Abstract
Aims/hypothesis
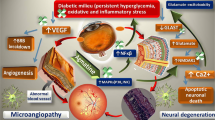
Diabetic retinopathy is characterised by retinal neurodegeneration and retinal vascular abnormalities, affecting one third of diabetic patients with disease duration of more than 10 years. Accumulated evidence suggests that serine racemase (SR) and D-serine are correlated with the pathogenesis of diabetic retinopathy and the deletion of the Srr gene reverses neurovascular pathologies in diabetic mice. Since D-serine content is balanced by SR synthesis and D-amino acid oxidase (DAAO) degradation, we examined the roles of DAAO in diabetic retinopathy and further explored relevant therapy.
Methods
Rats were used as a model of diabetes by i.p. injection of streptozotocin at the age of 2 months and blood glucose was monitored with a glucometer. Quantitative real-time PCR was used to examine Dao mRNA and western blotting to examine targeted proteins in the retinas. Bisulphite sequencing was used to examine the methylation of Dao mRNA promoter in the retinas. Intravitreal injection of DAAO-expressing adenovirus (AAV8-DAAO) was conducted one week before streptozotocin administration. Brain specific homeobox/POU domain protein 3a (Brn3a) immunofluorescence was conducted to indicate retinal ganglion cells at 3 months after virus injection. The permeability of the blood–retinal barrier was examined by Evans blue leakage from retinal capillaries. Periodic acid–Schiff staining and haematoxylin counterstaining were used to indicate retinal vasculature, which was further examined with double immunostaining at 7 months after virus injection.
Results
At the age of 12 months, DAAO mRNA and protein levels in retinas from diabetic animals were reduced to 66.2% and 70.4% of those from normal (control) animals, respectively. The Dao proximal promoter contained higher levels of methylation in diabetic than in normal retinas. Consistent with the observation, DNA methyltransferase 1 was increased in diabetic retinas. Injection of DAAO-expressing virus completely prevented the loss of retinal ganglion cells and the disruption of blood–retinal barrier in diabetic rats. Diabetic retinas contained retinal ganglion cells at a density of 54 ± 4/mm2, which was restored to 68 ± 9/mm2 by DAAO overexpression, similar to the levels in normal retinas. The ratio between the number of endothelial cells and pericytes in diabetic retinas was 6.06 ± 1.93/mm2, which was reduced to 3.42 ± 0.55/mm2 by DAAO overexpression; the number of acellular capillaries in diabetic retinas was 10 ± 5/mm2, which was restored to 6 ± 2/mm2 by DAAO overexpression, similar to the levels in normal retinas. Injection of the DAAO-expressing virus increased the expression of occludin and reduced gliosis, which were examined to probe the mechanism by which the disrupted blood–retinal barrier in diabetic rats was rescued and retinal neurodegeneration was prevented.
Conclusions/interpretation
Altogether, overexpression of DAAO before the onset of diabetes protects against neurovascular abnormalities in retinas from diabetic rats, which suggests a novel strategy for preventing diabetic retinopathy.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Diabetic retinopathy is a common complication of diabetes mellitus and manifests as non-proliferative diabetic retinopathy at the early stages and proliferative diabetic retinopathy at later stages. The typical pathologies of diabetic retinopathy include retinal neurodegeneration [1, 2] and retinal vascular abnormalities at the early stages, including increased permeability of the blood–retinal barrier, loss of retinal vascular endothelial cells and pericytes, and microaneurysm formation. Retinal haemorrhage, fibrovascular membrane formation and retinal neovascularisation are characteristics of late-stage diabetic retinopathy [3, 4].
d-Serine is degraded by d-amino acid oxidase (DAAO) and synthesized by serine racemase (SR), although SR also degrades d-serine through an α,β-elimination reaction in the cerebrum of adult rodents, in which DAAO is not expressed [5]. d-Serine is involved in neurotransmission, synaptic plasticity, cell migration and long-term potentiation in the brain [6,7,8]. In rodents, the neonatal retina is a two-layered structure consisting of an inner and an outer neuroblastic layer separated by the inner plexiform layer (IPL), develo** into three cell layers at 2 weeks, with a structure similar to the adult retina [9]. Compatible with the developmental pattern, neonatal d-serine in the retina is at peak level (at days 1–3) and gradually declines to adulthood, suggesting that d-serine plays a role in retinal development [10]. The high level of d-serine in the neonatal retina inhibits α-amino-3-hydroxyl-5-methyl-4-isoxazole propionic acid receptor (AMPAR) and kainite receptor and determines the constituents of N-methyl-d-aspartate receptor (NMDAR) and AMPAR, increasing the ratio of NMDAR/AMPAR [11, 12]. Thus, exogenous application of d-serine and modulation of d-serine level change the amplitude of NMDA-mediated light response in whole-cell patch-clamp recording of retinal ganglion cells (RGCs), using mice with deletion of Srr or Dao [11, 13, 14].
DAAO is a flavine adenine dinucleotide-containing enzyme that metabolizes d-amino acids to ammonia, hydrogen peroxide and keto acids [15]. In vertebrates, DAAO is expressed in the kidney and in the liver, acting as a detoxifying agent in aged animals, whereas in microorganisms it is used to generate energy [16, 17]. DAAO activity is at relatively higher level in the IPL of the retina [11], a region rich in NMDARs. In Dao mutant mice, d-serine in the retina is twofold higher than in wild-type retinas and application of d-serine potentiates NMDAR-mediated light response in RGCs from wild-type but not from Dao mutant mice, suggesting that DAAO mediates subsaturation of the NMDAR glycine site in wild-type retinas through degradation of d-serine [10].
SR is widely distributed in the retina, in which RGCs, Müller cells, astrocytes and retinal pigment epithelial cells all express SR [13, 18, 19]. Recent studies indicate that SR and its product, d-serine, are linked to diabetic retinopathy. For example, SR is increased in the retina of diabetic rats and d-serine is increased in the aqueous humour of diabetic rats and humans [20, 21]; deficiency of SR mitigates loss of RGCs and retinal vascular pathology in diabetic mice [22, 23]. These data suggest that overexpression of SR promotes, and deletion of SR attenuates, the pathologies of diabetic retinopathy. Since DAAO degrades d-serine whereas SR synthesises d-serine, we hypothesise that DAAO expression may prevent neurovascular abnormalities in diabetic retinopathy. Intraperitoneal injection of streptozotocin (STZ) has been widely used to generate a rat model of type 1 diabetes, in which the rats subsequently develop diabetic retinopathy. Thus, we examined DAAO expression in diabetic retinas by use of STZ-injected diabetic rats and further explored intervention strategies.
Methods
Materials
The following reagents were used: Triton X-100, paraformaldehyde, sucrose, Evans blue powder, formamide, xylazine hydrochloride (Sigma, St Louis, MO, USA); BSA (Shanghai Biotechnology, Shanghai, China); antibodies: SR (BD Biosciences, RRID: AB_399439, San Jose, CA, USA), occludin (BD Biosciences, RRID: AB_398403), DAAO (Abcam, cat. no. ab187525, Cambridge, MA, USA), collagen IV (Abcam, RRID: AB_445160), DNA methyltransferase (DNMT)1 (Cell Signaling Technology, RRID: AB_10545765, Beverly, MA, USA), DNMT3A (Cell Signaling Technology, RRID: AB_2263617), α-Tubulin (Cell Signaling Technology, RRID: AB_2210548), brain specific homeobox/POU domain protein 3a (Brn3a; Santa Cruz Biotechnology, RRID: AB_2167511, Santa Cruz, CA, USA), DNMT3B (Santa Cruz Biotechnology, RRID: AB_2094125), occludin (Abcam, cat. no. 216327, RRID: AB_2737295), DAAO antibody for immunofluorescence (Beyotime Institute of Biotechnology, Bei**g, cat. no. AF6663), p-extracellular signal-regulated kinase (ERK)1/ERK2 rabbit polyclonal antibody (Beyotime Institute of Biotechnology, cat. no. AF5818), ERK1/2 rabbit monoclonal antibody (Beyotime Institute of Biotechnology, cat. no. AF1051), glial fibrillary acidic protein (GFAP; Beyotime Institute of Biotechnology, cat. no. AF0156), Trizol reagent (Invitrogen, Shanghai, China), Alexa Fluor 594-conjugated isolectin GS-IB4 (isolectin-B4, Invitrogen, Shanghai, China); chloral hydrate (Shanghai Pharmaceutical Company, Shanghai, China); eye drops consisting of a mixture of 1% tropicamide and 1% phenylephrine hydrochloride (Santen Pharmaceutical, Jiangsu Province, China); VECTASHIELD antifade mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA); anti-rabbit Alexa Fluor 488 or anti-mouse Alexa Fluor 594 (Invitrogen, Shanghai, China); Takara Ex Taq, PrimeScript RT reagent kit with gDNA Eraser (Takara, Dalian, China); EZ DNA methylation-Gold kit (Zyme Research, Irvine, CA, USA); Go-Taq hot start polymerase (Promega, Madison, WI, USA); Power SYBR Green PCR master mixture (Life Technology, NY, USA). Periodic acid–Schiff (PAS) reagents (Bei**g Solarbio Science and Technology, China).
Study design
Male rats were used to generate the diabetic model. All the data were included for analysis except that the deceased rats (12 rats died at 3 months after STZ injection and 10 died at 7 months after injection; totally, 100 rats were used in this study) before the measurement were excluded for analysis. Quantification of endothelial cells, pericytes and acellular capillaries were conducted in a blinded fashion.
Establishment of diabetic animal model
Sprague–Dawley rats were purchased from the Shanghai Animal Experimental Center, Chinese Academy of Sciences and fed ad libitum in a pathogen-free standard animal facility at Wenzhou Medical University. All experiments were conducted in accordance with ARRIVE guidelines for the Use and Care of Animals and approved by the Wenzhou Medical University Committee on the Use and Care of Animals (approval number: wydw2020-0528). Male rats at the age of 2 months were randomly assigned to control groups receiving an i.p. injection of saline (0.9% NaCl) or to experimental groups receiving a single i.p. injection of STZ (70 mg/kg body weight). The blood glucose levels were monitored with a glucometer once a week after injection, and rats with random glucose level higher than 16.6 mmol/l in the three measurements were designated as diabetic rats.
Quantitative real-time PCR
RNA was extracted and cleaned and cDNA was synthesised as described [24]. The cDNA product (2 ng) was used as a template for PCR reaction with forward primer: CAGCCCAACCTCTGCACATGAAGAT spanning 73–98 bp and reverse primer: TGCTGGTTCCACTCCGCCTCCTG spanning two adjacent exons at 184–191 bp and at 2166–2181 bp, relative to the translation start site (TSS) of the gene, Dao (GenBank no. 114027). Amplification of the α-tubulin gene and PCR procedures and quantification followed our previous protocol [24].
Western blots
The harvested retinal homogenates were resolved with 12% SDS-PAGE electrophoresis and transferred to a nitrocellular membrane that was blocked with 5% skimmed milk. The membrane was cut in two pieces and the upper portion was incubated with α-tubulin antibody (1:1000) and the lower portion with DAAO antibody (1:500) overnight at 4°C. The membranes were incubated at room temperature for 1 h with a horseradish peroxidase-conjugated secondary antibody (Bei**g Zhongshan Golden Bridge Biotechnology, China). Following the incubation, the membrane was developed with enhanced chemiluminescence substrate (SuperSignal West Femto Maximum Sensitivity Substrate, Thermo Scientific). Subsequently, the membrane was re-probed with SR antibody (1:500). The immunoblotting procedure for occludin used a similar protocol. Images were acquired with FluorChem E Systems (Proteinsimple, Santa Clara, MA, USA).
Bisulphite sequencing
Retinas from wild-type (n = 15) or diabetic rats (n = 15) at the age of 12 months was used to extract genomic DNA. Modification of the genomic DNA with bisulphite was performed with an EZ DNA Methylation-Gold kit. With the modified DNA as a template, a PCR reaction spanning the particular CpG island adjacent to the Dao mRNA TSS was performed. The primers used for bisulphite sequencing were designed with Methprimer 2.0 software (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi) [25] with forward primer: TTTTTTTGTAGGTTGGGGTGAT and reverse primer: CAAAAAAATAAAACTACCAAAATCCC. The PCR products were subcloned and subject to sequencing (Invitrogen, Shanghai) as in our described protocol [24].
Intravitreal injection of AAV8 virus
The adeno-associated virus (AAV) viruses incorporated coding sequences of fluorescent protein ZsGreen and Dao; the sequence of internal ribosomal entry site (IRES) is located between ZsGreen and Dao encoding sequence, thereby allowing independent transcription of ZsGreen and Dao. The vehicle virus (AAV8-IRES-ZsGreen) and DAAO-expressing virus (AAV8-DAAO-IRES-ZsGreen) were packaged and generated by Biowit Biotechnologies (Shenzhen, China). Intravitreal injection was conducted following previous procedures [26]. Briefly, rats were randomly assigned to four groups with 25 rats in each group: normal rats with intravitreal injection of PBS, diabetic rats with intravitreal injection of PBS, or injection of AAV8-IRES-ZsGreen or of AAV8-DAAO-IRES-ZsGreen. Before intravitreal injection, the rats were anaesthetised with i.p. injection of 3% chloral hydrate and 0.18% xylazine hydrochloride, followed by eye dilation with eye drops consisting of a mixture of 1% tropicamide and 1% phenylephrine hydrochloride. Under an eye surgery microscope, a Hamilton needle was used to perforate in the sclera at the superior temporal quadrant,1 mm posterior to limbus. After withdrawing the needle, a microsyringe was used to gradually inject 4 μl virus (1 × 1013 viral genomes [vg]/ml) or equal volume of PBS through the perforation. After injection, the needle was kept in situ for 30 s before withdrawal.
Eyeball cryosectioning and immunostaining
The procedures to make cryosections and to conduct subsequent Brn3a immunostaining followed our previous protocol [26]. The frozen tissue block was incubated with a Brn3a antibody (1:200) in PBS containing 0.1% Triton X-100 at 4°C overnight. After being washed, the sections were incubated with a 594-conjugated secondary antibody (1:500, Invitrogen). Finally, the slides were loaded with VECTASHIELD antifade mounting medium with DAPI (Vector Laboratories) to indicate nuclei. Three slides chosen from every other slide for each eyeball were used for quantifying Brn3a-staining of RGCs, which were bilaterally counted at 250 μm straight-line distance from the optic disc.
Examination of the permeability of blood–retinal barrier with Evans blue
The procedure followed a previous protocol with modifications [27]. Evans blue dye (Sigma) was prepared by dissolving it in 0.9% NaCl (30 mg/ml) and injected via the iliac vein (45 mg/kg) after rats were anaesthetised. The injected Evans blue was circulated for 2 h; blood samples (300 μl) were collected every half hour via the tail vein to measure Evans blue concentration. At the end of circulation, the rats were perfused with 0.05 mol/l citrate buffer containing 1% paraformaldehyde. After perfusion, the eyes were isolated and the retina was removed from the eyecup under a dissecting microscope. The isolated retina was dried under 45°C for 5 h and dry weight was recorded. Each pair of dried retinas was dissolved in 150 μl formamide and incubated in a thermostat water bath at 70°C for 18 h. After incubation, the sample was subject to centrifugation at 18,000 g at 4°C for 50 min, and the supernatants were used for measuring Evans blue concentration with a spectroscope. The initially collected blood samples were subject to centrifugation at 18,000 g at 4°C for 15 min and the supernatants were diluted in formamide to measure Evans blue concentration. The quantifications were calculated according to the following equation, with results expressed as μl plasma × g retinal dry weight −1 h−1: retinal Evans blue content (μg)/retina dry weight (g)/averaged plasma Evans blue concentration (μg/μl) × circulation time (h).
Trypsin digestion of retinas
As described in the previous protocol [28], the enucleated eyeballs were fixed in 4% paraformaldehyde for 48 h. The retina was gently peeled off and washed in double distilled (dd)H2O in a shaker overnight. The retina was cut in half along the optic disc; one half was subject to PAS staining after trypsin digestion and the other half for double staining. The digestion was conducted in 3% trypsin dissolved in 0.2 mol/l Tris buffer (pH 7.4) at 37°C for 1 h and the residual neural tissue and inner limiting membrane were gently washed off from the retina until the retina became transparent. The retinal vessels were transferred to slides and dried at room temperature for PAS staining.
PAS staining and haematoxylin counterstaining
As described [29, 30], PAS staining and haematoxylin counterstaining were used to indicate retinal vasculature. The retinal vasculature from digested retinas was fixed in Carnoy’s solution for 10 min, washed and further dipped in 0.5% periodic acid solution for 10 min. The slides were gently and extensively washed and subsequently dipped in Schiff solution for 20 min in the dark and then dipped into 0.15% sodium pyrosulphite solution twice for 1 min each. The slides were washed and counterstained with Schiff solution for 3 min. After washing, the slides were treated for 3 s with solution: 1% concentrated hydrogen chloride (1 ml) dissolved in 75% ethanol solution (100 ml). Following the discoloration, the slides were abundantly washed and treated with 1% NH3.H2O2 for 5 s. Finally, the slides were dehydrated with graded ethanol, cleaned with xylene and sealed with neutral balsam. Under phase-contrast microscopy, endothelial cells and pericytes were identified and counted according to morphology from randomly selected fields. A ratio between the number of endothelial cells and pericytes was given to each counted field and the ratio for each retina was averaged from 6–8 fields, 5–8 retinas for each group.
Double immunostaining
As described, double immunostaining against isolectin-B4 and collagen IV was used to indicate retinal vasculature [31]. The retinas following trypsin digestion were flattened on slides and fixed in 4% paraformaldehyde for 10 min. After washing, the retina was permeabilised with 0.1% Triton X-100 for 30 min and blocked with 3% BSA for 30 min at room temperature. Subsequently, the retinas were incubated with isolectin B-4 and collagen IV antibody (1:200) at 4°C overnight, washed, and incubated with an Alexa Fluor 488-conjugated secondary antibody (Invitrogen) for 1 h at room temperature. Finally, the slides were loaded with DAPI containing antifade solution (Vector Laboratories). Under fluorescence microscopy, quantification was conducted by randomly choosing 16 images per retina, 6–8 retinas randomly selected for each group.
Determination of d-serine with reverse-phase HPLC
Aqueous humour was collected from the anterior chamber of the eyes of rats and d-serine levels were determined by HPLC (Agilent 1100), following our previous protocol [19, 20].
Statistics
The results are all indicated as mean ± SD. For data showing a normal distribution, Student’s t test was used to compare the difference between two groups and one-way ANOVA followed by Bonferroni’s post hoc test was used for multiple groups; otherwise, the Mann–Whitney U test was used to compare the difference between two groups and Kruskal–Wallis for comparisons among multiple groups (SPSS.15.0.1; SPSS, Chicago, IL, USA). F values, together with two critical parameters including degrees of freedom (df) in the numerator and the denominator were indicated; df in the numerator was calculated as: group number minus 1 whereas df in the denominator as: the number of all samples minus group number. Differences were considered statistically significant if p < 0.05.
Results
DAAO was reduced in retinas from diabetic rats, which was correlated with Dao mRNA promoter hypermethylation
Initially, we examined the expression pattern of DAAO in wild-type rat retinas and identified that DAAO was expressed in RGC and IPL layers with higher expression in RGCs and in astrocytes; DAAO expression was decreased in diabetic retinas compared with normal retinas (electronic supplementary material [ESM] Fig. 1). Next, Dao mRNA in the retina was examined by quantitative real-time PCR and DAAO protein was assessed by western blotting from non-diabetic and STZ-induced diabetic rats at the age of 12 months. Both Dao mRNA and DAAO protein levels were significantly reduced in diabetic retinas, 66.2% and 70.4% relative to normal retina, respectively (p = 0.007 for mRNA comparison; p = 0.002 for protein comparison) (Fig. 1a–c). Consistent with our previous study [20], SR protein levels in diabetic retinas were significantly increased compared with normal retinas (p = 0.005) (Fig. 1b, c). DNA methylation regulates gene expression and has been linked with gene silencing. We therefore explored whether methylation was related to decreased transcription of Dao mRNA. Using methprimer, we scanned ~10,000 bp upstream of the TSS of Dao and the adjacent exon1. Using this screening, four CpG islands were found relative to TSS: −6605 to −6483 bp, −5491 to −5371 bp, −3222 to −3102 bp, and −47 to +166 bp. With respect to the location relative to the TSS, we analysed the CpG island located between −47 bp and +166 bp and found 12 CpG dinucleotides in this island (Fig. 2a). Using genomic DNA isolated from the retinas of non-diabetic or diabetic rats at the age of 12 months, the CpG island was amplified by PCR with a pair of bisulphite-specific primers as described in the materials and methods. The methylation level of the CpG islands from non-diabetic rats was 96.1%, which was lower than 97.6% from diabetic rats (p = 0.041). Notably, the methylation values for the 9th CpG dinucleotides was 93.55% for non-diabetic rat retinas, which was significantly lower than the value 98.79% for diabetic rat retinas (p = 0.023); for the 10th dinucleotides, the value was 94.83% for non-diabetic retinas relative to 100% for diabetic retinas (p = 0.017) (Fig. 2b). We further investigated the protein levels of DNMT in retinas from rats at the age of 5 and 12 months: retinal DNMT1 in non-diabetic rat retinas was significantly lower than that from diabetic rat retinas (p = 0.012 at 5 months; p = 0.001 for 12 months); by contrast, there were no differences for DNMT3A or 3B between normal and diabetic retinas (Fig. 3a–d).
Dao mRNA and DAAO protein levels were reduced in diabetic retinopathy. (a) RNA was isolated from the retinas of non-diabetic (control [Con], n = 5) or diabetic (DR) rats (n = 5) at the age of 12 months and were subject to reverse-transcription quantitative real-time PCR amplification of Dao mRNA. α-Tubulin was used as a reference gene. Student’s t test was used to compare differences (**p = 0.007). (b) Retinal homogenates from non-diabetic or diabetic rats at the age of 12 months were harvested. Each well was loaded with 60 μg protein and subject to immunoblotting against DAAO (n = 8 for each group) and SR (n = 4 for each group). α-Tubulin was used as an internal loading control. (c) The densitometry ratios of protein bands of DAAO and SR/α-tubulin were quantified. The value in controls was set as 1 and the ratios from treatments were normalised accordingly. Student’s t test was used to compare differences: **p = 0.002 for the difference in DAAO; **p = 0.005 for the difference in SR
Dao mRNA proximal promoter contained CpG islands in which two dinucleotides were hypermethylated. (a) The CpG island (−47 to +166 bp) adjacent to the TSS (ATG) is indicated and the CpG dinucleotides (numbered 1–12) are in red. (b) Genomic DNA was extracted from the retinas of non-diabetic (control [Con], n = 15) and diabetic (DR) rats (n = 15) at the age of 12 months and subjected to bisulphite treatment, PCR amplification and methylation determination. The methylation levels of the 12 CpG dinucleotides in the CpG island were compared between non-diabetic (n = 15,225 clones) and diabetic rats (n = 15,225 clones). Mann–Whitney U test was used to compare the differences. *p = 0.023 for the methylation difference in the ninth CpG base pair (S9); *p = 0.017 for the methylation difference in the tenth CpG base pair (S10) between retinas from non-diabetic and diabetic rats
Increased DNMT1 protein levels in retinas from diabetic rats relative to control rats. (a) Retinal homogenates were harvested from non-diabetic (control [Con], n = 4) and diabetic (DR) rats (n = 4) at the age of 5 months, followed by western blot detection of DNMT1, DNMT3A and DNMT3B; α-tubulin was used as a loading control. (b) The densitometry ratios of protein bands of DNMT1, DNMT3A, and DNMT3B/α-tubulin are indicated. Student’s t test was used to compare differences: **p = 0.005. (c) Retinal homogenates were harvested from non-diabetic (n = 8) and diabetic rats (n = 8) at the age of 12 months, followed by western blot detection of DNMT1, DNMT3A and DNMT3B. (d) The densitometry ratios of DNMT1 and DNMT3A/α-tubulin are indicated (there were no DNMT3B bands on the blot). Student’s t test was used to compare differences: **p = 0.001. In (b, d) The value in controls was set as 1 and the values from treatments were normalised accordingly
Intravitreal injection of AAV8-DAAO virus before STZ administration prevented RGC loss in diabetic rats
We next examined whether overexpression of DAAO in diabetic retinas before STZ administration prevented retinal neuropathology. Initially, we tested the transduction efficiency of AAV8 virus in normal rats and tested it at one month after virus injection. Injection of AAV8-DAAO-IRES-ZsGreen virus (AAV8-DAAO) increased fluorescence in RGCs and the outer nuclear layer (ONL) (ESM Fig. 2a) and significantly increased DAAO protein level (ESM Fig. 2b) in retinal homogenates compared with injection of AAV8-IRES-ZsGreen virus (AAV8-ZsGreen), which suggests that injection of AAV8-DAAO virus increases DAAO expression in the retina. To test the effect of DAAO expression on retinal neuropathy, we injected AAV8-ZsGreen virus or AAV8-DAAO virus at one week before i.p. injection of STZ and further examined the effects at 3 months after virus injection. As expected, STZ injection induced diabetes in rats, as can be seen from the differences in blood glucose and body weight (p < 0.001 for all comparisons vs control; Fig. 4a, b). To confirm the DAAO expression in diabetic rats after virus injection, we randomly chose rats from each group to assay DAAO expression at 3 months after virus injection: DAAO expression in diabetic rats (treated with PBS) was reduced relative to the control group (p < 0.001) and injection of AAV8-DAAO in diabetic rats significantly increased DAAO expression compared with injection of AAV8-ZsGreen (p = 0.015) (F[3, 12] = 5.513) (Fig. 4c, d). We also examined SR expression in the retina: SR expression in diabetic rats was increased relative to the control group (p = 0.017) and injection of AAV8-DAAO in diabetic rats did not change SR expression compared with injection of AAV8-ZsGreen (F[3, 12] = 11.460) (Fig. 4c, d).
Injection of AAV8-DAAO virus prevented RGC loss in retinas from diabetic rats. (a) Virus or PBS injection was conducted at one week before STZ injection. The body weights were monitored once a week for non-diabetic rats (control [Con]), diabetic rats with intravitreal injection of PBS (PBS), or of AAV8-ZsGreen virus (AAV8-ZsGreen), or of AAV8-DAAO virus (AAV8-DAAO). The values at 1, 2, 3, and 7 months (M) after STZ injection are indicated. The comparison is ***p < 0.001 for PBS vs Con, †††p < 0.001 for AAV8-ZsGreen vs Con, and ‡‡‡p < 0.001 for AAV8-DAAO vs Con (Kruskal–Wallis for comparisons at 1, 2 and 3 months and one-way ANOVA for comparisons at 7 months). (b) The random blood glucose levels were documented once a week with similar protocol as for body weights. The values at 1, 2, 3 and 7 months after STZ injection are indicated. The comparisons are ***p < 0.001 for PBS vs Con, †††p < 0.001 for AAV8-ZsGreen vs Con, and ‡‡‡p < 0.001 for AAV8-DAAO vs Con (one-way ANOVA for comparisons at 1, 2 and 3 months and Kruskal–Wallis for comparisons at 7 months). (c) Retinal tissues from the four groups of rats (n = 4 randomly selected for each group) were harvested and subjected to western blot detection of DAAO or SR at 3 months after virus injection. (d) The densitometry ratios of protein bands are indicated. The value in controls was set as 1 and the values from treatments were normalised accordingly. (e) Retinas from the four groups of rats were processed for cryosections with which RGC immunolabeling was conducted against Brn3a; DAPI was used to indicate nuclei; and the Brn3a and DAPI images were merged. Scale bar, 50 μm. (f) Quantification for RGC from (e). RGC count is averaged from 5–8 rats with 6–8 optical fields for each retina. In (d, f) one-way ANOVA was used to compare the differences: *p < 0.05, **p < 0.01, ***p < 0.001 for comparisons shown. INL, inner nuclear layer; ONL, outer nuclear layer; RGCL, RGC layer
Simultaneously, we examined the effect of DAAO expression on RGC with its specific marker, Brn3a, a class IV POU domain containing transcription factor. The density of RGCs in retinas from diabetic rats was 54 ± 4/mm2, significantly lower than 62 ± 5/mm2 in the control group (p = 0.034) and the reduction was significantly mitigated by injection of AAV8-DAAO at 68 ± 9/mm2 compared with injection of AAV8-ZsGreen, at the value of 54 ± 9/mm2 (p = 0.001) (F[3, 28] = 6.520) (Fig. 4e, f). Gliosis is a response to various injuries including neurodegeneration, such as in glaucoma and diabetic retinopathy. Gliosis was obvious in diabetic retinas and was dramatically mitigated by DAAO overexpression (Fig. 5). Activation of the extracellular-signal-regulated kinase (ERK) pathway underlies inflammation in the diabetic retina [32] and inhibition of ERK is shown to provide neuroprotection in diabetic retinopathy [33]. Consistent with these observations, the ERK pathway was activated in diabetic retinas, and this effect was reduced by DAAO overexpression when examined at 7 months after virus injection (ESM Fig. 3).
Injection of AAV8-DAAO virus reduced gliosis in retinas from diabetic rats. (a) The retinas from control (Con), and PBS-, AAV8-ZsGreen- and AAV8-DAAO-treated diabetic rats at 3 months after injection were processed for cryosections with which immunolabeling against GFAP (1:100) was conducted; DAPI was used to indicate nuclei; and the GFAP and DAPI images were merged. The images were typical of 2 cryosections for each retina, 3 retinas for each group. Scale bar, 50 μm. (b) The intensities of GFAP staining were quantified from 2 cryosections for each retina, 3 retinas for each group. One-way ANOVA was used to compare the differences: ***p < 0.001. The fluorescence intensity in controls was set as 1 and the values from treatments were normalised accordingly. INL, inner nuclear layer; ONL, outer nuclear layer; RGCL, RGC layer
Intravitreal injection of AAV8-DAAO virus before STZ administration prevented retinal vascular pathology in diabetic rats
Since retinal neurodegeneration and disruption of retinal vasculature are mutually aggravated in diabetic retinopathy [34], we further investigated whether the neuroprotective effect of DAAO expression in diabetic retinopathy had effects on retinal vascular pathology. To test the effect of DAAO expression on retinal vascular pathology in diabetic rats, we injected virus one week before injection of STZ and examined the effect on the blood–retinal barrier at 3 months or on retinal vasculature at 7 months after virus injection. The leakage of Evans Blue dye was used as an indicator of disruption of the blood–retinal barrier. In diabetes (treated with PBS), the values of leakage were higher than in the control group (p = 0.022), whereas injection of AAV8-DAAO significantly reversed the leakage compared with injection of AAV8-ZsGreen (p = 0.001) (F[3, 31] = 6.718) (Fig. 6a). To explore the mechanism by which the blood–retinal barrier was disrupted, we examined occludin, a tight-junction protein in retinal endothelial cells. The retinal homogenates at 3 months after virus injection were used to examine occludin expression: the expression levels in diabetic retinas were decreased compared with normal retinas (p = 0.004) whereas injection of AAV8-DAAO significantly reversed occludin expression compared with injection of AAV8-ZsGreen virus (p = 0.008) (F[3, 12] = 6.044) (Fig. 6b, c). To examine the effect on retinal vasculature after DAAO expression, we randomly chose rats from each group to examine DAAO expression at 7 months after virus injection: DAAO expression in diabetic rats (treated with PBS) was reduced relative to the control group and injection of AAV8-DAAO virus significantly increased DAAO expression compared with injection of AAV8-ZsGreen virus (Fig. 7a, b). Simultaneously, we examined the effect on retinal vascular pathology. The ratio of endothelial cells/pericytes in retinas of diabetic animals was 6.06 ± 1.93/mm2, significantly higher than 3.94 ± 0.93/mm2 in controls (p = 0.013). Injection of AAV8-DAAO virus significantly reduced the ratios to 3.42 ± 0.55/mm2 compared with injection of AAV8-ZsGreen virus (5.81 ± 1.55/mm2) (p = 0.016) (F[3, 20] = 5.090) (Fig. 7c, d). We also examined the amount of acellular capillaries: the number of acellular capillaries in diabetic retinas was 9 ± 3/mm2, which was numerically higher but not significantly different from the control group at 4 ± 3/mm2 (p = 0.058), and the loss of capillaries was reversed by injection of AAV8-DAAO to a level of 6 ± 1/mm2, which was not different from those treated with injection of AAV8-ZsGreen (9 ± 7/mm2) (p = 0.180) (F[3, 20] = 2.056) (Fig. 7c, e). To further confirm the quantification of acellular capillary, we conducted double staining with isolectin-B4 as a marker of retinal endothelial cells and collagen IV as an indicator for basal membrane. Consistent with the PAS–haematoxylin counterstaining, the number of acellular capillaries in diabetic retinas was 10 ± 5/mm2, higher than the control group (6 ± 1/mm2) and the difference reached significance (p = 0.043) (Fig. 8a, b). The loss of capillaries was reversed by injection of AAV8-DAAO to a value of 6 ± 2/mm2 compared with injection of AAV8-ZsGreen (10 ± 3/mm2 and the difference also reached significance (p = 0.018) (F[3, 22] = 3.738) (Fig. 8a, b).
Injection of AAV8-DAAO virus significantly improved the blood–retinal barrier in retinas from diabetic rats. (a) The four groups of rats (control [Con] and PBS-, AAV8-ZsGreen- and AAV8-DAAO-treated diabetic rats) were subject to blood–retinal barrier assay by use of Evans blue leakage at 3 months after virus injection, as described in the Methods. The Evans blue leakage was averaged from 8–10 rats for each group. (b) The retinal homogenates from the four groups of rats were subjected to western blot detection of occludin at 3 months after virus injection. (c) The quantification from (b) was averaged for 4 rats for each group. In (a, c) one-way ANOVA was used to compare the differences: *p < 0.05, **p < 0.01 for comparisons shown
Injection of AAV8-DAAO virus prevented disruption of retinal vasculature in diabetic retina. (a) Retinal tissues from the four groups of rats (control [Con] and PBS-, AAV8-ZsGreen- and AAV8-DAAO-treated diabetic rats) were harvested and subjected to western blot detection of DAAO at 7 months after virus injection. (b) Densitometry ratios of protein bands were averaged from (a) and the value in controls was set at 1 and the values in treatments were normalised accordingly, n = 4 for each group. (c) The retinas from the four groups of rats were subjected to trypsin digestion, PAS staining and haematoxylin counterstaining. Scale bar, 50 μm. Red arrows indicated pericytes and blue arrows for endothelial cells and black arrows for acellular capillary. (d) The retinas subjected to PAS–haematoxylin staining were used for quantifying retinal endothelial cells (E), pericytes (P), and acellular capillaries. (e) The densities of acellular capillaries in diabetic retinas. (d, e) The quantification was averaged from 5–8 rats with 6–8 optical fields for each retina. (b, d, e) One-way ANOVA was used to compare the differences: *p < 0.05, **p < 0.01 for comparisons shown
Injection of AAV8-DAAO virus prevented acellular capillaries in retinas from diabetic rats. (a) The retinas from the four groups of rats (control [Con] and PBS-, AAV8-ZsGreen- and AAV8-DAAO-treated diabetic rats) at 7 months after virus injection were processed into cryosections and subjected to double staining with collagen IV and isolectin-B4; the images were merged. Scale bar, 50 μm. (b) The densities of acellular capillaries in diabetic retinas. The numbers of acellular capillaries were averaged from 6–8 retinas and 16 optical fields for each retina. One-way ANOVA was used to compare the differences: *p < 0.05
Discussion
In this study, we demonstrated that DAAO expression was reduced in retinas from diabetic rats relative to non-diabetic control rats and delivery of DAAO-expressing AAV8 virus significantly prevented neurovascular abnormalities in diabetic retinopathy. To our knowledge, this is the first report to explore the roles of DAAO in diabetic retinopathy. Notably, delivery of DAAO-expressing virus was conducted one week before STZ administration. However, it is unclear whether DAAO expression after diabetes onset still provides protection at this moment.
Inflammation, oxidative stress, and decreased production of neurotrophic factor have been connected to retinal neurodegeneration in diabetic retinopathy [35, 36]. SR is upregulated and d-serine production is increased by inflammation [20, 37]. d-Serine overproduction in the retina is linked with RGC death and depletion of SR significantly reduces RGC death and retinal vascular abnormalities in diabetic animal models as well as inhibiting angiogenesis in a choroidal neovascularisation model [19, 22, 23, 26]. By ligating to the NMDAR glycine site, d-serine enhances NMDAR-dependent excitotoxicity and depletion of d-serine significantly reduces neurotoxicity [38, 39]. Extracellular d-serine level is affected by the activity of the neuron–glial network and transporters on the membrane. For example, d-serine release in neurons may occur following depolarisation via the alanine–serine–cysteine transporter, while astroglial d-serine release may occur through exocytosis following activation of receptors at the plasma membrane of astrocytes [40]. Overexpression of DAAO is projected to degrade d-serine in the neuron–glial network in the retina, thus reducing intracellular d-serine and the d-serine pool for release. Surprisingly, the d-serine level in the aqueous humour of DAAO-overexpressing diabetic rats was decreased but was not different from that in vehicle virus-injected diabetic rats at the time point assayed, i.e. 3 months after virus injection (ESM Fig. 4). A possible explanation for this is that SR elevation possibly complemented d-serine deficiency due to DAAO overexpression since SR was already increased in diabetic retinas at the examined time point (Fig. 4c, d), which was consistent with our previous study [20]. Another possibility is that this may be due to the detection limit of HPLC (Agilent 1100) in our laboratory, which is less sensitive than other d-serine detection methods such as capillary electrophoresis [10]. Notably, in this study, we examined total d-serine in aqueous humour, in which it is impossible to identify the differences in d-serine adjacent to RGCs (which is critical for RGC survival) among the tested groups. However, DAAO overexpression reduced gliosis and inhibited p-ERK, suggesting its neuroprotection, most likely by degrading d-serine.
In diabetic retinopathy, degeneration of retinal neurons and damage of retinal vessels form a vicious cycle and are mutually exacerbated in diabetic retinopathy. For example, degeneration of retinal neurons produces proinflammatory cytokines and angiogenic factors promoting disruption of retinal vasculature and angiogenesis whereas disruption of retinal vasculature leads to extravasation of fluids and leucocytes to the retina [41], inducing neurotoxicity [34]. Considering the effect of DAAO overexpression on RGC loss and occludin expression, the protective effect following DAAO expression may result from its neuroprotection at the early stage of diabetic retinopathy, possibly before SR elevation. Currently, we do not know where the transitional point lies between SR increase and DAAO decrease in the retina of this diabetic rat model, so more work is needed to clarify this.
Importantly, d-aspartate has been shown to potentiate excitotoxicity by competitively inhibiting glutamate uptake by astroglia [42, 43]; high glucose and diabetes increase d-aspartate release from retinal tissue [44]. It has been shown that d-aspartate is also a substrate of DAAO degradation [16]. Thus, expression of DAAO may lead to lower levels of d-aspartate, resulting in neuroprotection; this is what we initially assumed. However, we did not find d-aspartate in aqueous humour from diabetic rats using HPLC in our laboratory, suggesting that d-aspartate possibly exerts a marginal effect.
Notably, SR has been demonstrated to be downregulated in the ageing cerebellum and this downregulation is associated with SR promoter hypermethylation [24]. DNMT1 was elevated in the retina of diabetic rats, similar to the effect observed in ageing cerebellum [24], but elevation of DNMT1 did not lead to a reduction of SR expression in the diabetic retina in this study. On the contrary, SR expression in diabetic rat retinas is increased, as shown in our previous study [20] and in this study, which suggests that positive regulators may override the silencing effect of methylation in the diabetic retina. For example, SR regulation has been shown to be positively regulated by inflammatory stimuli [37, 45, 46], which is a known pathogenic factor for diabetic retinopathy [47].
In summary, we demonstrated that overexpression of DAAO in the retina could prevent retinal neurovascular pathologies in diabetic retinopathy, which suggests a novel strategy for the prevention of diabetic retinopathy.
Data availability
Data will be provided by the corresponding author upon reasonable request.
Abbreviations
- AAV:
-
Adeno-associated virus
- AAV8:
-
Adeno-associated virus serotype 8
- AMPAR:
-
α-Amino-3-hydroxyl-5-methyl-4-isoxazole propionic acid receptor
- Brn3a:
-
Brain specific homeobox/POU domain protein 3a
- DAAO:
-
d-Amino acid oxidase
- DNMT:
-
DNA methyltransferase
- ERK:
-
Extracellular signal-regulated kinase
- GFAP:
-
Glial fibrillary acidic protein
- IPL:
-
Inner plexiform layer
- IRES:
-
Internal ribosomal entry site
- NMDAR:
-
N-Methyl-d-aspartate receptor
- ONL:
-
Outer nuclear layer
- PAS:
-
Period acid–Schiff
- RGC:
-
Retinal ganglion cell
- SR:
-
Serine racemase
- STZ:
-
Streptozotocin
- TSS:
-
Translation start site
References
Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW (1998) Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 102:783–791. https://doi.org/10.1172/JCI2425
Sohn EH, van Dijk HW, Jiao C et al (2016) Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A 113:E2655–E2664. https://doi.org/10.1073/pnas.1522014113
Cogan DG, Toussaint D, Kuwabara T (1961) Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol 66:366–378. https://doi.org/10.1001/archopht.1961.00960010368014
Adamis AP, Altaweel M, Bressler NM et al (2006) Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology 113:23–28. https://doi.org/10.1016/j.ophtha.2005.10.012
Foltyn VN, Bendikov I, De Miranda J et al (2005) Serine racemase modulates intracellular D-serine levels through an alpha,beta-elimination activity. J Biol Chem 280:1754–1763. https://doi.org/10.1074/jbc.M405726200
Wolosker H, Blackshaw S, Snyder SH (1999) Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci U S A 96:13409–13414
Zhang H, Song L, Chang Y et al (2017) Potential deficit from decreased cerebellar granule cell migration in serine racemase-deficient mice is reversed by increased expression of GluN2B and elevated levels of NMDAR agonists. Mol Cell Neurosci 85:119–126. https://doi.org/10.1016/j.mcn.2017.09.005
Turpin FR, Potier B, Dulong JR et al (2009) Reduced serine racemase expression contributes to age-related deficits in hippocampal cognitive function. Neurobiol Aging 32:1495–1504
Huang AS, Lee DA, Blackshaw S (2008) D-Aspartate and D-aspartate oxidase show selective and developmentally dynamic localization in mouse retina. Exp Eye Res 86:704–709. https://doi.org/10.1016/j.exer.2008.01.015
Romero GE, Lockridge AD, Morgans CW, Bandyopadhyay D, Miller RF (2014) The postnatal development of D-serine in the retinas of two mouse strains, including a mutant mouse with a deficiency in D-amino acid oxidase and a serine racemase knockout mouse. ACS Chem Neurosci 5:848–854. https://doi.org/10.1021/cn5000106
Gustafson EC, Morgans CW, Tekmen M et al (2013) Retinal NMDA receptor function and expression are altered in a mouse lacking D-amino acid oxidase. J Neurophysiol 110:2718–2726. https://doi.org/10.1152/jn.00310.2013
Daniels BA, Wood L, Tremblay F, Baldridge WH (2012) Functional evidence for D-serine inhibition of non-N-methyl-D-aspartate ionotropic glutamate receptors in retinal neurons. Eur J Neurosci 35:56–65. https://doi.org/10.1111/j.1460-9568.2011.07925.x
Stevens ER, Esguerra M, Kim PM et al (2003) D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci U S A 100:6789–6794
Sullivan SJ, Esguerra M, Wickham RJ, Romero GE, Coyle JT, Miller RF (2011) Serine racemase deletion abolishes light-evoked NMDA receptor currents in retinal ganglion cells. J Physiol 589:5997–6006. https://doi.org/10.1113/jphysiol.2011.217059
Pollegioni L, Molla G, Sacchi S, Rosini E, Verga R, Pilone MS (2008) Properties and applications of microbial D-amino acid oxidases: current state and perspectives. Appl Microbiol Biotechnol 78:1–16. https://doi.org/10.1007/s00253-007-1282-4
D’Aniello A, D’Onofrio G, Pischetola M et al (1993) Biological role of D-amino acid oxidase and D-aspartate oxidase. Effects of D-amino acids. J Biol Chem 268:26941–26949
Gabler M, Hensel M, Fischer L (2000) Detection and substrate selectivity of new microbial D-amino acid oxidases. Enzym Microb Technol 27:605–611. https://doi.org/10.1016/S0141-0229(00)00262-3
Dun Y, Duplantier J, Roon P, Martin PM, Ganapathy V, Smith SB (2008) Serine racemase expression and D-serine content are developmentally regulated in neuronal ganglion cells of the retina. J Neurochem 104:970–978. https://doi.org/10.1111/j.1471-4159.2007.05015.x
Jiang H, Wu M, Liu Y et al (2017) Serine racemase deficiency attenuates choroidal neovascularization and reduces nitric oxide and VEGF levels by retinal pigment epithelial cells. J Neurochem 143:375–388. https://doi.org/10.1111/jnc.14214
Jiang H, Fang J, Wu B et al (2011) Overexpression of serine racemase in retina and overproduction of D-serine in eyes of streptozotocin-induced diabetic retinopathy. J Neuroinflammation 8:119. https://doi.org/10.1186/1742-2094-8-119
Jiang H, Du J, He T, Qu J, Song Z, Wu S (2014) Increased D-serine in the aqueous and vitreous humour in patients with proliferative diabetic retinopathy. Clin Exp Ophthalmol 42:841–845. https://doi.org/10.1111/ceo.12329
Jiang H, Du J, Song J et al (2018) Loss-of-function mutation of serine racemase attenuates retinal ganglion cell loss in diabetic mice. Exp Eye Res 175:90–97. https://doi.org/10.1016/j.exer.2018.06.017
Ozaki H, Inoue R, Matsushima T, Sasahara M, Hayashi A, Mori H (2018) Serine racemase deletion attenuates neurodegeneration and microvascular damage in diabetic retinopathy. PLoS One 13:e0190864. https://doi.org/10.1371/journal.pone.0190864
Zhang H, Kuang XL, Chang Y, Lu J, Jiang H, Wu S (2015) Reduced serine racemase expression in aging rat cerebellum is associated with oxidative DNA stress and hypermethylation in the promoter. Brain Res 1629:221–230. https://doi.org/10.1016/j.brainres.2015.10.034
Li LC, Dahiya R (2002) MethPrimer: designing primers for methylation PCRs. Bioinformatics 18:1427–1431. https://doi.org/10.1093/bioinformatics/18.11.1427
Jiang H, Wang X, Zhang H, Chang Y, Feng M, Wu S (2016) Loss-of-function mutation of serine racemase attenuates excitotoxicity by intravitreal injection of N-methyl-D-aspartate. J Neurochem 136:186–193. https://doi.org/10.1111/jnc.13400
Xu Q, Qaum T, Adamis AP (2001) Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci 42:789–794
Chou JC, Rollins SD, Fawzi AA (2013) Trypsin digest protocol to analyze the retinal vasculature of a mouse model. J Vis Exp 76:e50489
Guo C, Zhang Z, Zhang P et al (2013) Novel transgenic mouse models develop retinal changes associated with early diabetic retinopathy similar to those observed in rats with diabetes mellitus. Exp Eye Res 119:77–87
Luo D, Fan Y, Xu X (2012) The effects of aminoguanidine on retinopathy in STZ-induced diabetic rats. Bioorg Med Chem Lett 22:4386–4390. https://doi.org/10.1016/j.bmcl.2012.04.130
McVicar CM, Hamilton R, Colhoun LM et al (2011) Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes 60:2995–3005. https://doi.org/10.2337/db11-0026
Ibrahim AS, El-Remessy AB, Matragoon S et al (2011) Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes 60:1122–1133. https://doi.org/10.2337/db10-1160
Long L, Li Y, Yu S et al (2019) Scutellarin prevents angiogenesis in diabetic retinopathy by downregulating VEGF/ERK/FAK/Src pathway signaling. J Diabetes Res 2019:4875421. https://doi.org/10.1155/2019/4875421
Antonetti DA, Barber AJ, Bronson SK et al (2006) Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes 55:2401–2411. https://doi.org/10.2337/db05-1635
Kern TS (2007) Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007:95103. https://doi.org/10.1155/2007/95103
Bikbova G, Oshitari T, Baba T, Yamamoto S (2014) Neurotrophic factors for retinal ganglion cell neuropathy - with a special reference to diabetic neuropathy in the retina. Curr Diabetes Rev 10:166–176. https://doi.org/10.2174/1573399810666140508121927
Wu S, Barger SW (2004) Induction of serine racemase by inflammatory stimuli is dependent on AP-1. Ann N Y Acad Sci 1035:133–146
Wu SZ, Bodles AM, Porter MM, Griffin WS, Basile AS, Barger SW (2004) Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide. J Neuroinflammation 1:2. https://doi.org/10.1186/1742-2094-1-2
Inoue R, Hashimoto K, Harai T, Mori H (2008) NMDA- and beta-amyloid1-42-induced neurotoxicity is attenuated in serine racemase knock-out mice. J Neurosci 28:14486–14491. https://doi.org/10.1523/JNEUROSCI.5034-08.2008
Martineau M, Parpura V, Mothet JP (2014) Cell-type specific mechanisms of D-serine uptake and release in the brain. Front Synaptic Neurosci 6:12
Leal EC, Martins J, Voabil P et al (2010) Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes 59:2637–2645. https://doi.org/10.2337/db09-1421
Balcar VJ, Johnston GA (1972) The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. J Neurochem 19:2657–2666. https://doi.org/10.1111/j.1471-4159.1972.tb01325.x
Ishida AT, Fain GL (1981) D-aspartate potentiates the effects of L-glutamate on horizontal cells in goldfish retina. Proc Natl Acad Sci U S A 78:5890–5894
Santiago AR, Pereira TS, Garrido MJ, Cristovao AJ, Santos PF, Ambrosio AF (2006) High glucose and diabetes increase the release of [3H]-D-aspartate in retinal cell cultures and in rat retinas. Neurochem Int 48:453–458. https://doi.org/10.1016/j.neuint.2005.10.013
Sasabe J, Chiba T, Yamada M et al (2007) D-serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J 26:4149–4159. https://doi.org/10.1038/sj.emboj.7601840
Yoshihisa Y, Rehman MU, Nakagawa M et al (2018) Inflammatory cytokine-mediated induction of serine racemase in atopic dermatitis. J Cell Mol Med 22:3133–3138
Tang J, Kern TS (2011) Inflammation in diabetic retinopathy. Prog Retin Eye Res 30:343–358. https://doi.org/10.1016/j.preteyeres.2011.05.002
Acknowledgements
The authors appreciate the support of G. Shan, School of Life Science, University of Science and Technology in China, Hefei.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
The study is supported by the National Natural Science Foundation of China for Youth (81600755), by the Zhejiang Province Natural Science Foundation (LY19H090011 and LY18H1200030), by the Wenzhou Medical University (QTJ100001) and by the Scientific Funding of Wenzhou city (Y20180173 and Y20180083).
Author information
Authors and Affiliations
Contributions
HJ, HZ and XJ designed and conducted experiments, acquired, analysed, and interpreted the data, and revised the article critically for intellectual content. SW conceptualised and designed experiments, analysed and interpreted data and drafted the article. All authors approved of the published version. SW is responsible for the integrity of the work as a whole.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM
(PDF 279 kb)
Rights and permissions
About this article
Cite this article
Jiang, H., Zhang, H., Jiang, X. et al. Overexpression of d-amino acid oxidase prevents retinal neurovascular pathologies in diabetic rats. Diabetologia 64, 693–706 (2021). https://doi.org/10.1007/s00125-020-05333-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-020-05333-y