Abstract
Long COVID is an emerging problem in the current health care scenario. It is a syndrome with common symptoms of shortness of breath, fatigue, cognitive dysfunction, and other conditions that have a high impact on daily life. They are fluctuating or relapsing states that occur in patients with a history of SARS-CoV-2 infection for at least 2 months. They are usually conditions that at 3 months after onset cannot be explained by an alternative diagnosis. Currently very little is known about this syndrome. A thorough review of the literature highlights that the cause is attributable to deposits of tau protein. Massive phosphorylation of tau protein in response to SARS-CoV-2 infection occurred in brain samples from autopsies of people previously affected with COVID-19. The neurological disorders resulting from this clinical condition are termed tauopathies and can give different pathological symptoms depending on the involved anatomical region of the brain. Peripheral small-fiber neuropathies are also evident among patients with Long COVID leading to fatigue, which is the main symptom of this syndrome. Certainly more research studies could confirm the association between tau protein and Long COVID by defining the main role of tau protein as a biomarker for the diagnosis of this syndrome that is widespread in the post-pandemic period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
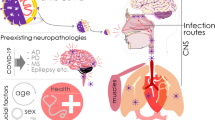
COVID-19 (coronavirus disease 2019) has changed the lives and customs of the world’s population. Recent data show that it has affected nearly 600 million people causing more than 6 million deaths worldwide. Major studies have focused on resolving the acute symptomatology produced by COVID-19, but just past the emergency period, health care is turning its attention to how to calm the long-term impact of this disease that has affected a substantial number of people with various symptoms. Complications and long-term adverse impacts affect various organs and systems and vary depending on the severity of the initial pathology as well as pre-existing risk factors (**e Y et al. 2022). Long COVID is recognized as a new medical syndrome with a broad clinical spectrum recognized in patients affected by the virus. The World Health Organization (WHO) has described this disease with so many symptoms present in various systems and organs and ranging from systemic symptoms of shortness of breath, fatigue, neurological dysfunction, and others that last for more than 2 months with a fluctuating course over time and that cannot be explained by a different diagnosis, regardless of the severity of the initial pathology or hospitalization status (WHO, 2022). Many studies in the literature recognize this as a syndrome characterized by a constellation of symptoms with a duration of more than 28 days. It is that condition of persistence of signs and symptoms that continue or develop after acute SARS-CoV-2 (severe acute syndrome coronavirus-2) infection. If symptoms continue beyond 4 weeks after infection to 12 weeks, it is called symptomatic persistent COVID-19 disease; if symptoms continue for more than 12 weeks and cannot be explained by any other condition, it is called post-COVID-19 syndrome. Long COVID includes both of these conditions (CDC, 2022). Those most affected by Long COVID are women, those of advanced age, those who are obese or overweight, and those who have been hospitalized for COVID-19. In the latter case, there is an apparent correlation with the number of pre-existing chronic conditions and the severity of interventions required (e.g., intensive care unit admission). Susceptibility seems, in addition, to increase with the number of symptoms in the acute phase (particularly with dyspnea) but the association with their severity is not yet clearly defined. Symptomatology also experienced in the pediatric population is also not uncommon (Raveendran AV et al. 2021; Castanares-Zapatero D et al. 2022; Harari S et al. 2022). Nearly 6% of children who arrive at the emergency department with COVID-19 report symptoms of Long COVID within the next 90 days. A study shows that manifesting four or more symptoms on arrival at the emergency department and being 14 years of age and older are all factors associated with Long COVID. The research included 1884 children with COVID-19 who underwent a 90-day follow-up. Long COVID was evidenced in about 10% of children admitted and 5% of children discharged from emergency departments. The most frequently reported symptoms of pediatric patients were weakness, cough, difficulty breathing, or shortness of breath. COVID-19 was observed to be associated with persistent symptoms in some children after 3 months. Appropriate management and follow-up are critical and crucial, especially in children at increased risk of Long COVID (Funk AL et al. 2022).
This study aims to demonstrate how Long COVID phenomena can be caused by the development of tau protein causing a variety of disorders at various levels in tissues and organs. This review intends to summarize the existing literature about the correlation of tauopathies with infection due to COVID-19.
The pathogenesis of Long COVID
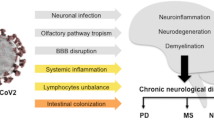
“Long COVID” syndrome is a multi-organ disorder, which occurs in a percentage of individuals infected and cured by COVID-19. Long COVID occurs in 30–50% of COVID-19 patients (Nalbandian A et al. 2021). Long COVID syndrome is characterized by the manifestation of a broad spectrum of clinical, neurological, cardiovascular, metabolic, renal, and respiratory symptoms, and which may exacerbate underlying pathologies already present in the aforementioned systems. To date, there are no established and universally accepted diagnostic criteria for the diagnosis of “Long COVID.” Clinical evidence indicates that the most commonly encountered neurological manifestations of “Long COVID” include panic attacks, anxiety, fatigue, sleep, and mood disorders. The pathophysiological mechanisms underlying Long COVID neurological symptoms are still being discussed and researched, it is believed that altered neuroinflammatory and redox balance dysregulation processes resulting in oxidative stress may play an important role (Stefanou MI et al. 2022). Neurological damage can be caused by direct entry of SARS-CoV-2 into the brain, causing damage to cerebral vessels and brain cells, triggering neuroimmune and neuroinflammatory responses. The brain damage then may manifest in the COVID-19 infection phase or the Long COVID phase (Lee MH et al. 2021; Magro CM et al. 2021). Long COVID syndrome is also characterized by clinical manifestations in the cardiovascular system. In particular, several pathophysiological mechanisms have been considered to underlie cardiovascular damage, such as dysregulation of the renin-angiotensin system, direct cardiac inflammation caused by ACE-2-mediated endocellular viral penetration into cardiocytes. An interesting epidemiological study of 47,780 showed that a diagnosis of COVID-19 correlated with a 3-fold increased risk of major adverse CV events up to 4 months after diagnosis (compared with non-hospitalized controls) (Ayoubkhani D et al., 2022). Underlying the organ damage that can occur during COVID-19 infection with symptoms that can also occur in Long COVID, there may be endotheliitis. Specifically, the vessel endothelium regulates numerous functions such as permeability, cell growth and migration, platelet function, and inflammation. Based on this, it is normal to think that inflammation of the endothelium may be responsible for COVID-19 tissue and organ damage (Varga et al. Lancet 2020; Calabretta et al. Br J Haemtol; 2021; Hattori et al. Biochem Pharmacol 2022). Neuroinflammation in particular, in COVID-19 patients without neurodegeneration, can cause p-tau deposition, degenerating neurons, microglia activation, and increased cytokines, in some cases with Aβ plaques and p-tau pretangles, generating an Alzheimer-like syndrome. Probably neuroinflammation underlies the formation of Aβ plaques and p-tau pretangles. This leads us to ask, could the new anti-Alzheimer monoclonal antibodies be effective in Long COVID neurological symptoms? And also is there a difference in Long COVID symptoms caused by the various SARS-CoV-2 variants? In this regard, the evidence is still limited. In addition to this, it should be noted that Long COVID symptoms may intensify a pre-existing condition such as diabetes, further complicating the clinical scenario (**e Y & Al-Aly Z. 2022). In addition, we emphasize that collaborative research initiatives are urgently needed to accelerate the development of preventive and therapeutic strategies for the neurological sequelae of “Long COVID.”
Clinical symptoms
Many studies and projects have been conducted to delineate the clinical picture of Long COVID and its prevalence. Many people reported experiencing post-COVID-19 symptoms from 28 to 84 days. These symptoms occur in various tissues and organs: 97.70% of people surveyed said they experienced fatigue, 91.20% headache, 72% loss of sense of smell, 70.80% shortness of breath, 68.20% persistent cough, 60% chest pain, 51% diarrhea, and 30% delirium at the end of 28 days (Sudre et al. 2021). The clinical manifestations of Long COVID are heterogeneous, and a person with this condition may present with one or more general symptoms and/or symptoms affecting specific organs and systems. General symptoms include persistent fatigue/asthenia, excessive tiredness, fever, muscle weakness, widespread pain, muscle and joint pain, worsening of perceived health status, anorexia, and reduced appetite. Specific symptoms may be (Davis HE et al. Liu X et al. 2020; Kucirkaet al. 20202021; Arevalo-Rodriguez et al. 2020) of various natures. Neurological and psychological/psychiatric symptoms such as headache may present as a new symptom or as a worsening of preexisting symptoms. Attacks may be more frequent or pain may last longer than usual. Other neurological afflictions may include cognitive impairment, which manifests as difficulty in concentration and attention, memory problems, and difficulty in executive functions (especially in the elderly and/or those with existing cognitive deficits); peripheral neuropathy and dysautonomia, which is the malfunction of the autonomic or vegetative nervous system that controls involuntary bodily functions; poor and non-restorative sleep, chronic malaise, mood depression (feeling sad, irritable and impatient toward others, losing interest in activities previously enjoyed, having difficulty making decisions, having negative thoughts), anxiety, delirium, and psychosis. Compulsory social distance has undoubtedly exacerbated these disorders. Some patients may present with symptoms related to post-traumatic stress disorder.
Alterations in the sense of smell, taste, and hearing can lead to olfactory disturbances, such as parosmia or hyposmia, swallowing and taste dysfunction (food may taste salty, bland, metallic, or sweet), otalgia, tinnitus, dysphonia, and sore throat (complaints such as pain, a wheezing cough, a sensation of mucus stagnation in the throat, and a feeling of needing to clear the throat). In respiratory persistent cough, dyspnea and diminished capability of the rib cage to expand. Gastrointestinal symptoms may lead to loss of appetite, nausea, vomiting, abdominal pain, diarrhea, dyspepsia, gastroesophageal reflux, belching, and abdominal distension. Several studies are currently evaluating the long-term gastrointestinal consequences of COVID-19, including post-infectious irritable bowel syndrome. Other very common symptoms are muscle aches, joint pain, fever, and fatigue; the most common skin manifestation is erythema pernio (vulgarly called “chilblain”) with papulo-squamous (i.e., characterized by redness, swelling, and scaly blisters) eruptions and rashes. Other consequences may be alopecia, which usually lasts about 6 months. In relation to immune-mediated diseases with dermatologic manifestations, cases of flare-ups of psoriasis and latent forms have been described; thyroiditis, new-onset diabetic ketoacidosis (in the absence of a previous diagnosis of diabetes mellitus). Cardiovascular and hematologic symptoms may occur such as chest pain and tightness, palpitations and tachycardia at the slightest exertion, change in blood pressure, and arrhythmias; especially in the post-acute phase of COVID-19, the development of venous thromboembolic disease has been observed. Consequences on the reproductive system include sexual dysfunction, irregular menstrual cycles, heavy menstrual cycles, and testicular pain. The clinical spectrum of Long COVID is shown in Table 1 with a description of symptoms commonly manifested in the various organ systems of the body.
Tauopathies and COVID-19
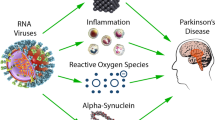
SARS-CoV-2 virus is descended from the large family of Coronaviridae and has started its propagation around the world as of 2019 with whole genome sequencing had in January 2020 (Vitiello A et al. 2022; Ferrara F, 2020; Hu B et al. 2021). SARS-CoV-2 penetrates inside the cell via the ACE-2 receptor, and WHO has defined this disease as COVID-19 (Ferrara F & Vitiello A, 2021). Blood vessels, cardiac pericytes, respiratory system cells, cerebral cortex, kidneys, and the hypothalamus in the brainstem have these receptors. Thus, there is a multiorgan phase where SARS-CoV-2 through these receptors produces acute symptomatology with inflammatory and oxidative stress and following dysregulation of immunoinflammatory pathways. Many inflammatory markers have been found in the brain from COVID-19 patients causing extensive neurological symptomatology (Ferrara F et al. 2020). Hyposmia and hypogeusia are two clinical consequences that frequently occur in COVID-19 patients. Hyposmia is a clinical condition that occurs early in Alzheimer’s disease (AD) with elevated expression of tau protein deposits. Recently, it has been shown how (Vitiello A & Ferrara F, 2021a) oxidative stress and inflammatory pathways triggered by the virus can cause ryanodine receptor (RYR) leakage with dysregulation of intracellular calcium levels, activation of calcium-dependent enzymes, and massive hyperphosphorylation of tau protein. COVID-19 leads to 3.8-fold increased oxidative stress with increased glutathione disulfide (GSSG)/glutathione (GSH) expression in brain cortex. Also increased are levels of circulating kynurenine, which is also a marker of inflammation with increased action of protein kinase A (PKA) and calmodulin-dependent protein kinase II (CaMKII) binding. Hence, we come to determine hyperphosphorylation of tau protein on multiple residues thus demonstrating that we are dealing with tau pathology.
With the full activity of the inflammatory/oxidative pathway, there is elevated production of transforming growth factor-β (TGF-β) leading to increased NADPH oxidase 2 (NOX2) activity and decreased calstabin 2 with activation of low cytosolic Ca2+ channels and pathological Ca2+ leakage into the endoplasmic reticulum. Altered calcium regulation thus causes an increase in Ca2+/cAMP/PKA signaling resulting in an increased state of neuronal activation and phosphorylation of tau demonstrated on human brain models with many cascading events that can lead to neuronal cell death for which local inflammatory pathways are responsible. Oxidative stress in neurons leads to elevated phosphorylation of p231T and its mislocalization in the neuron (Ramani et al. 2020). The tau protein physiologically is a protector against damage caused to DNA by possible peroxidation, regulating the proper conformation of DNA by binding directly to it (Hua Q et al. 2003; Vitiello A et al. 2021). In contrast, pathological tau promotes filamentous actin production causing oxidative stress with disruption of the nucleoskeleton leading to apoptotic cell death (Fulga T A et al. 2007; Frost B et al. 2016, 2014; Arendt T et al. 2010). SARS-CoV-2 infection leads to high inflammatory activation with dysregulation of immune pathways (Tay et al. 2020; Vardhana S A & Wolchok 2020). In COVID-19 patients, there are elevated levels of pro-inflammatory cytokines such as IL-1, IL-2, IL-4, IL-6, IL-7, IL-10, IL-13, IL-17, G-CSF, GM-CSF, M-CSF, and IP-10. NOD like receptor protein 3 (NLRP-3) and an inflammasome that stimulates with multi-organ effect through dysregulated inflammatory and immunological pathways caused by SARS-CoV-2. NLRP-3 also influences the phosphorylation of tau protein and therefore plays a crucial role in tauopathies (Costela-Ruiz V J et al. 2020) precisely because the inflammasome is a component of innate immunity by regulating the inflammatory-immunological response in infectious states and stresses (Ising C et al. 2019; Mangan et al. 2018).
The tau protein was discovered in 1975 by Weingarten and his team noting that together with microtubules it plays a key role in the stability of the cell and for this it is also called “microtubule-associated tau protein.” Thus, “tauopathies” are all those neurodegenerative diseases related to the accumulation of tau protein. These diseases include progressive supranuclear palsy, Alzheimer’s disease, frontotemporal dementias, corticobasal syndrome, and chronic traumatic encephalopathy, caused pathologically by tau protein deposits in the brain. The symptoms caused by tauopathies reflect the involved anatomical areas of the brain. Diagnosis is made through history taking and translational research to try to make an earlier diagnosis. The tau protein by converting the 6S dimers of tubulin into 36 s rings plays a crucial role in the polymerization of microtubules located physiologically in the axons of neurons (Weingarten MD et al. 1975). In addition, other interactions with tubulins are carried out dynamically by regulating various features of neuronal growth with influence on neurite polarity, axonal sprouting, and morphogenesis (Drubin DG et al. 1985; Liu CWA et al. 1999; Takei Y et al. 2000). Tau protein also plays a very important role in the interaction between the with tyrosine kinase and the plasma membrane (Brandt R et al. 1995; Jensen PH et al. 1999; Hanger DP et al. 2019).
Tau protein when it has pathological forms is found in multiple states where the main one is aberrant phosphorylation with aggregation into larger insoluble filaments (Orr ME et al. 2017). There are studies in the literature where pathogenic tau in one cell can induce additional pathogenic tau in neighboring cells by synaptic insemination with extracellular microvesicles called “exosomes” leading to further progression of pathology (Frost B et al. 2009; De Calignon A et al. 2012). Exosomes filled with tau protein are present in the brain fluid of patients with mild Alzheimer and neurological dementia (Goetzl EJ et al. 2016). Massive hyperphosphorylation can lead to memory deficits associated with peripheral neuropathy accompanied by small-fiber (A-δ and C-fiber) and major-fiber neuropathy with tactile allodynia, impaired thermoregulation, slowed motor nerve conduction, and decreased intraepidermal fiber density (Marquez A et al. 2021). There are studies in the literature that are trying to understand the role of tau protein as a biomarker to diagnose most neurodegenerative diseases by comparison with imaging techniques. PET imaging studies have shown the correlation between tau pathology and Alzheimer’s disease with respect to Aβ plaque deposition (Calcutt NA et al. 2008; Akihiko A et al., 2021), so we rely on monitoring phosphorylated cerebrospinal fluid (CSF) (p-tau), total tau (T-tau), and amyloid-β 42 (Aβ42) to judge the progress of Alzheimer’s disease.
The neuropathies involved following COVID-19 infection affect myelinated Aδ fibers and unmyelinated C fibers. The variability of pathology is very high, and data attested between 11.7/100,000 and 13.3/10,000, but these are probably underestimates. Neuropathy can also vary in type and can be mixed, sensory only, or autonomic only, depending on the nerve fibers involved (Brier MR 2016). Immunological and histochemical studies have shown that small fibers affect microvascular tone (Ferrara F & Vitiello A, 2020; Vitiello A & Ferrara F, 2021b; Bitirgen G et al., 2022).
Conclusions
Complications following COVID-19 represent a significant expense commitment for the health service, and a recent literature review states that such complications are 20–25% among COVID-19 patients (62). Many literature studies show that tau protein accumulation is common to all symptoms of Long COVID. Also confirming this is an in vitro model on brain organs infected with SARS-CoV-2 virus where there was massive phosphorylation of tau protein, pTau 231, with neuronal cell death. Viral infection can lead to triggering a pathological tau protein that spreads healthier naive cells across synapses, with subsequent functional and structural changes in the autonomic and peripheral nervous system. Peripheral neuropathy that is triggered at the small fiber level can lead to a wide range of clinical symptoms. Patients with Long COVID present with well-defined symptoms of cognitive dysfunction, peripheral neuropathy, fatigue, postexertional malaise, and autonomic dysfunction, potentially attributable to taupetic neuropathy including the peripheral neuropathy of small fibers discussed earlier. The role of tau protein seems well defined in the symptomatology of Long COVID. Deposits of such protein can lead to peripheral fatigue and different pathologies depending on the organ involved. Further studies could confirm this hypothesis of massive neuronal accumulation of such protein in COVID-19 patient so as to define a reliable biomarker. Certainly then should the mechanisms of the aberrant phosphorylation that occurs be known, other therapeutic targets could be targeted to limit symptoms due to Long COVID.
Data availability
Full availability of data and materials. All stated data can be provided on request to the reader.
Code availability
Not applicable.
References
Ando A, Miyamoto M, Saito N, Kotani K, Kamiya H, Ishibashi S, Tavakoli M (2021) Small fibre neuropathy is associated with impaired vascular endothelial function in patients with type 2 diabetes. Front Endocrinol (lausanne). 12:653277. https://doi.org/10.3389/fendo.2021.653277
Arendt T, Brückner MK, Mosch B, Lösche A (2010) Selective cell death of hyperploid neurons in Alzheimer’s disease. Am J Pathol 177(1):15–20. https://doi.org/10.2353/ajpath.2010.090955
Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I et al (2021) Post-COVID-19 syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. BMJ 372:n693
Bitirgen G, Korkmaz C, Zamani A, Ozkagnici A, Zengin N, Ponirakis G, Malik RA (2022) Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with Long COVID. Br J Ophthalmol 106(12):1635–1641. https://doi.org/10.1136/bjophthalmol-2021-319450
Brandt R, Léger J, Lee G (1995) Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J Cell Biol 131(5):1327–1340. https://doi.org/10.1083/jcb.131.5.1327
Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TL, Ances BM (2016) Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 8(338):338ra66. https://doi.org/10.1126/scitranslmed.aaf2362
Calabretta E, Moraleda JM, Iacobelli M, Jara R, Vlodavsky I, O’Gorman P, Pagliuca A, Mo C, Baron RM, Aghemo A, Soiffer R, Fareed J, Carlo-Stella C, Richardson P (2021) COVID-19-induced endotheliitis: emerging evidence and possible therapeutic strategies. Br J Haematol 193(1):43–51. https://doi.org/10.1111/bjh.17240
Calcutt NA, Jolivalt CG, Fernyhough P (2008) Growth factors as therapeutics for diabetic neuropathy. Curr Drug Targets 9(1):47–59. https://doi.org/10.2174/138945008783431727
Castanares-Zapatero D, Chalon P, Kohn L, Dauvrin M, Detollenaere J, Maertens de Noordhout C, Primus-de Jong C, Cleemput I, Van den Heede K (2022) Pathophysiology and mechanism of Long COVID: a comprehensive review. Ann Med 54(1):1473–1487. https://doi.org/10.1080/07853890.2022.2076901
CDC. Healthcare workers [Internet] Centers for Disease Control and Prevention. 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-Covid-19-conditions.html [Accessed October 2022]
Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L (2020) SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev 54:62–75. https://doi.org/10.1016/j.cytogfr.2020.06.001
Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, Redfield S, Austin JP, Akrami A (2021) Characterizing Long COVID in an international cohort 7 months of symptoms and their impact. EClinicalMedicine 38:101019. https://doi.org/10.1016/j.eclinm.2021.101019
de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT (2012) Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73(4):685–697. https://doi.org/10.1016/j.neuron.2011.11.033.Erratum.In:Neuron.2012Oct18;76(2):461
Drubin DG, Feinstein SC, Shooter EM, Kirschner MW (1985) Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol 101(5 Pt 1):1799–1807. https://doi.org/10.1083/jcb.101.5.1799
Ferrara F, Porta R, Santilli P, D’Aiuto V, Vitiello A (2020) Are multiple sclerosis therapies safe in severe acute respiratory syndrome coronavirus 2 times? Indian J Pharmacol 52(5):441–442. https://doi.org/10.4103/ijp.IJP_417_20
Ferrara F, Vitiello A. 2020 Potential pharmacological approach in the regulation of angiotensin-II conversion enzyme and dipeptidyl-peptidase 4 in diabetic COVID-19 patients. Italian Journal of Medicine 15(1) https://doi.org/10.4081/itjm.2020.1435
Ferrara F, Vitiello A (2021) Scientific hypothesis for treatment of COVID-19’s lung lesions by adjusting ACE/ACE2 imbalance. Cardiovasc Toxicol 21(6):498–503. https://doi.org/10.1007/s12012-021-09649-y
Ferrara F (2020) Antirheumatic in SARS-CoV-2: benefit or risk? Italian Journal of Medicine 14(2):114–115. https://doi.org/10.4081/itjm.2020.1290
Frost B, Bardai FH, Feany MB (2016) Lamin dysfunction mediates neurodegeneration in tauopathies. Curr Biol 26(1):129–136. https://doi.org/10.1016/j.cub.2015.11.039
Frost B, Hemberg M, Lewis J, Feany MB (2014) Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci 17(3):357–366. https://doi.org/10.1038/nn.3639
Frost B, Jacks RL, Diamond MI (2009) Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284(19):12845–12852. https://doi.org/10.1074/jbc.M808759200
Fulga TA, Elson-Schwab I, Khurana V, Steinhilb ML, Spires TL, Hyman BT, Feany MB (2007) Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol 9(2):139–148. https://doi.org/10.1038/ncb1528
Funk AL, Kuppermann N, Florin TA, Tancredi DJ, **e J, Kim K, Finkelstein Y, Neuman MI, Salvadori MI, Yock-Corrales A, Breslin KA, Ambroggio L, Chaudhari PP, Bergmann KR, Gardiner MA, Nebhrajani JR, Campos C, Ahmad FA, Sartori LF, Navanandan N, Kannikeswaran N, Caperell K, Morris CR, Mintegi S, Gangoiti I, Sabhaney VJ, Plint AC, Klassen TP, Avva UR, Shah NP, Dixon AC, Lunoe MM, Becker SM, Rogers AJ, Pavlicich V, Dalziel SR, Payne DC, Malley R, Borland ML, Morrison AK, Bhatt M, Rino PB, Beneyto Ferre I, Eckerle M, Kam AJ, Chong SL, Palumbo L, Kwok MY, Cherry JC, Poonai N, Waseem M, Simon NJ, Freedman SB (2022) Pediatric Emergency Research Network–COVID-19 Study Team Post-COVID-19 conditions among children 90 days after SARS-CoV-2 infection. JAMA Netw Open 5(7):e2223253. https://doi.org/10.1001/jamanetworkopen.2022.23253
Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, Schwartz JB, Miller BL (2016) Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J 30(11):3853–3859. https://doi.org/10.1096/fj.201600756R
Hanger DP, Goniotaki D, Noble W (2019) Synaptic localisation of tau. Adv Exp Med Biol 1184:105–112. https://doi.org/10.1007/978-981-32-9358-8_9
Harari S, Ripamonti L, Marveggio P, Mannucci PM (2022) Long COVID: a patient perspective. Eur J Intern Med 95:104–105. https://doi.org/10.1016/j.ejim.2021.10.023
Hattori Y, Hattori K, Machida T, Matsuda N. 2022 Vascular endotheliitis associated with infections: its pathogenetic role and therapeutic implication. Biochem Pharmacol :114909
Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021 Mar;19(3):141–154. doi: https://doi.org/10.1038/s41579-020-00459-7. Epub 2020 Oct 6. Erratum in: Nat Rev Microbiol. 2022 May;20(5):315 https://doi.org/10.1038/s41579-020-00459-7
Hua Q, He RQ, Haque N, Qu MH, del Carmen AA, Grundke-Iqbal I, Iqbal K (2003) Microtubule associated protein tau binds to double-stranded but not single-stranded DNA. Cell Mol Life Sci 60(2):413–421. https://doi.org/10.1007/s000180300034
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, Schwartz S, Albasset S, McManus RM, Tejera D, Griep A, Santarelli F, Brosseron F, Opitz S, Stunden J, Merten M, Kayed R, Golenbock DT, Blum D, Latz E, Buée L, Heneka MT (2019) NLRP3 inflammasome activation drives tau pathology. Nature 575(7784):669–673. https://doi.org/10.1038/s41586-019-1769-z
Jensen PH, Hager H, Nielsen MS, Hojrup P, Gliemann J, Jakes R (1999) Alpha-synuclein binds to tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J Biol Chem 274(36):25481–25489. https://doi.org/10.1074/jbc.274.36.25481
Kanji JN, Zelyas N, MacDonald C, Pabbaraju K, Khan MN, Prasad A, Hu J, Diggle M, Berenger BM, Tipples G (2021) False negative rate of COVID-19 PCR testing: a discordant testing analysis. Virol J 18(1):13. https://doi.org/10.1186/s12985-021-01489-0
Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J (2020) Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med 173(4):262–267. https://doi.org/10.7326/M20-1495
Lee MH, Perl DP, Nair G et al (2021) Microvascular injury in the brains of patients with COVID-19. N Engl J Med 384(5):481–483. https://doi.org/10.1056/NEJMc2033369
Liu CW, Lee G, Jay DG (1999) Tau is required for neurite outgrowth and growth cone motility of chick sensory neurons. Cell Motil Cytoskeleton 43(3):232–242. https://doi.org/10.1002/(SICI)1097-0169(1999)43:3%3c232::AID-CM6%3e3.0.CO;2-7
Liu X, Feng J, Zhang Q, Guo D, Zhang L, Suo T, Hu W, Guo M, Wang X, Huang Z, **ong Y, Chen G, Chen Y, Lan K (2020) Analytical comparisons of SARS-COV-2 detection by qRT-PCR and ddPCR with multiple primer/probe sets. Emerg Microbes Infect 9(1):1175–1179. https://doi.org/10.1080/22221751.2020.1772679
Magro CM, Mulvey J, Kubiak J, et al. Severe COVID-19: a multifaceted viral vasculopathy syndrome. Ann Diagn Pathol. 2021;50:151645. doi: https://doi.org/10.1016/j.anndiagpath.2020.151645
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E (2018) Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17(8):588–606. https://doi.org/10.1038/nrd.2018.97
Marquez A, Guernsey LS, Frizzi KE, Cundiff M, Constantino I, Muttalib N, Arenas F, Zhou X, Lim SH, Ferdousi M, Ponirakis G, Silverdale M, Kobylecki C, Jones M, Marshall A, Malik RA, Jolivalt CG (2021) Tau associated peripheral and central neurodegeneration: identification of an early imaging marker for tauopathy. Neurobiol Dis. 151:105273. https://doi.org/10.1016/j.nbd.2021.105273
Nalbandian A, Sehgal K, Gupta A et al (2021) Post-acute COVID-19 syndrome. Nat Med 27(4):601–615. https://doi.org/10.1038/s41591-021-01283-z
Orr ME, Sullivan AC, Frost B (2017) A brief overview of tauopathy: causes, consequences, and therapeutic strategies. Trends Pharmacol Sci 38(7):637–648. https://doi.org/10.1016/j.tips.2017.03.011
Ramani A, Müller L, Ostermann PN, Gabriel E, Abida-Islam P, Müller-Schiffmann A, Mariappan A, Goureau O, Gruell H, Walker A, Andrée M, Hauka S, Houwaart T, Dilthey A, Wohlgemuth K, Omran H, Klein F, Wieczorek D, Adams O, Timm J, Korth C, Schaal H, Gopalakrishnan J (2020) SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J 39(20):106230
Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr. 2021 May-Jun;15(3):869–875 https://doi.org/10.1016/j.dsx.2021.04.007. Erratum In: Diabetes Metab Syndr. 2022 May;16(5):102504.
Stefanou MI, Palaiodimou L, Bakola E, Smyrnis N, Papadopoulou M, Paraskevas GP, Rizos E, Boutati E, Grigoriadis N, Krogias C, Giannopoulos S, Tsiodras S, Gaga M, Tsivgoulis G (2022) Neurological manifestations of Long COVID syndrome: a narrative review. Ther Adv Chronic Dis 17(13):20406223221076890. https://doi.org/10.1177/20406223221076890
Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, Pujol JC, Klaser K, Antonelli M, Canas LS, Molteni E, Modat M, Jorge Cardoso M, May A, Ganesh S, Davies R, Nguyen LH, Drew DA, Astley CM, Joshi AD, Merino J, Tsereteli N, Fall T, Gomez MF, Duncan EL, Menni C, Williams FMK, Franks PW, Chan AT, Wolf J, Ourselin S, Spector T, Steves CJ (2021) Attributes and predictors of Long COVID. Nat Med 27(4):626–631. https://doi.org/10.1038/s41591-021-01292-y
Takei Y, Teng J, Harada A, Hirokawa N (2000) Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J Cell Biol 150(5):989–1000. https://doi.org/10.1083/jcb.150.5.989
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20(6):363–374. https://doi.org/10.1038/s41577-020-0311-8
Vardhana SA, Wolchok JD (2020) The many faces of the anti-COVID-19 immune response. J Exp Med 217:e20200678
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395(10234):1417–1418. https://doi.org/10.1016/S0140-6736(20)30937-5
Vitiello A, Ferrara F (2021) Brief review of the mRNA vaccines COVID-19. Inflammopharmacology 29(3):645–649. https://doi.org/10.1007/s10787-021-00811-0
Vitiello A, Ferrara F (2021) Pharmacological agents modifying the renin angiotensin and natriuretic peptide systems in COVID-19 patients. Wien Klin Wochenschr 133(17–18):983–988. https://doi.org/10.1007/s00508-021-01855-6
Vitiello A, La Porta R, Ferrara F (2021) Scientific hypothesis and rational pharmacological for the use of sacubitril/valsartan in cardiac damage caused by COVID-19. Med Hypotheses 147:110486. https://doi.org/10.1016/j.mehy.2021.110486
Vitiello A, Porta R, Pianesi L, Ferrara F (2022) COVID-19 pandemic: vaccine and new monoclonal antibodies, point of view. Ir J Med Sci 191(1):487–488. https://doi.org/10.1007/s11845-021-02584-5
Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 72:1858–1862
World Health Organization (WHO). https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Accessed October 2022]
**e Y, Al-Aly Z (2022) Risks and burdens of incident diabetes in Long COVID: a cohort study. Lancet Diabetes Endocrinol 10(5):311–321. https://doi.org/10.1016/S2213-8587(22)00044-4
**e Y, Xu E, Bowe B (2022) Long-term cardiovascular outcomes of COVID-19. Nat Med 28:583–590. https://doi.org/10.1038/s41591-022-01689-3
Funding
No funding was received to conduct this study.
Author information
Authors and Affiliations
Contributions
FF: writing—original draft, methodology; AZ: conceptualization, supervision, validation; MM: conceptualization, writing—original draft; RL: conceptualization, supervision, validation; UT: supervision, validation; MB: supervision, validation; AV: writing—review and editing, supervision, validation.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors consent to the publication of the manuscript.
Competing interests
The authors declare no competing interests.
Other
The authors declare that the opinions expressed are of a personal nature and do not in any way commit the responsibility of the administrations to which they belong.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Long COVID is an unknown emerging problem in the current global health care scenario, of which the cause is attributable to deposits of tau protein.
• In brain samples from previously COVID-19-affected and deceased individuals was found an intense phosphorylation of the tau protein in response to SARS-CoV-2 infection, as well as peripheral small fiber neuropathies are also evident among patients who express Long COVID.
• In the post-pandemic era, further research studies are needed to establish the association between tau protein and Long COVID.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferrara, F., Zovi, A., Masi, M. et al. Long COVID could become a widespread post-pandemic disease? A debate on the organs most affected. Naunyn-Schmiedeberg's Arch Pharmacol 396, 1583–1589 (2023). https://doi.org/10.1007/s00210-023-02417-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02417-5