Abstract
Pulmonary hypertension (PH) is characterized by vascular remodeling predominantly driven by a phenotypic switching in pulmonary artery smooth muscle cells (PASMCs). However, the underlying mechanisms for this phenotypic alteration remain incompletely understood. Here, we identified that RNA methyltransferase METTL3 is significantly elevated in the lungs of hypoxic PH (HPH) mice and rats, as well as in the pulmonary arteries (PAs) of HPH rats. Targeted deletion of Mettl3 in smooth muscle cells exacerbated hemodynamic consequences of hypoxia-induced PH and accelerated pulmonary vascular remodeling in vivo. Additionally, the absence of METTL3 markedly induced phenotypic switching in PASMCs in vitro. Mechanistically, METTL3 depletion attenuated m6A modification and hindered the processing of pri-miR-143/145, leading to a downregulation of miR-143-3p and miR-145-5p. Inhibition of hnRNPA2B1, an m6A mediator involved in miRNA maturation, similarly resulted in a significant reduction of miR-143-3p and miR-145-5p. We demonstrated that miR-145-5p targets Krüppel-like factor 4 (KLF4) and miR-143-3p targets fascin actin-bundling protein 1 (FSCN1) in PASMCs. The decrease of miR-145-5p subsequently induced an upregulation of KLF4, which in turn suppressed miR-143/145 transcription, establishing a positive feedback circuit between KLF4 and miR-143/145. This regulatory circuit facilitates the persistent suppression of contractile marker genes, thereby sustaining PASMC phenotypic switch. Collectively, hypoxia-induced upregulation of METTL3, along with m6A mediated regulation of miR-143/145, might serve as a protective mechanism against phenotypic switch of PASMCs. Our results highlight a potential therapeutic strategy targeting m6A modified miR-143/145-KLF4 loop in the treatment of PH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary hypertension (PH) is a severe vascular lung disease characterized by vascular remodeling, predominantly attributed to abnormalities in pulmonary artery smooth muscle cells (PASMCs) [1]. Under typical physiological conditions, PASMCs exhibit a quiescent and differentiated phenotype, expressing contractile proteins such as α-SMA, SM22, Smoothelin and Calponin. However, in response to pathological stimuli like hypoxia and vascular injury, PASMCs undergo a phenotypic switching characterized by heightened proliferation, migration, and a decrease in contractile markers [2].
N6-methyladenosine (m6A) is a prevalent RNA modification implicated in various biological processes including mRNA splicing [3], stability [4], nuclear translocation [5], and translation initiation [6]. RNA m6A methylation encompasses a range of effector proteins: 'writers' like METTL3/14, Wtap, and KIAA1429; 'erasers' such as FTO and Alkbh5; and 'readers' including YTHDF1/2/3 and YTHDC1/2 [7]. RNA modification has been identified as a key player in the regulation of VSMCs-associated phenotypic alterations. For instance, METTL3 knockdown impedes the differentiation of adipose-derived stem cells (ADSCs) into VSMCs through regulating the secretion of specific paracrine factors [8]. Under the stimulation of indoxyl sulfate, METTL14 installs m6A on vascular osteogenic transcript Klotho, inducing its degradation and thereby promoting calcification of primary human artery smooth muscle cells (HASMCs) [9]. In PH samples and hypoxic PASMCs, elevated YTHDF1 accelerates PASMC proliferation, phenotype switch and PH progression by recognizing and boosting translational efficiency of m6A-modified MAGED1 [10]. Despite existing evidence on m6A involvement in VSMCs phenotype transformation, its role in mediating the phenotypic changes of PASMCs via microRNA remains inadequately explored.
microRNAs (miRNAs) are small, single-stranded, non-coding RNAs that negatively regulate gene expression [11]. Research highlights the pivotal role of miRNAs in the differentiation of vascular smooth muscle cells (VSMCs), especially miR-143/145 [12]. miR-143/145 are co-transcribed from a bicistronic transcript and are primarily expressed in VSMCs [13,14,15]. Their overexpression enhances the expression of VSMCs differentiation marker genes [15, 16]. Conversely, the absence of miR-143/145 results in a shift from a contractile to a synthetic phenotype in VSMCs [14, miRNA transfection The Chemically synthesized miRNA mimics or inhibitors for miR-143-3p, miR-145-5p and the corresponding negative control (miR-Con or anti-Con), were acquired from GenePharma (Shanghai, China). rPASMCs at 70% confluence on 10-cm culture dishes were transfected with 400 pmol of either miRNA mimic, mimic control (miR-Con), or miR-143/-145 inhibitors (anti-miR-143, anti-miR-145), inhibitor control (anti-Con) using TurboFect Transfection Reagent (Thermo Scientific). After of transfection, cells were harvested for further analysis at 48 h post-transfection. Total RNA of rPASMC infected with either shNC or shMETTL3 lentiviruses was subjected to methylated RNA Immunoprecipitation based qPCR (MeRIP-qPCR). Briefly, 10 μg of total RNA was incubated with 1 μg anti-m6A antibody (Synaptic Systems, Cat. No. 202 003) or a corresponding control IgG (ab172730, abcam) in 200 μL 1 × IP buffer at 4 °C for 2 h, followed by incubation of protein A/G magnetic beads (GE17152104010150, Merck, USA) at 4 °C for another 2 h. Immunoprecipitated RNA was eluted using Thermolabile Proteinase K (#P8111S, NEB) in reverse transcription buffer, first at 37 °C for 30 min and then at 55 °C for 10 min to inactivate the enzyme. The eluted RNA was directly subjected to RT and qPCR analysis. We also saved 0.5 μg of the same total RNA as input. The m6A enrichment in each sample was calculated by normalizing to input. The quantification of RNA methylation was conducted on purified RNA samples using the EpiQuik m6A RNA Methylation Quantification Kit (P-9005, EpiGentek), following the manufacturer's instructions. The data were analyzed using GraphPad Prism version 8.3.0 (GraphPad Software, Inc., San Diego, CA). All data are presented as mean value ± standard deviation (Mean ± SD). The differences between two groups were assessed using a two-tailed unpaired t test, while those among three or more groups were analyzed using one-way ANOVA followed by Tukey's multiple comparisons test. A P value less than 0.05 was considered statistically significant.MeRIP-qPCR
RNA methylation quantification
Statistical analysis
Results
METTL3 protein is upregulated in HPH animals
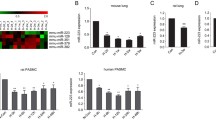
To reveal the role of RNA methylation in the progression of PH, we prepared hypoxic mouse models of PH (Fig. 1A, B), and assessed the expression of key enzymes associated with RNA methylation, including METTL3, METTL14, Wtap, FTO and Alkbh5. Among the three 'writer' proteins, METTL3 was the most significantly upregulated one in the lungs of hypoxic pulmonary hypertension (HPH) mice, while METTL14 and Wtap exhibited smaller increases in expression (Fig. 1C). Oppositely, the m6A 'eraser' protein, FTO, was significantly decreased in the HPH mouse lung, whereas Alkbh5 levels remain unchanged. Elevated levels of METTL3 protein were also observed in lung tissues and pulmonary arteries (PAs) of hypoxic PH rats (Fig. 1D, E), indicating the potential regulatory significance of METTL3 in PH progression. Consequently, we focused on METTL3 in following investigation.
METTL3 protein is upregulated during PH development. A, B Male C57BL/6 mice (6-week-old) were subjected to either normoxia or hypoxia for 3 weeks. The right ventricular systolic pressure (RVSP) (A) and right ventricular hypertrophy index (RVHI) (B) for both PH and control animals were assessed (n = 5). C The protein levels of METTL3, METTL14, Wtap, FTO and Alkbh5 in the lungs of PH and control animals were detected by western blotting (n = 3). D, E The protein levels of METTL3 in rat lung (rLung, D) and pulmonary artery (rPA, E) were also examined by western blotting (n = 3). β-actin was used as a loading control for western blotting. Nor: normoxia; Hyp: hypoxia. Data were analyzed by a two-tailed unpaired t test. Statistical significance is denoted by *P < 0.05, **P < 0.01 and ***P < 0.001. ns non-significance
Smooth muscle-specific knockout of Mettl3 aggravates hypoxia-induced PH in mouse
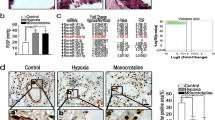
To elucidate the role of METTL3 in PH progression in vivo, we developed smooth muscle-specific Mettl3 knockout mice (Fig. 2A). The knockout mice of SMMHC-CreERT2;Mettl3fl/fl (Mettl3SMCKO) and the control mice of Mettl3fl/fl were subjected to either hypoxia (10% O2) or normoxia (21% O2) exposure for 3 weeks. Hemodynamic analysis revealed that right ventricular systolic pressure (RVSP) and right ventricular hypertrophy index (RVHI) in Mettl3fl/fl mice were considerably increased under hypoxia compared to those under normoxia (Fig. 2B, C). Moreover, hypoxic Mettl3SMCKO mice exhibited an even greater elevation in RVSP and RVHI than Mettl3fl/fl mice under hypoxia (Fig. 2B, C). This suggests that smooth muscle-specific knockout of Mettl3 exaggerates hypoxia-induced PH hemodynamic changes. In addition, histological analysis revealed a significant augmentation of pulmonary arterial wall thickness and remodeling in Mettl3SMCKO mice compared to Mettl3fl/fl mice (Fig. 2D–F). Western blotting analysis confirmed a significant reduction of METTL3 in the PAs of Mettl3SMCKO mice (Fig. S1), thereby validating the effective knockout of Mettl3. Immunostaining also confirmed the lack of METTL3 in the PAs of Mettl3SMCKO mice, associated with an increase in pulmonary arterial wall thickness, as indicated by anti-α-SMA staining (Figs. 2G, S2). Enhanced pulmonary vascular remodeling in Mettl3SMCKO mice under both normoxia and hypoxia was further confirmed by anti-Calponin immunostaining, which demonstrated pronounced co-localization with α-SMA (Fig. S2).
Smooth muscle-specific knockout of Mettl3 promotes the progression of PH. A Schematic illustrating conditional smooth muscle-specific Mettl3 loss-of-function mouse model. B, C The RVSP B was measured in mmHg by right heart catheterization, and the RVHI C was determined as the ratio of the weight of RV to the sum of LV plus ventricular septum (RV/(LV + S)) for knockout mice SMMHC-CreERT2;Mettl3fl/fl (Mettl3SMCKO) and the control mice Mettl3fl/fl (n = 6). D Representative images of hematoxylin and eosin (HE)-stained lung sections in both Mettl3SMCKO and Mettl3fl/fl mice. Scale bars, 100 μm. E, F The wall thickness of the PAs was calculated for 6 mice, with n = 120 per group. The relative wall thickness was given by (outer perimeter—inside perimeter)/outer perimeter (E), and the relative wall area by (outer area—inside area)/outer area (F). G Representative double-labeled immunostaining with antibodies against METTL3 and α-SMA in the PAs of SMMHC-CreERT2;Mettl3fl/fl (Mettl3SMCKO) and the control mice Mettl3fl/fl. Scale bars, 50 μm (merged) and 20 μm (magnified). H The mRNA levels of METTL3 and PCNA in mouse PAs were evaluated by qRT-PCR (n = 5). β-actin was used as an internal reference for qRT-PCR. I The m6A levels in total RNA from mouse PAs were analyzed using methylation quantification kit (P-9005, EpiGentek) (n = 6). Nor: normoxia; Hyp: hypoxia. Data were analyzed by using a one-way ANOVA followed by Tukey's multiple comparisons test. Statistical significance is denoted by * P < 0.05, ** P < 0.01 and *** P < 0.001
The qRT-PCR assay and m6A methylation quantification revealed a significant upregulation in METTL3 expression (Fig. 2H) and m6A level (Fig. 2I) in the PAs of Mettl3fl/fl mice under hypoxia compared to normoxia. Conversely, a significant reduction in METTL3 expression and m6A level was found in the PAs of Mettl3SMCKO mice relative to Mettl3fl/fl mice under both normoxia and hypoxia. Trace amounts of METTL3 were detected in the PAs of knockout mice, potentially originating from tissues such as pulmonary artery endothelial cells (PAECs) and pulmonary artery fibroblasts (PAFs) where METTL3 was not knocked out. Another possibility is the detection of truncated transcripts resulting from exon deletion (including exons 2 and 3, and potentially 4 through alternative splicing) (Figs. S3, S4; Table S3). Sequence analysis revealed that frameshift mutations in these truncated transcripts introduce premature termination codons, precluding the synthesis of functional METTL3 protein. Additionally, the qRT-PCR assay highlighted an elevated level of PCNA in the PAs of Mettl3SMCKO mice compared to Mettl3fl/fl mice under hypoxia (Fig. 2H). Furthermore, Ki67 expression significantly increased in both normoxic and hypoxic conditions following Mettl3 knockout, suggesting that METTL3 plays a broad regulatory role in cell proliferation mechanisms, extending beyond those induced by hypoxia alone (Fig. S5). Overall, our findings suggest that Mettl3 deletion might aggravate the development of PH in mice, possibly through altering cellular proliferative phenotype.
METTL3 deficiency drives phenotypic switching in PASMCs
To clarify the role of METTL3 in influencing PASMCs phenotype, lentivirus-mediated METTL3-specific shRNA was employed to silence METTL3 in rat PASMCs (rPASMCs). Three days after infection, METTL3 expression was dramatically repressed at both mRNA and protein levels in rPASMCs treated with shMETTL3 compared to the shNC group (Fig. 3A, B). Subsequent RNA sequencing (RNA-seq) was performed to analyze the transcriptional shifts in rPASMCs exposed to shMETTL3 versus the control group. Quantitative analysis revealed 1506 differentially expressed transcripts in METTL3-silenced rPASMCs [padj-value < 0.001; fold change (FC) ≥ 2], consisting of 656 up- and 850 down-regulated genes (Fig. S6A). Kyoto encyclopedia of genes and genomes (KEGG) analysis of these differentially expressed genes (DEGs) highlighted pathways impacted by METTL3 inhibition, including vascular smooth muscle contraction, cell adhesion, and calcium signaling (Fig. S6B).
Elimination of METTL3 induces a phenotypic switch in PASMCs from contractile to synthetic. A, B METTL3 expression levels were assessed in rPASMCs infected with shNC or shMETTL3 lentiviruses by qRT-PCR (A) and western blotting (B), respectively. The bar chart depicts the relative METTL3 protein level (n = 3). C A heatmap displays the expression of VSMCs markers in transcriptome sequencing (n = 3). D–H The RNA levels of α-SMA, SM22, Smoothelin, Calponin, PCNA and MMP2 in rPASMCs (D) and hPASMCs (G) infected with shNC or shMETTL3 were determined by qRT-PCR (n = 3). The protein levels of α-SMA, SM22, Smoothelin and Calponin in rPASMCs (E) and hPASMCs (H) infected with shNC or shMETTL3 were assessed by western blotting (n = 3). The protein levels of METTL3 in shNC or shMETTL3 hPASMCs were assessed (F) (n = 3). I The mRNA levels of SM22, α-SMA, Smoothelin and Calponin in mouse PAs were determined by qRT-PCR (n = 5). J Representative images of EdU labeling depicts the proliferation of rPASMCs upon inhibition and overexpression of METTL3. EdU-positive cells was quantified across 10 random fields, with DAPI staining highlighting all cells (n = 3). Scale bar represents 200 μm. The bar chart illustrates the proportion of EdU-positive cells. K Representative images from wound healing assay display the migration of rPASMCs following inhibition and overexpression of METTL3 (n = 3). Scale bar represents 1000 μm. Bar chart elucidates the changes in wound width at 72 h. β-actin was used as an internal reference for qRT-PCR and as a loading control for western blotting. Data were analyzed by a two-tailed unpaired t test, except the expression levels in PAs were analyzed by a one-way ANOVA followed by Tukey's multiple comparisons test. Statistical significance is denoted by * P < 0.05, ** P < 0.01 and *** P < 0.001
Among the DEGs, contractile marker genes such as α-SMA (Acta2), SM22 (Tagln), Smoothelin (Smtn) and Calponin (Cnn1) were significantly downregulated upon METTL3 inhibition (Fig. 3C). This downregulation was further confirmed by qRT-PCR and western blotting in rPASMCs (Fig. 3D, E) and human PASMCs (hPASMCs) (Fig. 3F–H). Conversely, overexpression of METTL3 (Fig. S7A) led to upregulation of α-SMA, SM22, Smoothelin and Calponin in rPASMCs (Fig. S7B) and hPASMCs (Fig. S7C). Similarly, a downregulation of SM22, α-SMA, Smoothelin and Calponin was observed in the PAs of Mettl3SMCKO mice relative to Mettl3fl/fl mice under hypoxia (Fig. 3I). The proliferative marker, PCNA, exhibited heightened levels after METTL3 silencing (Fig. 3D). Simultaneously, the migration marker MMP2 also upregulated following METTL3 inhibition (Fig. 3D) but downregulated with METTL3 overexpression (Fig. S7B-C). In addition, EdU incorporation and wound healing assay further demonstrated that METTL3 knockdown facilitated, whereas its overexpression reduced, the proliferation and migration of rPASMCs (Fig. 3J, K).
Collectively, these findings demonstrate that METTL3 deficiency led to a significant shift in PASMCs from a contractile to a synthetic phenotype, thereby potentiating PH progression.
Knockdown of METTL3 supresses miR-143/145 expression via m6A-dependent impairment of miRNA maturation
To explore the mechanism by which METTL3 mediates phenotypic transition of PASMCs, small RNA sequencing was conducted in rPASMCs treated with shNC or shMETTL3 lentiviruses. Inhibition of METTL3 resulted in a disordered miRNA expression profile, with a marked decrease of multiple miRNAs including miR-204-5p, miR-129-2-3p, miR-149-5p, miR-28-5p, miR-184, miR-425-5p, miR-145-5p, miR-23a-3p, miR-129-5p, miR-143-3p and miR-328a-3p (Fig. 4A, B). Among them, miR-143-3p and miR-145-5p have been identified as pivotal regulators of VSMCs phenotype [23]. Similarly, we verified that knockout of Mettl3 markedly reduced the levels of miR-143-3p and miR-145-5p in the PAs of Mettl3SMCKO mice compared with Mettl3fl/fl mice under both normoxia and hypoxia conditions (Fig. 4C). Furthermore, METTL3 overexpression resulted in elevated miR-143-3p and miR-145-5p levels in rPASMCs (Fig. S8A) and hPASMCs (Fig. S8B), suggesting that METTL3 plays a regulatory role in the expression of miR-143-3p and miR-145-5p.
Loss of METTL3 impairs miR-143/145 cluster processing in an m6A-dependent manner. A A heatmap displays differentially expressed miRNAs in rPASMCs following infection with shNC or shMETTL3 lentiviruses based on small RNA sequencing (n = 3). B Differentially expressed miRNAs in rPASMCs were validated by qRT-PCR (n = 3). C The expression levels of miR-143-3p and miR-145-5p were detected in the PAs of either Mettl3SMCKO or Mettl3fl/fl mice by qRT-PCR (n = 6). snoRNA202 was used as an internal reference in qRT-PCR for miRNA. D–I HEK293T cells infected with shNC or shMETTL3 lentiviruses were further transfected with pri-miR-143 or pri-miR-145 overexpression plasmids. The inhibition of METTL3 was verified by qRT-PCR (D, G). The pri-miR-143, miR-143-3p, miR-143-5p (E), pri-miR-145, miR-145-3p, and miR-145-5p (H) were detected by qRT-PCR (n = 3). The m6A enrichment of pri-miR-143 (F) or pri-miR-145 (I) was ascertained by MeRIP-qPCR (n = 3). J–L The shNC and shMETTL3 HEK293T cells were transfected with plasmids overexpressing either the wild-type or m6A-mutant versions (mut, A-to-T mutation) of human pri-miR-143 and pri-miR-145 (J), and the pri-miR-143, miR-143-3p (K), pri-miR-145, and miR-145-5p (L) were detected by qRT-PCR (n = 3). β-actin or snoRNA202 was used as an internal reference in qRT-PCR for pri-RNA or miRNA (B, C), respectively. Green fluorescent proteins (GFP) encoded by the pri-miRNA overexpression vector was used as an internal reference in qRT-PCR to evaluate pri-miRNA processing. A two-tailed unpaired t test (B–I), or one-way ANOVA followed by Tukey's multiple comparisons test (K, L), were used to estimate the significance. Statistical significance is denoted by * P < 0.05, ** P < 0.01 and *** P < 0.001. ns: non-significance
We subsequently explore how METTL3 regulates the expression of miR-143-3p and miR-145-5p. Previous studies have reported that METTL3 can modulate pri-miRNA processing through m6A modification [7). Our findings unmask a promising therapeutic approach via targeting m6A modified miR-143/145-KLF4 loop for PH treatment.
Within PASMCs, METTL3 depletion reduces m6A modification, subsequently diminishing miR-143/145 expression via impeding miRNA processing in an m6A-dependent manner. The reduction in mature miR-145-5p and miR-143-3p enhances their respective targets, KLF4 and FSCN1. Augmented KLF4 in turn represses the transcription of miR-143/145 cluster, establishing a positive feedback loop with miR-143/145. This perpetuating cycle suppresses contractile markers, facilitating the phenotypic switch in PASMCs and pulmonary vascular remodeling. Hypoxia-induced upregulation of METTL3 and consequent increase of miR-143/145 may serve as a compensatory and protective agent against the progression of PH
Discussion
Pulmonary hypertension (PH) is a progressive vascular lung disease characterized by vascular remodeling primarily attributed to phenotypic transformation of PASMCs [1, 34,35,36]. However, the underlying mechanisms of PASMCs dysregulation in PH are not fully understood.
Accumulating evidence suggests that RNA methylation plays a crucial role in PASMCs phenotypic switching. METTL14 mediated m6A methylation leads to mRNA decay of Grb-2-related adaptor protein (GRAP), thus promoting the proliferation and migration of hypoxic human PASMCs via Ras/ERK signaling pathway [37]. Similarly, YTHDF1 identified the m6A mark on Foxm1 mRNA, facilitating hypoxia-induced PASMCs proliferation and the expression of proliferative markers [38]. Conversely, deletion of YTHDF1 alleviated phenotype switch of PASMCs through recognizing m6A modified MAGED1 mRNA [10]. Our experiments confirmed that METTL3 was upregulated in lungs and PAs of PH animal. METTL3 depletion induced a marked phenotypic switch in PASMCs in vitro and enhanced pulmonary vascular remodeling in vivo. The deficiency of METTL3 significantly mitigated the expression of miR-143/145 cluster and elevated the levels of their target genes, FSCN1 and KLF4. The reduction of miR-143/145 upon METTL3 deficiency is due to the impediment of m6A-mediated miRNA processing. Consequently, our findings reveal the essential role of METTL3-mediated m6A modification on miRNA-143/145 in phenotypic switching of PASMCs and PH progression.
Many studies suggest that miR-143/145 play a key role in maintaining contractile activity of VSMCs [14,15,16,17]. In this study, miR-143/145 exhibit a pronounced elevation in hypoxic mouse PAs. Furthermore, miR-143/145 are regulated by METTL3 and play crucial roles in PASMCs under normoxic and hypoxic conditions. The introduction of miR-143/145 maintains the contractile phenotype of PASMCs. Additionally, these miRNAs regulate cell proliferation and migration, with miR-145-5p directly targeting KLF4, and miR-143-3p targeting FSCN1. These findings highlight the importance of miR-143-3p and miR-145-5p in PASMC function and their potential involvement in PH and vascular diseases.
Mechanistically, METTL3 deficiency decreases the level of m6A modification on pri-miR-143/145, impeding miR-143/145 maturation and leading to significant downregulation of mature miR-143/145. Multiple reports have described the effects of m6A on miRNA processing. For instance, METTL3 silencing inhibited m6A level on pri-miR-375, reducing miR-375-3p expression [39]. Peng et al. demonstrated that KIAA1429/ALKBH5-mediated m6A modifications control the processing of pri-miR-143-3p through interacting with the microprocessor protein DGCR8 [40]. In human bronchial epithelial 16HBE cells, METTL3 silencing led to reduced m6A modification and miR-143-3p expression, thus promoting airway epithelial cells epithelial mesenchymal transition (EMT) and increasing the production of extracellular matrix (ECM) in lung fibroblasts through targeting Smad3 [41]. We, therefore, conclude that METTL3 is capable of controlling the expression of a series of miRNAs including miR-143/145 cluster in an m6A-dependent manner, and that hypoxia-induced METTL3 upregulation, along with the increase of miR-143/145, could serve as a protective mechanism against PH progression under hypoxia.
Transcription factor KLF4 is instrumental in controlling VSMC phenotypic switching [12, 29, 32, 42, 43]. Through binding to the G/C repressive element and the transforming growth factor-β (TGF-β) control element (TCE), KLF4 significantly decreases contractile marker genes in VSMCs [31, 44]. In this study we unmask that genetic deletion and silencing of METTL3 significantly upregulate KLF4 in rPASMCs. Enhanced KLF4 represses contractile proteins, resulting in a remarkable shift of PASMCs phenotype. We verified that KLF4 can be directly targeted by miR-145-5p in rPASMCs, resembling findings in human embryonic stem cells [27]. Using a luciferase reporter assay, we demonstrate that KLF4 binds to the promoter of miR-143/145 cluster and represses its transcriptional activity, thereby establishing a positive feedback loop between KLF4 and miR-143/145. While KLF4 has been reported to inhibit miR-143 transcription by binding to its upstream motifs [45], to our knowledge, this study pioneered the identification of a feedback loop between KLF4 and miR-143/145 cluster. Similarly, miR-145 and its target, the transcription factor OCT4, form a double-negative feedback loop that modulates the transition between self-renewal and differentiation in human embryonic stem cells (hESCs) [27]. We therefore hypothesize that this regulatory circuit involving miR-143/145 and its transcription factor target could represent a fundamental mechanism governing cellular phenotype transformation.
Contrary to our findings, Qin et al. reported that increased METTL3 under hypoxia enhanced m6A-mediated degradation of PTEN, promoting PASMC proliferation and migration [46]. The discrepancy may arise from differences in experimental models and METTL3 targets. Our study used a SMC-specific Mettl3 knockout model to investigate METTL3 effects on PASMCs in vivo, while Qin's study used wild-type rats under hypoxia, reflecting outcomes involving various cell types such as PASMCs, PAECs, and PAFs. Additionally, we focused on the role of METTL3 in miR-143/145 expression, whereas Qin's study examined PTEN modulation, potentially resulting in different PASMC phenotypes. In line with our findings, Lin et al. reported that METTL3 inhibition in adipose-derived stem cells (ADSCs) suppressed the expression of VSMC-specific marker genes such as α-SMA, SM22, Calponin, and SMMHC [8], suggesting a protective role of METTL3 in preserving the differentiated phenotype of VSMCs. Collectively, these findings emphasize the context-dependent and target-specific effects of METTL3 in biological process and diseases like PH.
Consistent with our observations, Caruso et al. [47] and Deng et al. [48] have also reported elevated expression of miR-143 and miR-145 in hypoxic animal models of PH. However, their studies concluded that miR-143 and miR-145 have detrimental effects on PH progression, contrasting to our findings. This divergence may be attributable to variations in experimental models and techniques, the involvement of different cell types in PH, and the specific downstream targets and signaling pathways modulated by miR-143/145. Our investigation employed the SMC-specific Mettl3 knockout approach to specifically elucidate the roles of miR-143 and miR-145 in the smooth muscle layer in PH. In contrast, Caruso and Deng utilized global knockout models and administered anti-miRs subcutaneously in animals. Consequently, their studies may reflect the combined effects of miR-143 and miR-145 on various cell types involved in PH, including PASMCs, PAECs and PAFs. Additionally, our results suggest that miR-145 and miR-143 influence PH development through their impact on KLF4 and FSCN1, respectively. In contrast, Caruso's study implicated the Wnt/β-catenin signaling pathway in the action of miR-145, whereas Deng's study did not identify a specific target for miR-143. The variability in downstream targets and pathways modulated by miR-143 and miR-145 could result in differing outcomes regarding their impact on PH. Similar inconsistency in miR-143/145 functionality is also observed in atherosclerosis research. Overexpression of miR-145 exacerbates inflammation in cell and animal models of atherosclerosis through NF-κB pathway activation, suggesting a detrimental impact on the disease [49, 50]. However, miR-145 expression is reduced in human atherosclerotic aortas, associated with decreased SMC contractile markers such as Calponin, α-SMA, and myocardin, implying that loss of miR-145 may exacerbate atherosclerosis [51]. Taken together, these discrepancies underscore the complexity and dynamic nature of miRNA regulation in cardiovascular diseases. A comprehensive understanding of the context-dependent effects, downstream regulatory pathways, epigenetic and post-genomic modulation of miR-143/145 in SMC proliferation, arterial remodeling, and inflammation is essential to unravel the intricate molecular mechanisms underlying PH and other vascular diseases [52].
Claudio et al. reported that hnRNPA2B1 functions as an m6A reader in pri-miRNAs, enhancing their maturation into mature miRNAs [26]. However, Wu et al. later revealed through crystal structure analysis of hnRNPA2B1 with various RNA substrates that m6A likely increases the accessibility of hnRNPA2B1 to pri-miRNAs rather than directly binding to it, thereby supporting pri-miRNAs maturation [53]. Our research corroborates the vital role of hnRNPA2B1 as an m6A mediator in miRNA processing, demonstrating that its deficiency leads to impaired maturation of pri-miR-143/145.
In this study, we use whole lung tissue instead of isolated PASMCs for assessing Mettl3 knockout in mRNA transcripts, as depicted in Figure S4B. This approach was chosen due to the challenges associated with isolating PASMCs from small mouse PAs. Future research incorporating isolated PASMCs from Mettl3 knockout mice will provide clearer insights and reduce confounding influences from other cell types, as recommended by established protocols [54]. Additionally, we conducted transcriptome analysis and qRT-PCR assays to identify potential targets of miR-143/145, such as KLF4 and FSCN1, in METTL3-silenced rPASMCs and Mettl3SMCKO mice under hypoxic conditions. While these approaches have provided valuable insights, we recognize that proteomic methods, including mass spectrometry and advanced protein arrays, would offer a more direct assessment of the impact of miR-143/145 on protein expression and could reveal additional targets that may be overlooked in transcriptomic analyses. It is worth noting that proteomic analysis might also have limitations in detecting proteins with low expression abundance. Future studies could benefit from integrating proteomic approaches with transcriptomic analyses to obtain a comprehensive understanding of the molecular mechanisms through which miR-143/145 influence PASMC phenotype.
Conclusion
This study unveils a previously unidentified m6A-regulated miR-143/145-KLF4 positive feedback circuit essential for determining the PASMCs phenotype. Our insights highlight a potential therapeutic avenue by targeting the m6A-modification pathway linked to miR-143/145 cluster for PH management.
Availability of data and materials
NGS data have been deposited in NCBI Sequence Read Archive (SRA) and are available through SRA accession number PRJNA1018125.
Abbreviations
- m6A:
-
N6-methyladenosine
- FSCN1:
-
Fascin actin-bundling protein 1
- HPH:
-
Hypoxic pulmonary hypertension
- KLF4:
-
Krüppel-like factor 4
- LV:
-
Left ventricle
- miRNAs:
-
MicroRNAs
- PA:
-
Pulmonary artery
- PAECs:
-
Pulmonary artery endothelial cells
- PAFs:
-
Pulmonary artery fibroblasts
- PASMCs:
-
Pulmonary artery smooth muscle cells
- PH:
-
Pulmonary hypertension
- RV:
-
Right ventricle
- RVHI:
-
Right ventricular hypertrophy index
- RVSP:
-
Right ventricular systolic pressure
- VSMCs:
-
Vascular smooth muscle cells
- MeRIP:
-
Methylated RNA Immunoprecipitation
References
Gong J et al (2019) Long non-coding RNA CASC2 suppresses pulmonary artery smooth muscle cell proliferation and phenotypic switch in hypoxia-induced pulmonary hypertension. Respir Res 20:53
Ma B et al (2023) Pulmonary artery smooth muscle cell phenotypic switching: a key event in the early stage of pulmonary artery hypertension. Drug Discov Today 28:103559
Cowling VH (2009) Regulation of mRNA cap methylation. Biochem J 425:295–302
Boo SH, Kim YK (2020) The emerging role of RNA modifications in the regulation of mRNA stability. Exp Mol Med 52:400–408
Shen EC et al (1998) Arginine methylation facilitates the nuclear export of hnRNP proteins. Genes Dev 12:679–691
Zaccara S, Ries RJ, Jaffrey SR (2019) Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol 20:608–624
Shi H, Wei J, He C (2019) Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell 74:640–650
Lin J et al (2020) Hypoxia promotes vascular smooth muscle Cell (VSMC) differentiation of adipose-derived stem cell (ADSC) by regulating Mettl3 and paracrine factors. Stem Cells Int 2020:2830565
Chen J et al (2019) METTL14-dependent m6A regulates vascular calcification induced by indoxyl sulfate. Life Sci 239:117034
Hu L et al (2021) YTHDF1 regulates pulmonary hypertension through translational control of MAGED1. Am J Resp Crit Care 203:1158–1172
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Yap C et al (2021) Six shades of vascular smooth muscle cells illuminated by KLF4 (Kruppel-Like Factor 4). Arterioscler Thromb Vasc Biol 41:2693–2707
Weber C, Schober A, Zernecke A (2010) MicroRNAs in arterial remodelling, inflammation and atherosclerosis. Curr Drug Targets 11:950–956
Boettger T et al (2009) Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest 119:2634–2647
Cordes KR et al (2009) miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460:705–710
Cheng Y et al (2009) MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res 105:158–166
**n M et al (2009) MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev 23:2166–2178
Vacante F et al (2019) The function of miR-143, miR-145 and the MiR-143 host gene in cardiovascular development and disease. Vascul Pharmacol 112:24–30
Deng L et al (2022) LncPTSR triggers vascular remodeling in pulmonary hypertension by regulating [Ca(2+)](i) in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol 66:524–538
Niu YQ et al (2015) An improved method for detecting circulating microRNAs with S-Poly(T) Plus real-time PCR. Scientific Rep 5:15100
Kang K et al (2012) A novel real-time PCR assay of microRNAs using S-Poly(T), a specific Oligo(dT) reverse transcription primer with excellent sensitivity and specificity. PLoS ONE 7:e48536-48545
Kang K et al (2019) An improved Tet-on system in microRNA overexpression and CRISPR/Cas9-mediated gene editing. J Anim Sci Biotechnol 10:43–54
Rangrez AY et al (2011) miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet 4:197–205
**ao L et al (2020) METTL3 promotes IL-1beta-induced degeneration of endplate chondrocytes by driving m6A-dependent maturation of miR-126-5p. J Cell Mol Med 24:14013–14025
Pan X et al (2021) METTL3 promotes adriamycin resistance in MCF-7 breast cancer cells by accelerating pri-microRNA-221-3p maturation in a m6A-dependent manner. Exp Mol Med 53:91–102
Alarcon CR et al (2015) HNRNPA2B1 Is a mediator of m(6)A-dependent nuclear RNA processing events. Cell 162:1299–1308
Xu N et al (2009) MicroRNA-145 regulates OCT4, SOX2, and KLF2 and represses pluripotency in human embryonic stem cells. Cell 137:647–658
Liu R et al (2012) The cluster of miR-143 and miR-145 affects the risk for esophageal squamous cell carcinoma through co-regulating fascin homolog 1. PLoS ONE 7:e33987
Shankman LS et al (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21:628–637
Deaton RA, Gan Q, Owens GK (2009) Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol 296:H1027-1037
Kawai-Kowase K, Owens GK (2007) Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am J Physiol Cell Physiol 292:C59-69
Liu Y et al (2005) Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem 280:9719–9727
Yoshida T, Gan Q, Owens GK (2008) Kruppel-like factor 4, Elk-1, and histone deacetylases cooperatively suppress smooth muscle cell differentiation markers in response to oxidized phospholipids. Am J Physiol Cell Physiol 295:C1175-1182
Lechartier B et al (2022) Phenotypic diversity of vascular smooth muscle cells in pulmonary arterial hypertension. Chest 161:219–231
Chakraborty R et al (2021) Targeting smooth muscle cell phenotypic switching in vascular disease. JVS Vascular Sci 2:79–94
Wang G et al (2015) Origin and differentiation of vascular smooth muscle cells. J Physiol 593:3013–3030
Liu P et al (2022) m(6)A Modification-mediated GRAP regulates vascular remodeling in hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol 67:574–588
Kang T et al (2023) Inhibition of YTHDF1 prevents hypoxia-induced pulmonary artery smooth muscle cell proliferation by regulating Foxm1 translation in an m6A-dependent manner. Exp Cell Res 424:113505
Chen JQ et al (2023) Silencing METTL3 stabilizes atherosclerotic plaques by regulating the phenotypic transformation of vascular smooth muscle cells via the miR-375-3p/PDK1 axis. Cardiovasc Drug Ther 37:471–486
Wang P et al (2021) KIAA1429 and ALKBH5 oppositely influence aortic dissection progression via regulating the maturation of Pri-miR-143-3p in an m6A-dependent manner. Front Cell Develop Biol 9:668377
Wang J et al (2023) m(6)A-modified miR-143–3p inhibits epithelial mesenchymal transition in bronchial epithelial cells and extracellular matrix production in lung fibroblasts by targeting Smad3. Pulm Pharmacol Ther 83:102251
Wang C et al (2008) Kruppel-like factor 4 is required for the expression of vascular smooth muscle cell differentiation marker genes induced by all-trans retinoic acid. J Biochem 144:313–321
Bulut GB et al (2021) KLF4 (Kruppel-Like Factor 4)-dependent perivascular plasticity contributes to adipose tissue inflammation. Arterioscler Thromb Vasc Biol 41:284–301
Zheng B, Han M, Wen JK (2010) Role of Kruppel-like factor 4 in phenotypic switching and proliferation of vascular smooth muscle cells. IUBMB Life 62:132–139
Liu H et al (2013) miR-145 and miR-143 regulate odontoblast differentiation through targeting Klf4 and Osx genes in a feedback loop. J Biol Chem 288:9261–9271
Qin YH et al (2021) The m(6)A methyltransferase METTL3 promotes hypoxic pulmonary arterial hypertension. Life Sci 274:119366
Caruso P et al (2012) A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res 111:290–300
Deng L et al (2015) MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res 117:870–883
Sheng L et al (2018) Microrna-145 accelerates the inflammatory reaction through activation of NF-κB signaling in atherosclerosis cells and mice. Biomed Pharmacother 103:851–857
Riches-Suman K (2021) Diverse roles of microRNA-145 in regulating smooth muscle (dys)function in health and disease. Biochem Soc Trans 49:353–363
Zhang YN et al (2016) Phenotypic switching of vascular smooth muscle cells in the “normal region” of aorta from atherosclerosis patients is regulated by miR-145. J Cell Mol Med 20:1049–1061
Danckwardt S, Trégouët DA, Castoldi E (2023) Post-transcriptional control of haemostatic genes: mechanisms and emerging therapeutic concepts in thrombo-inflammatory disorders. Cardiovasc Res 119:1624–1640
Wu BX et al (2018) Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun 9:420
Lee KJ et al (2013) Isolation of pulmonary artery smooth muscle cells from neonatal mice. J Vis Exp. https://doi.org/10.3791/50889
Acknowledgements
We would like to thank the editors and reviewers for their constructive comments. Also, we thank the Instrument Analysis Center of Shenzhen University and the Public service platform for large-scale instruments and equipment of the College of Life Sciences and Oceanography for their assistance in instruments and equipment.
Funding
This work was supported by National Natural Science Foundation of China (82270054, 82370065, 82170070, 82241022, 82200067, 91739109, 81970053, 82300076 and 89202586); Joint project of basic research and applied basic research in Yunnan Province (202201AY070001-224); Key Basic Research Projects of Shenzhen (JCYJ20210324120206017); Shenzhen-Hong Kong Jointly Funded Project (SGDX20201103095404019); Yunnan Provincial People's Hospital Cooperation Project (202201AY070001-224); Open Project of Respiratory Disease Clinical Medical Center of Yunnan Province (2022LCZXKF-HX03 and 2022LCZXKF-HX04); Shenzhen stable support for general projects (8940317–0109); Shenzhen Medical Research Fund [B2302015]; Medicine Plus Program of Shenzhen University [2024YG001] and Guangdong Provincial Key Laboratory of Regional Immunity and Diseases [2019B030301009].
Author information
Authors and Affiliations
Contributions
D.G. and K.K. designed the research; C.S., H.L., **aojia L., J.D., S.C., L.Z., J.Chen, **nyi L., J.Cheng, **aoyun L., M.L., X.Z., C.Z., S.Liu., C.Liang, J.X., J.Z., S.Liang and H.T. carried out experiments; J.W. and J. K. analyzed data; Y.N., C.Liu, J.Z., S.Liang and H.T. interpreted results of experiments; C.S., M.L. prepared figures; K.K., C.S. drafted manuscript; D.G. approved final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical statement
All experiments were carried out in accordance with China Council on Animal Care and Basel Declaration, and the animal procedures were approved by the Animal Care and Use Committee of Shenzhen University, China (Ethical number: 2021003; Date: 2021/05/17).
Consent for publication
All authors read and approved the submission and final Apublication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, K., Sun, C., Li, H. et al. N6-methyladenosine-driven miR-143/145-KLF4 circuit orchestrates the phenotypic switch of pulmonary artery smooth muscle cells. Cell. Mol. Life Sci. 81, 256 (2024). https://doi.org/10.1007/s00018-024-05304-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05304-1