong DSM, Liebl D, Ng CP, Henry CJ (2021) Physicochemical, textural and structural characteristics of wheat gluten-soy protein composited meat analogues prepared with the mechanical elongation method. Food Struct 28:100183. https://doi.org/10.1016/J.FOOSTR.2021.100183 Saldanha do Carmo C, Knutsen SH, Malizia G, Dessev T, Geny A, Zobel H, Myhrer KS, Varela P, Sahlstrøm S (2021) Meat analogues from a faba bean concentrate can be generated by high moisture extrusion. Future Foods 3:100014. https://doi.org/10.1016/J.FUFO.2021.100014 Kamani MH, Meera MS, Bhaskar N, Modi VK (2019) Partial and total replacement of meat by plant-based proteins in chicken sausage: evaluation of mechanical, physico-chemical and sensory characteristics. J Food Sci Technol 56(5):2660–2669. https://doi.org/10.1007/S13197-019-03754-1 Bedin E, Torricelli C, Gigliano S, De Leo R, Pulvirenti A (2018) Vegan foods: mimic meat products in the Italian market. Int J Gastron Food Sci 13:1–9. https://doi.org/10.1016/J.IJGFS.2018.04.003 Karefyllakis D, van der Goot AJ, Nikiforidis CV (2019) Multicomponent emulsifiers from sunflower seeds. Curr Opin Food Sci 29:35–41. https://doi.org/10.1016/j.cofs.2019.07.005 Devnani B, Ong L, Kentish S, Gras S (2020) Heat induced denaturation, aggregation and gelation of almond proteins in skim and full fat almond milk. Food Chem 325:126901. https://doi.org/10.1016/J.FOODCHEM.2020.126901 Zheng L, Teng F, Wang N, Zhang XN, Regenstein JM, Liu JS, Li Y, Wang ZJ (2019) Addition of salt ions before spraying improves heatand cold-induced gel properties of Soy Protein Isolate (SPI). Appl Sci. https://doi.org/10.3390/APP9061076 Pietrasik Z, Sigvaldson M, Soladoye OP, Gaudette NJ (2020) Utilization of pea starch and fibre fractions for replacement of wheat crumb in beef burgers. Meat Sci. https://doi.org/10.1016/j.meatsci.2019.107974 Rios-Mera JD, Saldaña E, Cruzado-Bravo MLM, Martins MM, Patinho I, Selani MM, Valentin D, Contreras-Castillo CJ (2020) Impact of the content and size of NaCl on dynamic sensory profile and instrumental texture of beef burgers. Meat Sci. https://doi.org/10.1016/j.meatsci.2019.107992 Cornet SHV, Snel SJE, Schreuders FKG, van der Sman RGM, Beyrer M, van der Goot AJ (2022) Thermo-mechanical processing of plant proteins using shear cell and high-moisture extrusion cooking. Crit Rev Food Sci Nutr 62(12):3264–3280. https://doi.org/10.1080/10408398.2020.1864618 Giezen FE, Jansen WWJT, Willemsen JHA (2013) Method of Making Structured Protein Composition. Available online: https://patents.google.com/patent/WO2012158023A1/en (accessed on 14 December 2020). Abdel-Naeem HHS, Mohamed HMH (2016) Improving the physico-chemical and sensory characteristics of camel meat burger patties using ginger extract and papain. Meat Sci 118:52–60. https://doi.org/10.1016/J.MEATSCI.2016.03.021 Lorenzo JM, Pateiro M, Domínguez R, Barba FJ, Putnik P, Kovačević DB, Shpigelman A, Granato D, Franco D (2018) Berries extracts as natural antioxidants in meat products: A review. Food Res Int 106:1095–1104. https://doi.org/10.1016/J.FOODRES.2017.12.005 Naveena BM, Vaithiyanathan S, Muthukumar M, Sen AR, Kumar YP, Kiran M, Shaju VA, Chandran KR (2013) Relationship between the solubility, dosage and antioxidant capacity of carnosic acid in raw and cooked ground buffalo meat patties and chicken patties. Meat Sci 95(2):195–202. https://doi.org/10.1016/j.meatsci.2013.04.043 Hong X, Zhao Q, Liu Y, Li J (2023) Recent advances on food-grade water-in-oil emulsions: Instability mechanism, fabrication, characterization, application, and research trends. Crit Rev Food Sci Nutr 63(10):1406–1436. https://doi.org/10.1080/10408398.2021.1964063 Bahmanyar F, Hosseini SM, Mirmoghtadaie L, Shojaee-Aliabadi S (2021) Effects of replacing soy protein and bread crumb with quinoa and buckwheat flour in functional beef burger formulation. Meat Sci 172:108305. https://doi.org/10.1016/J.MEATSCI.2020.108305 Rabadán A, Álvarez-Ortí M, Martínez E, Pardo-Giménez A, Zied DC, Pardo JE (2021) Effect of replacing traditional ingredients for oils and flours from nuts and seeds on the characteristics and consumer preferences of lamb meat burgers. LWT. https://doi.org/10.1016/j.lwt.2020.110307 Barros JC, Munekata PES, de Carvalho FAL, Domínguez R, Trindade MA, Pateiro M, Lorenzo JM (2021) Healthy beef burgers: Effect of animal fat replacement by algal and wheat germ oil emulsions. Meat Sci 173:108396. https://doi.org/10.1016/J.MEATSCI.2020.108396 Kumar M, Tomar M, Potkule J, Reetu Punia S, Dhakane-Lad J, Singh S, Dhumal S, Chandra Pradhan P, Bhushan B, Anitha T, Alajil O, Alhariri A, Amarowicz R, Kennedy JF (2022) Functional characterization of plant-based protein to determine its quality for food applications. Food Hydrocolloids. https://doi.org/10.1016/j.foodhyd.2021.106986 Zhang J, Chen Q, Kaplan DL, Wang Q (2022) High-moisture extruded protein fiber formation toward plant-based meat substitutes applications: Science, technology, and prospect. Trends Food Sci Technol 128:202–216. https://doi.org/10.1016/j.tifs.2022.08.008 Webb D, Li Y, Alavi S (2023) Chemical and physicochemical features of common plant proteins and their extrudates for use in plant-based meat. Trends Food Sci Technol 131:129–138. https://doi.org/10.1016/j.tifs.2022.11.006 Lee HW, Lu Y, Zhang Y, Fu C, Huang D (2021) Physicochemical and functional properties of red lentil protein isolates from three origins at different pH. Food Chem. https://doi.org/10.1016/j.foodchem.2021.129749 Alonso-Miravalles L, Zannini E, Bez J, Arendt EK, O’Mahony JA (2020) Physical and flow properties of pseudocereal-based protein-rich ingredient powders. J Food Eng 281:109973. https://doi.org/10.1016/J.JFOODENG.2020.109973 Özdemir EE, Görgüç A, Gençdağ E, Yılmaz FM (2022) Physicochemical, functional and emulsifying properties of plant protein powder from industrial sesame processing waste as affected by spray and freeze drying. LWT. https://doi.org/10.1016/j.lwt.2021.112646 Razavizadeh S, Alencikiene G, Vaiciulyte-Funk L, Ertbjerg P, Salaseviciene A (2022) Utilization of fermented and enzymatically hydrolyzed soy press cake as ingredient for meat analogues. LWT. https://doi.org/10.1016/j.lwt.2022.113736 Wang JS, Wang AB, Zang XP, Tan L, Xu BY, Chen HH, ** ZQ, Ma WH (2019) Physicochemical, functional and emulsion properties of edible protein from avocado (Persea americana Mill.) oil processing by-products. Food Chem 288:146–153. https://doi.org/10.1016/j.foodchem.2019.02.098 Li C, Yang J, Yao L, Qin F, Hou G, Chen B, ** L, Deng J, Shen Y (2020) Characterisation, physicochemical and functional properties of protein isolates from Amygdalus pedunculata Pall seeds. Food Chem 311:125888. https://doi.org/10.1016/j.foodchem.2019.125888 Li M, Wen X, Peng Y, Wang Y, Wang K, Ni Y (2018) Functional properties of protein isolates from bell pepper (Capsicum annuum L. var. annuum) seeds. LWT 97:802–810. https://doi.org/10.1016/j.lwt.2018.07.069 Liu FF, Li YQ, Wang CY, Zhao XZ, Liang Y, He JX, Mo HZ (2021) Impact of pH on the physicochemical and rheological properties of mung bean (Vigna radiata L.) protein. Process Biochem 111:274–284. https://doi.org/10.1016/j.procbio.2021.10.008 Tanger C, Müller M, Andlinger D, Kulozik U (2022) Influence of pH and ionic strength on the thermal gelation behaviour of pea protein. Food Hydrocolloids 123:106903. https://doi.org/10.1016/j.foodhyd.2021.106903 Cattan Y, Patil D, Vaknin Y, Rytwo G, Lakemond C, Benjamin O (2022) Characterization of Moringa oleifera leaf and seed protein extract functionality in emulsion model system. Innov Food Sci Emerg Technol 75:102903. https://doi.org/10.1016/J.IFSET.2021.102903 Li X, Ye C, Tian Y, Pan S, Wang L (2018) Effect of ohmic heating on fundamental properties of protein in soybean milk. J Food Process Eng 41(3):12660. https://doi.org/10.1111/JFPE.12660 Mohan N, Mellem JJ (2020) Functional properties of the protein isolates of hyacinth bean [Lablab purpureus (L.) Sweet]: An effect of the used procedures. LWT 129:109572. https://doi.org/10.1016/j.lwt.2020.109572 Zhang A, Wang L, Song T, Yu H, Wang X, Zhaohuai X (2022) Effects of high pressure homogenization on the structural and emulsifying properties of a vegetable protein: Cyperus esculentus L. LWT. https://doi.org/10.1016/j.lwt.2021.112542 Khoder RM, Yin T, Liu R, **ong S, You J, Hu Y, Huang Q (2020) Effects of nano fish bone on gelling properties of tofu gel coagulated by citric acid. Food Chem. https://doi.org/10.1016/j.foodchem.2020.127401 Omura MH, de Oliveira APH, de Soares L et al (2021) Effects of protein concentration during ultrasonic processing on physicochemical properties and techno-functionality of plant food proteins. Food Hydrocolloids. https://doi.org/10.1016/j.foodhyd.2020.106457 Mir NA, Riar CS, Singh S (2023) Effect of film forming solution pH on antibacterial, antioxidant and structural characteristics of edible films from modified quinoa protein. Food Hydrocolloids. https://doi.org/10.1016/j.foodhyd.2022.108190 Fathi N, Almasi H, Pirouzifard MK (2018) Effect of ultraviolet radiation on morphological and physicochemical properties of sesame protein isolate based edible films. Food Hydrocolloids 85:136–143. https://doi.org/10.1016/J.FOODHYD.2018.07.018 Moghadam M, Salami M, Mohammadian M, Khodadadi M, Emam-Djomeh Z (2020) Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocolloids. https://doi.org/10.1016/j.foodhyd.2020.105735 Jia Y, Yan X, Huang Y, Zhu H, Qi B, Li Y (2022) Different interactions driving the binding of soy proteins (7S/11S) and flavonoids (quercetin/rutin): Alterations in the conformational and functional properties of soy proteins. Food Chem. https://doi.org/10.1016/j.foodchem.2022.133685 Temthawee W, Panya A, Cadwallader KR, Suppavorasatit I (2020) Flavor binding property of coconut protein affected by protein-glutaminase: Vanillin-coconut protein model. LWT. https://doi.org/10.1016/j.lwt.2020.109676 Dou W, Zhang X, Zhao Y, Zhang Y, Jiang L, Sui X (2022) High moisture extrusion cooking on soy proteins: Importance influence of gums on promoting the fiber formation. Food Res Int 156:111189. https://doi.org/10.1016/J.FOODRES.2022.111189 Chen Q, Zhang J, Zhang Y, Wang Q (2022) Effect of fatty acid saturation degree on the rheological properties of pea protein and its high-moisture extruded product quality. Food Chem. https://doi.org/10.1016/j.foodchem.2022.133139 Zhang J, Liu L, Jiang Y, Faisal S, Wang Q (2020) A new insight into the high-moisture extrusion process of peanut protein: From the aspect of the orders and amount of energy input. J Food Eng. https://doi.org/10.1016/j.jfoodeng.2019.07.015 Hu Y, Yang S, Zhang Y, Shi L, Ren Z, Hao G, Weng W (2022) Effects of microfluidization cycles on physicochemical properties of soy protein isolate-soy oil emulsion films. Food Hydrocolloids 130:107684. https://doi.org/10.1016/j.foodhyd.2022.107684 Mozafarpour R, Koocheki A, Nicolai T (2022) Modification of grass pea protein isolate (Lathyrus sativus L.) using high intensity ultrasound treatment: Structure and functional properties. Food Res Int 158:111520. https://doi.org/10.1016/j.foodres.2022.111520 Wang Y, Liu J, Wei F, Liu X, Yi C, Zhang Y (2019) Improvement of the nutritional value, sensory properties and bioavailability of rapeseed meal fermented with mixed microorganisms. LWT 112:108238. https://doi.org/10.1016/J.LWT.2019.06.005 Day L, Cakebread JA, Loveday SM (2022) Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci Technol 119:428–442. https://doi.org/10.1016/J.TIFS.2021.12.020 Zhu R, Liu X, Li X, Zeng K, Yi L (2021) Transformation of Inferior Tomato into Preservative: Fermentation by Multi-Bacteriocin Producing Lactobacillus paracasei WX322. Foods 10(6):1278. https://doi.org/10.3390/FOODS10061278 Liu Z, Zheng Z, Zhu G, Luo S, Zhang D, Liu F, Shen Y (2021) Modification of the structural and functional properties of wheat gluten protein using a planetary ball mill. Food Chem. https://doi.org/10.1016/j.foodchem.2021.130251 Yang HJ, Lee JH, Won M, Song KB (2016) Antioxidant activities of distiller dried grains with solubles as protein films containing tea extracts and their application in the packaging of pork meat. Food Chem 196:174–179. https://doi.org/10.1016/j.foodchem.2015.09.020 Zhang J, Liu L, Jiang Y, Shah F, Xu Y, Wang Q (2020) High-moisture extrusion of peanut protein-/carrageenan/sodium alginate/wheat starch mixtures: effect of different exogenous polysaccharides on the process forming a fibrous structure. Food Hydrocolloids 99:105311. https://doi.org/10.1016/J.FOODHYD.2019.105311 Chen X, Zhao H, Wang H, Xu P, Chen M, Xu Z, Wen L, Cui B, Yu B, Zhao H, Jiao Y, Cheng Y (2022) Preparation of high-solubility rice protein using an ultrasound-assisted glycation reaction. Food Res Int 161:111737. https://doi.org/10.1016/J.FOODRES.2022.111737 Nikbakht Nasrabadi M, Sedaghat Doost A, Mezzenga R (2021) Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocolloids. https://doi.org/10.1016/j.foodhyd.2021.106789 Liu D, Zhang L, Wang Y, Li Z, Wang Z, Han J (2020) Effect of high hydrostatic pressure on solubility and conformation changes of soybean protein isolate glycated with flaxseed gum. Food Chem. https://doi.org/10.1016/j.foodchem.2020.127530 Hall AE, Moraru CI (2021) Structure and function of pea, lentil and faba bean proteins treated by high pressure processing and heat treatment. LWT. https://doi.org/10.1016/j.lwt.2021.112349 Hu C, **ong Z, **ong H, Chen L, Zhang Z (2021) Effects of dynamic high-pressure microfluidization treatment on the functional and structural properties of potato protein isolate and its complex with chitosan. Food Res Int. https://doi.org/10.1016/j.foodres.2020.109868 Peyrano F, Speroni F, Avanza MV (2016) Physicochemical and functional properties of cowpea protein isolates treated with temperature or high hydrostatic pressure. Innov Food Sci Emerg Technol 33:38–46. https://doi.org/10.1016/j.ifset.2015.10.014 Hu S, Zhu S, Luo J, Ouyang L, Feng J, Zhou J (2022) Effect of extrusion on physicochemical properties and antioxidant potential of protein isolate derived from Baijiu vinasse. Food Chem. https://doi.org/10.1016/j.foodchem.2022.132527 Gao K, Rao J, Chen B (2022) Unraveling the mechanism by which high intensity ultrasound improves the solubility of commercial pea protein isolates. Food Hydrocolloids 131:107823. https://doi.org/10.1016/J.FOODHYD.2022.107823 Yang ZH, Zhou HM, Bai YP (2021) Effects of vacuum ultrasonic treatment on the texture of vegetarian meatloaves made from textured wheat protein. Food Chem. https://doi.org/10.1016/j.foodchem.2021.130058 Guo J, He Z, Wu S, Zeng M, Chen J (2019) Binding of aroma compounds with soy protein isolate in aqueous model: Effect of preheat treatment of soy protein isolate. Food Chem 290:16–23. https://doi.org/10.1016/J.FOODCHEM.2019.03.126 Woo Choi H, Ryoo C, Hahn J, Choi YJ (2023) Development of a novel technology for high-moisture textured soy protein using a vacuum packaging and pressurized heat (vacuum-autoclaving) treatment. Food Chem 399:133887. https://doi.org/10.1016/J.FOODCHEM.2022.133887 Ertugrul U, Namli S, Tas O, Kocadagli T, Gokmen V, Sumnu SG, Oztop MH (2021) Pea protein properties are altered following glycation by microwave heating. LWT 150:111939. https://doi.org/10.1016/J.LWT.2021.111939 Zhang S, Huang W, Roopesh MS, Chen L (2022) Pre-treatment by combining atmospheric cold plasma and pH-shifting to prepare pea protein concentrate powders with improved gelling properties. Food Res Int. https://doi.org/10.1016/j.foodres.2022.111028 Qu Z, Chen G, Wang J, **e X, Chen Y (2023) Preparation, structure evaluation, and improvement in foaming characteristics of fibrotic pea protein isolate by cold plasma synergistic organic acid treatment. Food Hydrocolloids. https://doi.org/10.1016/j.foodhyd.2022.108057 Acosta-Domínguez L, Cocotle-Ronzón Y, Alamilla-Beltrán L, Hernandez-Martinez E (2021) Effect of a cryogenic treatment in the microstructure, functional and flow properties of soy protein isolate. Food Hydrocolloids 119:106871. https://doi.org/10.1016/J.FOODHYD.2021.106871 Burger TG, Singh I, Mayfield C, Baumert JL, Zhang Y (2022) The impact of spray drying conditions on the physicochemical and emulsification properties of pea protein isolate. LWT 153:112495. https://doi.org/10.1016/J.LWT.2021.112495 Liu G, Hu M, Du X, Liao Y, Yan S, Zhang S, Qi B, Li Y (2022) Correlating structure and emulsification of soybean protein isolate: Synergism between low-pH-shifting treatment and ultrasonication improves emulsifying properties. Colloids Surf, A. https://doi.org/10.1016/j.colsurfa.2022.128963 **ong D, Xu Q, Tian L, Bai J, Yang L, Jia J, Liu X, Yang X, Duan X (2023) Mechanism of improving solubility and emulsifying properties of wheat gluten protein by pH cycling treatment and its application in powder oils. Food Hydrocolloids 135:108132. https://doi.org/10.1016/j.foodhyd.2022.108132 Sánchez-Reséndiz A, Rodríguez-Barrientos S, Rodríguez-Rodríguez J, Barba-Dávila B, Serna-Saldívar SO, Chuck-Hernández C (2018) Phosphoesterification of soybean and peanut proteins with sodium trimetaphosphate (STMP): Changes in structure to improve functionality for food applications. Food Chem 260:299–305. https://doi.org/10.1016/j.foodchem.2018.04.009 Shen Y, Li Y (2021) Acylation modification and/or guar gum conjugation enhanced functional properties of pea protein isolate. Food Hydrocolloids. https://doi.org/10.1016/j.foodhyd.2021.106686 Meenmanee S, Rattananukrom A, Thaiphanit S, Suppavorasatit I (2022) Improvement of solubility, foaming, and emulsification properties of coconut (Cocos nucifera L.) protein by non-enzymatic deamidation. LWT 153:112493. https://doi.org/10.1016/j.lwt.2021.112493 Nesterenko A, Alric I, Silvestre F, Durrieu V (2014) Comparative study of encapsulation of vitamins with native and modified soy protein. Food Hydrocolloids 38:172–179. https://doi.org/10.1016/j.foodhyd.2013.12.011 Sun Q, Ma ZF, Zhang H, Ma S, Kong L (2019) Structural characteristics and functional properties of walnut glutelin as hydrolyzed: effect of enzymatic modification. Int J Food Prop 22(1):265–279. https://doi.org/10.1080/10942912.2019.1579738 Sorde KL, Ananthanarayan L (2019) Effect of transglutaminase treatment on properties of coconut protein-guar gum composite film. LWT. https://doi.org/10.1016/j.lwt.2019.108422 Pöri P, Nisov A, Nordlund E (2022) Enzymatic modification of oat protein concentrate with trans-and protein-glutaminase for increased fibrous structure formation during high-moisture extrusion processing. LWT. https://doi.org/10.1016/j.lwt.2021.113035 Meinlschmidt P, Ueberham E, Lehmann J, Schweiggert-Weisz U, Eisner P (2016) Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate. Food Chem 205:229–238. https://doi.org/10.1016/j.foodchem.2016.03.016 Dong S, Wang JM, Cheng LM, Lu YL, Li SH, Chen Y (2017) Behavior of Zein in aqueous ethanol under atmospheric pressure cold plasma treatment. J Agric Food Chem 65(34):7352–7360 Zhang S, Huang W, Feizollahi E, Roopesh MS, Chen L (2021) Improvement of pea protein gelation at reduced temperature by atmospheric cold plasma and the gelling mechanism study. Innov Food Sci Emerg Technol. https://doi.org/10.1016/j.ifset.2020.102567 Liu B, Wang H, Hu T, Zhang P, Zhang Z, Pan S, Hu H (2017) Ball-milling changed the physicochemical properties of SPI and its cold-set gels. J Food Eng 195:158–165. https://doi.org/10.1016/j.jfoodeng.2016.10.006 Liu J, Li P, Jiang Z, Yang R, Zhang W (2019) Characterisation of peanut protein concentrates from industrial aqueous extraction processing prepared by spray and freeze drying methods. Int J Food Sci Technol 54(5):1597–1608. https://doi.org/10.1111/IJFS.14028 Abd Rahim FN, Ibadullah WZW, Saari N, Brishti FH, Mustapha NA, Ahmad N, Arulrajah B (2023) The effect of alkaline extraction and drying techniques on the physicochemical, structural properties and functionality of rice bran protein concentrates. Int J Biol Macromol 242:124908 Zhao Q, **ong H, Selomulya C, Chen XD, Huang S, Ruan X, Zhou Q, Sun W (2013) Effects of spray drying and freeze drying on the properties of protein isolate from rice dreg protein. Food Bioprocess Technol 6(7):1759–1769. https://doi.org/10.1007/S11947-012-0844-3 Nissen SH, Schmidt JM, Gregersen S, Hammershøj M, Møller AH, Danielsen M, Stødkilde L, Nebel C, Dalsgaard TK (2021) Increased solubility and functional properties of precipitated Alfalfa protein concentrate subjected to pH shift processes. Food Hydrocolloids 119:106874. https://doi.org/10.1016/j.foodhyd.2021.106874 Open Access funding enabled and organized by Projekt DEAL. The open access publishing fee is covered under the agreement between the DEAL Consortium and Springer and Springer Nature upon acceptance due to Shahida Anusha Siddiqui being affiliated with the Technical University of Munich. Shahida Anusha Siddiqui—Conceptualization, Methodology, Writing—Original Draft, Writing—Review and Editing, Validation, Formal Analysis, Investigation, Software, Data Curation, Visualization, Resources, Project administration, Funding Acquisition, Supervision. Ibrahim Khalifa—Writing—Original Draft, Resources, Visualization. Tao Yin—Writing—Original Draft, Visualization. Mohamed K. Morsy—Writing—Original Draft, Visualization. Ramy M. Khoder—Writing—Original Draft, Visualization. Molla Salauddin—Writing—Original Draft, Visualization. Wasiya Farzana—Writing—Original Draft, Visualization. Sonu Sharma—Writing—Review and Editing, Visualization, Validation. Nauman Khalid—Conceptualization, Review and Editing, Supervision. The authors declare no conflict of interest. This article does not include any studies including human participants performed by any of the authors. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Siddiqui, S.A., Khalifa, I., Yin, T. et al. Valorization of plant proteins for meat analogues design—a comprehensive review.
Eur Food Res Technol (2024). https://doi.org/10.1007/s00217-024-04565-1 Received: Revised: Accepted: Published: DOI: https://doi.org/10.1007/s00217-024-04565-1Data availability
References
Funding
Author information
Authors and Affiliations
Contributions
Corresponding author
Ethics declarations
Conflict of interest
Compliance with ethics requirements
Additional information
Publisher's Note
Rights and permissions
About this article
Cite this article
Keywords