Abstract
Prolonged stimulation of β-adrenergic receptor (β-AR) can lead to sympathetic overactivity that causes pathologic cardiac hypertrophy and fibrosis, ultimately resulting in heart failure. Recent studies suggest that abnormal protein ubiquitylation may contribute to the pathogenesis of cardiac hypertrophy and remodeling. In this study, we demonstrated that deficiency of a deubiquitinase, Josephin domain-containing protein 2 (JOSD2), ameliorated isoprenaline (ISO)- and myocardial infarction (MI)-induced cardiac hypertrophy, fibrosis, and dysfunction both in vitro and in vivo. Conversely, JOSD2 overexpression aggravated ISO-induced cardiac pathology. Through comprehensive mass spectrometry analysis, we identified that JOSD2 interacts with Calcium–calmodulin-dependent protein kinase II (CaMKIIδ). JOSD2 directly hydrolyzes the K63-linked polyubiquitin chains on CaMKIIδ, thereby increasing the phosphorylation of CaMKIIδ and resulting in calcium mishandling, hypertrophy, and fibrosis in cardiomyocytes. In vivo experiments showed that the cardiac remodeling induced by JOSD2 overexpression could be reversed by the CaMKIIδ inhibitor KN-93. In conclusion, our study highlights the role of JOSD2 in mediating ISO-induced cardiac remodeling through the regulation of CaMKIIδ ubiquitination, and suggests its potential as a therapeutic target for combating the disease.
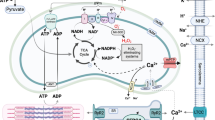
Graphical abstract

JOSD2 binds to the CaMKIIδ and deubiquitinates CaMKIIδ, thereby leading to CaMKIIδ phosphorylation, association with calcium homeostasis disorder and disrupting the function of myosin and actin, during the pathogenesis of ISO-induced heart hypertrophy, remodeling and dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial hypertrophy is an adaptive response to pathophysiological stressors that initially aids in maintaining cardiac output by augmenting myocardial contractility and wall thickness[1]. Under physiological conditions, such as during pregnancy or exercise, controlled sympathetic nerve stimulation is beneficial for cardiac function [2]. However, under pathological conditions, such as hypertension, atherosclerosis, and myocardial infarction, prolonged sympathetic nerve activity can lead to an overload of catecholamine or epinephrine secretion [3, 4]. Over-activation of the β-adrenergic receptor (β-AR) contributes to pathological fibrosis and disrupts myocardial structure and function, leading to heart failure [5]. Isoprenaline (ISO), a non-selective β-AR agonist, activates adenylate cyclase, producing cyclic adenosine monophosphate (cAMP), and the activation of the protein A (PKA) pathway. This pathway regulates phospholamban (PLB), sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA), and ryanodine receptor (RyR), thus influencing calcium ion[6] levels and ultimately contributing to heart failure. Although β-blockers remain a primary component of current heart failure therapy, they still have several limitations [7]. Therefore, understanding the mechanisms underlying chronic sympathetic nerve stimulation-induced heart failure and identifying new potential therapeutic targets is critical to address this urgent medical need.
Ubiquitination is a reversible protein post-translational modification controlled by ubiquitin ligases and deubiquitinases (DUBs). DUBs play a pivotal role in regulating protein stability and function, which are involved in many biological processes, contributing to the onset and progression of various diseases [8, 9]. Some DUBs, such as USP4, USP18, USP14, USP4, CYLD, and A20, have been reported to play a key role in cardiovascular diseases [10]. In recent years, UCHL1 has been reported to regulate cardiac hypertrophy [24]. However, the role of JOSD2 in the pathogenesis of cardiovascular diseases remains unclear.
In this study, we observed that JOSD2 was upregulated in mouse models of ISO- and myocardial infarction (MI)-induced cardiac injury. Intriguingly, JOSD2 deficiency was found to ameliorate ISO- and MI-induced myocardial hypertrophy, fibrosis, and cardiac dysfunction. In contrast, restoration of JOSD2 expression significantly exacerbated pathological cardiac dysfunction. Mechanistically, JOSD2 directly removed the K63-linked polyubiquitin chains on Calcium–calmodulin-dependent protein kinase IIδ (CaMKIIδ) to increase CaMKIIδ phosphorylation and then cause calcium mishandling in cardiomyocytes. Our findings demonstrate JOSD2 as a novel regulator of heart failure induced by chronic sympathetic nerve stimulation.
Materials and methods
Reagents
DAPI Flouromoust-G (DAPI; Cat# 36308ES20) was purchased from Yeasen Biotechnology Co., Ltd. (Shanghai, China). Lipofectamine 2000 (Cat# 11668019) and Lipofectamine 3000(Cat# L3000015) were purchased from Thermo Fisher (Waltham, MA, USA). Isoprenaline (Cat# HY-B0468) and KN-93 (Cat# HY-15465B) were purchased from Med Chem Express (MCE.NJ, USA). Antibodies against GAPDH (Cat# sc-365062) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies against α-actinin (Cat# ab9465), Vimentin (Cat# ab8978), goat anti-rabbit IgG H&L (Alexa Fluor 594; Cat# ab150080), and goat anti-mouse IgG H&L (Alexa Fluor 488; Cat# ab150117) were purchased from Abcam (Cambridge, MA). Antibodies against Calcium–calmodulin-dependent protein kinase II delta-Specific (CaMKIIδ; Cat# 20667-1-AP), Flag tag (Flag; Cat# 20543-1-AP), GFP tag (GFP; Cat# 66002-1-AP), beta-Myosin heavy chain (β-MyHC; Cat# 22280-1-AP), Collagen type I (COL-1; Cat# 14695-1-AP), transforming growth factor-beta1 (TGFβ-1; Cat# 21898-1-AP), and atrial natriuretic peptide (ANP; Cat# 27426-1-AP) were purchased from Proteintech (Wuhan, China). Phospho-CaMKII (Cat# 12716 s), horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Cat# 7076), and goat anti-rabbit IgG (Cat# 7074) were purchased from Cell Signaling Technology (CST, Danvers, MA, USA). The antibody against JOSD2 (orb 184482) was purchased from Biorbyt (Cambridge, UK). Small interfering RNA against JOSD2 and scrambled sequences were purchased from Genepharma (Shanghai, China). Flag-JOSD2, GFP-CaMKIIδ, Myc-Ub, Myc-K48, and Myc-K63 plasmids were obtained from Genechem (Shanghai, China).
Animal experiments
The whole-body JOSD2 knockout (JOSD2−/−) mice on the C57BL/6 J background were provided by Prof. Fu** You (Peking University). In the present study, heterozygous JOSD2 mice were bred to generate homozygous JOSD2 KO and wild-type (WT) littermate animals. The mice used in the experiments were littermates. JOSD2−/− mice and wild-type C57BL/6 J mice were maintained in an SPF environment with a twelve-hour light–dark cycle and ad libitum access to food and water. The mouse feeding and experimental procedures were approved by the Ethics Examination and Approval Committee of the Animal Experimental Center of The First Affiliated Hospital of Wenzhou Medical University (wydw-2021–347).
Two mouse models of heart failure, including ISO- and MI-induced cardiac dysfunction, were used. (1) ISO was dissolved in 0.002% ascorbic acid (Cat# HY-N6958, MedChemExpress, State of New Jersey, America) and administered via osmotic mini-pumps (Alzet MODEL 1002, Calif) at a dose of 30 mg/kg/day for 2 weeks, as previously described [25]. (2) Mice underwent a surgical procedure in which the left anterior descending (LAD) artery was ligated to induce myocardial infarction. All animal experiments were conducted by blinded experimenters, and randomization was used when assigning the groups. At the end of the study, mice were euthanized by dissecting the diaphragm under isoflurane (2%-3%) anesthesia, after which blood and heart tissues were collected for analysis.
For JOSD2 deletion in the ISO Model, eight-week-old C57BL/6 mice and JOSD2−/− mice were randomly divided into four groups: untreated C57BL/6 mice received osmotic pumps delivering 0.002% ascorbic acid (WT-Sham; n = 6); ISO-infused C57BL/6 mice (WT-ISO; n = 6); untreated JOSD2−/− mice received osmotic pumps delivering 0.002% ascorbic acid (JOSD2−/−-Sham; n = 6); and ISO-infused JOSD2−/− mice (JOSD2−/−-ISO; n = 6).
For JOSD2 overexpression in the ISO model, the mouse JOSD2 gene was cloned into AAV-9 by Genechem Co. LTD (Shanghai, China). WT mice were infected with adeno-associated virus serotype 9 (AAV9) encoding JOSD2 (AAV-JOSD2, 0.73 × 1013 particles/mouse; n = 6) or an empty vector (AAV-NC, 0.73 × 1013 particles/mouse; n = 6) through the tail vein. Four weeks post-injection, mice were infused with 0.002% ascorbic acid or ISO (AAV-NC + ISO; n = 6) (AAV-JOSD2 + ISO; n = 6) in the same way. During the ISO-infused period, KN-93 (a potent and selective inhibitor of CaMKII) was administered at 5 mg/kg/day (AAV9-JOSD2 + ISO + KN-93; n = 6).
For JOSD2 deletion in the MI Model, eight-week-old mice were randomly divided into four groups. C57BL/6 mice (WT-MI; n = 12) and JOSD2−/− mice (JOSD2−/−-MI; n = 12) were anesthetized by spontaneous inhalation and maintained under general anesthesia with 1–2% isoflurane while being mechanically ventilated via a rodent ventilator (HX-100E, Chengdu Technology & Market CO, LTD) through a tracheal cannula. The chest area of the mice was prepared by removing hair. A thoracotomy device was placed to prop the thoracic rib, and the heart was exposed through the left fourth intercostal space. The LAD was ligated with an 8–0 unabsorbable suture 1 mm from the apex of the normally positioned left auricle. Samples were analyzed 7 days after injury. The control mice underwent a sham operation (WT-Sham; n = 12) (JOSD2−/−-Sham; n = 12).
Cell culture and transfection
HEK-293 T cells (Cat# SCSP-502) were obtained from the Stem Cell Bank of the Chinese Academy of Sciences (Shanghai, China). HEK-293 T cells were cultured in DMEM medium (Cat# C11995500BT, Gibco, Bei**g, China) containing 4.5 g/L glucose, 10% fetal bovine serum (Cat# BC-SE-FBS07, Nan**g SenBeiJia Biological Technology Co., Ltd), and 1% penicillin/streptomycin (Cat# BC-CE-007 Nan**g SenBeiJia Biological Technology Co., Ltd). Cells were transfected with siRNA (siJOSD2: CTACTATAATCTGGACTCA) or expression plasmids using Lipofectamine 2000 or Lipofectamine 3000 following the manufacturer’s protocols.
Neonatal rat ventricular myocytes (NRVMs) were isolated following a published protocol[26]. Briefly, neonatal rats were sterilized and sacrificed. Heart tissues were removed, washed in PBS, and digested using a mixture of pancreatin (Cat# T8150, Solarbio, Bei**g, China) and collagenase (Cat# BY06021, Shanghai boyun biotech co., Ltd, Shanghai, China). Fibroblasts were removed by differential adherence culture for 1 h. NVRMs were cultured in DMEM medium containing 4.5 g/L glucose and 10% fetal bovine serum. After 48 h of culture, NVRMs exhibited regular pulsation and were used for experiments.
Primary mouse peritoneal macrophages (MPMs) were prepared from C57BL/6 wild-type mice. Mice were administered 1 ml of 4% thioglycolate (Cat# 70157, Sigma-Aldrich, Germany) in PBS intraperitoneally. Three days later, peritoneal cells were collected and incubated in RPMI-1640 medium (Cat# C11875500BT, Gibco; Eggenstein, Germany) supplemented with 10% fetal bovine serum at 37 °C for 6 h. The cells were then washed with PBS to remove non-adherent cells. The remaining adherent cells were used as the peritoneal macrophages described in the experiments.
Real-time quantitative PCR
Total RNA was isolated from cells and heart tissues using RNAiso Plus (Cat# 9109, TAKARA, Tokyo, Japan), followed by reverse-transcription to cDNA using cDNA Synthesis Super Mix (Cat# 11141ES60, Yeasen Biotechnology Co., Ltd. Shanghai, China). Quantitative polymerase chain reaction was performed using SYBR Green Master Mix (Cat# 11202ES08). The relative expression of target genes was normalized to Actb and analyzed using the 2−ΔΔCT method. The primer sequences are also listed in Supplementary Table S1.
Western blot and immunoprecipitation
Total proteins were extracted from myocardial tissues and NRVMS using RIPA buffer (Cat# P0013B, Beyotime, Shanghai, China) and quantified by the Bradford assay (Cat# 5000205, Bio-Rad Laboratories, Hercules, CA). After denaturation with 5X SDS loading buffer and boiling, the isolated proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The samples were transferred to a PVDF membrane which was blocked with 5% skim milk for one hour at room temperature. Subsequently, the membrane was incubated with primary antibodies overnight at 4℃. After washing three times with TBST, the membrane was incubated with the corresponding secondary antibody for 1 h at room temperature. Following three additional washes with TBST, images were obtained using ECL (Cat# P10300, NCM Biotech, Suzhou, China). The gray density of the bands was quantified using Image J analysis software.
Protein complexes were evaluated by co-immunoprecipitation (co-IP). Cell lysates from animal tissues and NRVMS were incubated with the target antibody overnight at 4 °C. Samples were immunoprecipitated with protein A + G Agarose beads (Cat# P2055-2 ml, beyotime, Shanghai, China) and shaken at room temperature for 4 h. Protein-bead mixtures were washed three times with PBS and used for subsequent western blot experiments.
Immunofluorescence staining
Frozen sections were used for staining with α-actinin, Vimentin, and JOSD2. The sections were fixed in cold methanol and permeabilized using 0.25% Triton X-100(Cat# P1080). Sections and cells were then blocked with 5% Bovine serum albumin (BSA, Cat# A8020) for 30 min, incubated with the primary antibody overnight at 4 °C. After washing, the sections were incubated with fluorescent-labeled secondary antibodies for 1 h and counterstained with DAPI Flouromoust-G for 10 min. The stained sections were observed using a fluorescence microscope (Nikon, Japan).
Histological analysis
The hearts were fixed in 4% paraformaldehyde (Cat# BL539A, biosharp, China) and embedded in paraffin. 5-μm thick sections were stained with hematoxylin and eosin (H&E) (Cat# G1120, Solarbio Life Sciences, Bei**g, China), Sirius Red (Cat# G1472, Solarbio Life Science, Bei**g, China), and Masson’s Trichrome (Cat# G1340, Solarbio Life Sciences, Bei**g, China) for routine histology and assessment of cardiac fibrosis. The stained images were observed using a light microscope (Nikon, Japan). 5 μm thick frozen sections were washed with PSB three times and then incubated with 5 μg/ml FITC-conjugated wheat germ agglutinin (WGA-FITC, Cat# GTX01502) staining.
Phalloidin-TRITC staining
NRVMS were seeded in glass-bottom dishes, washed twice with sterile PBS at 37℃, and then fixed with a 4% polyformaldehyde solution at room temperature for ten minutes. After removing the excess polyformaldehyde with PBS, the cells were permeabilized with a 0.5% Triton X-100 solution for five minutes. Subsequently, the NRVMS were incubated with a TRITC-labeled Phalloidin working solution (CA1610, Bei**g Solarbio Science & Technology Co., Ltd, Bei**g, China) at room temperature in the dark for thirty minutes. Finally, the nuclei were stained with DAPI Flouromoust-G, followed by fluorescence observation under a fluorescence microscope and photography.
Enzyme-linked immunosorbent assay
The cTnT levels in the serum were measured using ELISA kits (Cat# H149-4, Jiancheng, Nan**g, China). The ANP level in the serum was measured using ELISA kits (Cat# H180-96 T, Westang Bio-tech, Shanghai, China). The protein amounts were calculated using the standard curve method. All experiments followed the instructions provided in the protocol.
5-Triphenyl-2H-tetrazolium chloride (TTC) staining
The heart tissues were removed and washed with PBS to eliminate any excess blood or debris. Subsequently, the samples were sectioned into slices of uniform thickness using either a sharp blade or a specialized tissue slicer. The tissue sections were placed in a container containing a 2% TTC (Cat# T8170, Solarbio, Bei**g, China) staining solution and incubated at 37 °C for about 10–20 min. During this incubation period, the dehydrogenase enzymes present in the viable tissue reduced the TTC, resulting in the formation of a red-colored formazan. After incubation, the stained tissue sections were fixed in 4% paraformaldehyde and then subjected to imaging. The viable tissue was characterized by a red color, whereas nonviable tissue appeared as a pale, unstained area.
Echocardiography
Transthoracic echocardiography examinations were performed on mice using a Vevo 3100 (Fujifilm VisualSonics) ultrasound system with a 30-MHz linear array ultrasound transducer. The heart rate (HR) of anesthetized mice was maintained at 400–500 beats per min (bpm). After the mice were anesthetized with oxygen and 1%–2% isoflurane, ultrasound analysis was conducted to assess cardiac function. All measurements derived from echocardiography were obtained by averaging the readings of three consecutive and complete cardiac cycles.
Intracellular calcium ion detection
To load the fluorescence probe, NRVMs were incubated with a Fluo-4 (Cat# S1060, Beyotime, Shanghai, China) AM working solution (2 μM) at 37℃ for 20 min. The NRVMS were then washed three times with PBS, and incubated in DMEM medium. They were then placed in a spinning-disk confocal microscope (SpinSR, Olympus, Japan) to measure the transient changes in intracellular calcium ion concentration through fluorescence detection.
LC–MS/MS analysis
HEK-293 T cells were transfected with a plasmid expressing Flag-tagged JOSD2. After 24 h, the cells were lysed with a co-immunoprecipitation lysis buffer. The samples were then enriched with Flag antibody and protein A + G Agarose beads at 4 °C overnight before being eluted with PBS three times. Next, the samples were subjected to SDS-PAGE gel and Coomassie blue staining. The excised gel segments were subjected to mass spectrometry, with the analysis by Hwayen Biomedical Science and Technology Co Ltd (Shanghai, China).
Statistical analysis
Data presented in the present study were representative of at least 3 independent experiments and expressed as Mean ± SEM. To identify meaningful group differences, we used 2-tailed Student’s t-test for comparing two groups and a one-way analysis of variance (ANOVA) followed by a Tukey post-hoc test when comparing more than two groups of data. For samples that required a single individual to be measured more than once, data sets were analyzed independently using two-way repeated-measures ANOVA analysis with a single pooled variance and a Tukey correction for pairwise comparisons within groups for each data set. A statistically significant difference was obtained at p < 0.05. All data analyses were implemented in GraphPad Prism 8.0 software (San Diego, CA, USA).
Results
JOSD2 is upregulated in myocardial hypertrophy and fibrosis
We used recent transcriptome sequencing data (GSE123092) on heart tissues from mice induced with acute myocardial infarction (AMI). The results revealed a significant increase in JOSD2 mRNA levels compared to control samples (Fig. S1A). Then, we observed a remarkable increase in the protein levels of JOSD2 in ISO- and MI-induced mouse heart tissues (Fig. 1A–B and Fig. S1B-C). Notably, among the DUBs in the MJDs family, only JOSD2 mRNA levels in myocardial tissue from mice induced with ISO and MI showed an increase (Fig. S1D-E). These findings suggest that JOSD2 may play a role in the pathogenesis of ISO- or MI-induced cardiac remodeling. Considering that cardiac tissue mainly consists of cardiomyocytes, macrophages, and fibroblasts, we compared the protein levels of JOSD2 in these cell types through immunoblotting. Our results demonstrated the highest expression levels of JOSD2 in cardiomyocytes (Fig. 1C and Fig. S1F). Moreover, immunostaining of mouse heart tissues revealed that the increase in JOSD2 was primarily localized and elevated in cardiomyocytes, but not in primary fibroblasts, following ISO administration (Fig. 1D). Additionally, we observed a time-dependent increase in JOSD2 expression in NRVMs under prolonged ISO stimulation (Fig. 1E). These findings further suggest a positive correlation between JOSD2 levels and ISO challenge in cardiomyocytes.
JOSD2 is upregulated in myocardial hypertrophy and fibrosis. A Wildtype mice were infused with saline (Sham) or ISO for 2 weeks. One lane represents one mouse. Representative western blot analysis for JOSD2 levels in WT-Saline or ISO-induced (WT-ISO) mouse heart tissues. GAPDH was used as the loading control. B Wildtype mice were subjected to MI surgery for 1 week. One lane represents one mouse. Representative western blot analysis for JOSD2 levels in WT-Sham or MI-induced (WT-MI) heart tissues. GAPDH was used as the loading control. C Representative western blot analysis for JOSD2 protein levels in neonatal rat ventricular myocytes (NRVMs), mouse peritoneal macrophages (MPMs) and primary fibroblasts (PFs). GAPDH was used as the loading control. D Immunofluorescence staining of mouse heart tissues for JOSD2 (red), sarcomeric alpha-actinin (green), and vimentin (green). Sections were counterstained with DAPI (blue). (scale bar = 25 μm). E Representative western blot analysis for JOSD2 levels with prolonged ISO stimulation time
JOSD2 knockout alleviates ISO-induced cardiac remodeling
We constructed and identified the whole-body JOSD2−/− mice (Fig. S2A-B), which were infused with ISO for two weeks to induce cardiac injuries. The body weights of the mice did not significantly differ between WT and JOSD2−/− mice. Echocardiography revealed that the left ventricular systolic function of JOSD2−/−-ISO mice were superior to that of WT-ISO mice (Fig. 2A and Table 1). Parameters such as ejection fraction (EF%), fractional shortening (FS%), and thickening of the anterior left ventricular walls (LVAW) also indicated that JOSD2 knockout ameliorated ISO-induced cardiac dysfunction (Table 1). The size of the heart, as indicated by the HW/BW and HW/TL ratios, was markedly higher in WT-ISO mice than in JOSD2−/−-ISO mice (Fig. 2B and Table 1). Furthermore, WGA staining showed increased cardiomyocyte size in ISO mice, but not in JOSD2−/−-ISO mice (Fig. 2C–D). H&E staining of heart tissues demonstrated that JOSD2 knockout prevented ISO-induced impairment of myocardial morphology (Fig. 2E). Sirius Red and Masson’s Trichrome staining showed that ISO-induced myocardial fibrosis was mitigated in JOSD2−/−-ISO mice (Fig. 2F–I). These changes were associated with increased expression of injurious genes (such as Myh7 and Nppa) and profibrotic genes (COL-1 and TGF-β1). The mRNA (Fig. 2J) and proteins (Fig. 2K-L) levels of these genes and proteins in WT-ISO mice were remarkably increased compared to WT-Con mice. However, the levels of these genes were reversed in JOSD2−/−-ISO mice (Fig. 2J–L). Furthermore, the serum ANP levels also indicated excessive hypertrophy in WT-ISO mice but not in JOSD2−/−-ISO mice (Fig. 2M). Overall, these findings indicate that JOSD2 deficiency protects against ISO-induced morphological and functional abnormalities in mouse heart.
JOSD2 knockout alleviates ISO-induced cardiac remodeling. Wildtype and JOSD2 knockout mice were infused with saline (Sham) or ISO for 2 weeks. A Representative M-mode echocardiographic images from mice in each group. B Representative images from harvested mouse heart tissues (scale bar: 2.5 mm). C-I Representative images and quantification of heart tissues from WT and JOSD2−/− mice stained with WGA (wheat germ agglutinin, C and D), H&E (E), Sirius Red (F and G) and Masson (H and I) in sections of hearts (scale bar: 100 μm). J mRNA levels of hypertrophy-associated and fibrosis-associated genes in heart tissues of mice. Data was normalized to Actb. K Western blot analysis of hypertrophy-associated β-MyHC, ANP and fibrosis-associated COL-1, TGF-β1 in heart tissues. L Densitometric quantification of blots in panel K, GAPDH was used as the loading control. M The content of ANP in plasma. All quantitative data is presented as Mean ± SEM; n = 6; ns = not significant; * = p < 0.05
JOSD2 deficiency protects against myocardial injury induced by MI
To further investigate the role of JOSD2 in myocardial injury, we performed MI surgery on both WT and JOSD2−/− mice. Echocardiography revealed a significant reduction in left ventricular systolic function following MI in both groups (WT-MI and JOSD2−/−-MI). However, the JOSD2−/−-MI group showed a significant improvement in systolic function (Fig. 3A and Table 2). We further validated the effect of JOSD2 on MI-induced hypertrophy and fibrosis. WGA and Sirius Red staining demonstrated that JOSD2 deficiency resulted in a significant reduction in the degree of hypertrophy and fibrosis, respectively (Fig. 3B–E). Additionally, TTC staining revealed a smaller area of infarction in JOSD2-deficient mice (Fig. 3F–G). The JOSD2−/− mice exhibited a slower increase in serum cardiac injury markers, cTnT and ANP, compared to WT mice after MI surgery (Fig. 3H–I). Similarly, the levels of mRNA (Fig. 3J) and proteins (Fig. 3K–L) of injurious genes (Myh7 and Nppa) and profibrotic genes (COL-1, and TGF-β1) in mouse heart tissues followed similar trends. These findings demonstrate that JOSD2 deficiency also protects mice against MI-induced myocardial injury.
JOSD2 deficiency protects against myocardial injury induced by MI. Wildtype and JOSD2 knockout mice were subjected to MI surgery to model cardiac hypertrophy and fibrosis. Tissues from mice were analyzed after 1 week. A Representative M-mode echocardiographic images from mice in each group. B Representative images and quantification of heart tissues stained with WGA (B and C, scale bar: 50 μm), Sirius Red (D and E, scale bar: 100 μm), and TTC (F and G) in sections of hearts (scale bar: 1 mm). H-I The content of cTnT (H) and ANP (I) in plasma. J mRNA levels of hypertrophy-associated and fibrosis-associated genes in heart tissues of mice. Data was normalized to Actb. K Western blot analysis of hypertrophy-associated and fibrosis-associated proteins in heart tissues. L Densitometric quantification of blots in panel K, GAPDH was used as the loading control. All quantitative data is presented as Mean ± SEM; n = 6; ns = not significant; * = p < 0.05
JOSD2 regulates ISO-induced cardiomyocyte hypertrophy and fibrosis
To investigate the relationship between JOSD2 and ISO-induced hypertrophy and fibrosis in vitro, we conducted experiments using NRVMs. NRVMs with silenced or overexpressed JOSD2 were exposed to ISO for 24 h. Phalloidin-TRITC staining demonstrated that JOSD2 knockdown protected cardiomyocytes against ISO-induced increase in cell size (Fig. 4A–B). Furthermore, the levels of proteins and mRNA, such as β-MyHC, ANP, COL-1, and TGF-β supported the notion that JOSD2 deficiency alleviated ISO-induced hypertrophy and fibrosis in cardiomyocytes (Fig. 4C–D and Fig. S3A). Conversely, we confirmed in a similar experiment that JOSD2 overexpression aggravated ISO-induced hypertrophy and fibrosis in cardiomyocytes (Fig. 4E–H and Fig. S3B-D). These findings validated the crucial role of JOSD2 in ISO-challenged cardiomyocytes.
JOSD2 regulates ISO-induced cardiomyocyte hypertrophy and fibrosis. NVRMs were used to model ISO effects in culture. Cells were transfected with siRNA against JOSD2 (panels A-D) or JOSD2 overexpressing vectors (panels E–H). Scrambled siRNA and empty vectors were used as control. Following transfections, cells were exposed to 10 μM ISO for 24 h. A Following ISO exposure, cells were stained with Phalloidin-TRITC to assess hypertrophic responses [scale bar = 100 μm]. B Mean cell area quantification of image in panel A. C Representative western blot for β-MyHC, ANP, COL-1, TGF-β1 and JOSD2 in NVRMs. GAPDH was used as the loading control. D mRNA levels of hypertrophy-associated and fibrosis-associated genes were measured in NVRMs. Data was normalized to Actb. E Hypertrophic response to ISO in cells overexpressing JOSD2. Representative Phalloidin-TRITC staining image [scale bar = 100 μm]. F Mean cell area quantification of the image in panel E. G Representative western blot for β-MyHC, ANP, COL-1, TGF-β1 and JOSD2 in NVRMs. GAPDH was used as a loading control. H mRNA levels of hypertrophy-associated genes and fibrosis-associated were measured in NVRMs. Data was normalized to Actb. All quantitative data is presented as Mean ± SEM; n = 3; ns = not significant; * = p < 0.05
JOSD2 interacts with CaMKIIδ directly and promotes CaMKIIδ activation
To identify potential substrates of JOSD2, we performed immunoprecipitation in HEK-293 T cells transfected with the Flag-JOSD2 plasmid. The proteins eluted from the beads were then identified via HPLC-tandem mass spectrometry. Our results revealed that JOSD2 may interact with CaMKIIδ, a protein highly associated with cardiomyocyte hypertrophy and calcium handling, and is known to be activated upon ISO stimulation (Fig. 5A–B). We further confirmed this protein interaction by co-immunoprecipitation assay in both NRVMs transfected with Flag-JOSD2 plasmid (Fig. 5C) and HEK-293 T cells co-transfected with Flag-JOSD2 and GFP-CaMKIIδ plasmids (Fig. 5D). Phosphorylation of the tyrosine 287 residue of CaMKIIδ is a key molecular event in its activation. Recent studies have shown an increase in tyrosine 287 phosphorylation of CaMKIIδ in response to ISO in cardiomyocytes [27, 24]. In this study, we conducted whole-body JOSD2 knockout and heart-specific JOSD2 overexpression to investigate the key role of JOSD2 in ISO/MI-induced pathological cardiac hypertrophy by activating CaMKIIδ. A limitation of our study is the lack of myocardial-specific JOSD2 knockout mice.
Calcium mishandling has been implicated in the pathogenesis of various cardiac diseases, including arrhythmias, hypertrophic cardiomyopathy [34]. CaMKII, an enzyme involved in the regulation of receptors, transporters and various ion channels in cardiomyocytes, is activated by binding to calcium and calmodulin, which is sustained by autophosphorylation of the enzyme [35]. Research has confirmed that CaMKIIα and β isoforms are mainly expressed in neuronal tissues, whereas CaMKIIδ and γ can be detected in cells generally [36]. CaMKIIδ is the main isoform expressed in heart, with 11 different splice variants, among which the δA, δB, δC and δ9 are the most commonly expressed in the heart [37]. CaMKIIδC mainly induces cardiomyocyte death and mitochondrial dysfunction [38]. SERCA2 is responsible for the reuptake of Ca2+ into the SR, thereby enhancing the relaxation of cardiomyocytes. In contrast, the ryanodine receptor (RYR) is responsible for the release of Ca2+ from the SR, leading to contraction [39]. CaMKIIδ, through phosphorylation of phospholamban (PLN), regulates the activity of SERCA2 and also directly phosphorylates RYR to modulate its function [40, 41]. Dysregulation of CaMKIIδ activity has been linked to the pathogenesis of cardiac diseases [42,43,44]. For instance, excessive CaMKIIδ activation is associated with the development of arrhythmias [45], heart failure, and hypertrophic cardiomyopathy [46]. CaMKIIδ is activated upon Ca2+/CaM protein binding to the regulatory domain of CaMKIIδ, which leads to a conformational change of CaMKII to induce the autophosphorylation at Thr287 [37]. CaMKIIδ has been reported to be affected by the presence of many post-translational-modifications (PTM), which could be independent of or affecting each other. It has been shown previously that O-GlcNAcylation at S280 promotes the autophosphorylation of CaMKIIδ [47]. Nitrosylation of the Cys-273 site reduces Ca2+/CaM binding to CaMKIIδ and then CaMKIIδ autophosphorylation, perhaps through steric occlusion of the CaM-binding site [48]. Similar observation has been reported that phosphorylation at T306 on CaMKII blocks Ca2+/CaM binding, resulting in the inactivation of CaMKII [49]. Unfortunately, no previous studies reported the effect of CaMKII ubiquitination on its phosphorylation. In this study, we found that JOSD2 directly removes ubiquitin from CaMKIIδ at K227 and promotes CaMKIIδ phosphorylation. It is very interesting to explore the mechanism by which CaMKIIδ ubiquitination affects CaMKII phosphorylation. Similarly, the ubiquitination of YAP at the K252 residue interferes with the upstream protein binding with YAP, resulting in a decrease in YAP phosphorylation [50]. Therefore, we guess that the K227 ubiquitination of CaMKIIδ may prevent the interaction of CaMKIIδ with Ca2+/CaM via changing the spatial conformation, while JOSD2 deubiquitinates CaMKIIδ at K227, promoting Ca2+/CaM binding to CaMKIIδ to induce CaMKIIδ autophosphorylation.
The function of JOSD2 is influenced by the effects of its substrates. Studies have shown that JOSD2 can stably phosphoglycerate dehydrogenase (PHGDH) to promote lung adenocarcinoma subset [18] and hepatocellular carcinoma [20]. JOSD2 also inhibits CTNNB1 degradation to enhance hepatocellular carcinoma progression [19]. Research by Zhou’s group showed that JOSD2 removed the ubiquitination of NLRP3-R779C to promote inflammasome activation [22]. Other scholars have demonstrated that JOSD2 inhibits nuclear localization of PKM2 instead of affecting its protein stability to prevent the progression of acute myeloid leukemia [16]. Our data confirmed that JOSD2 can directly remove K63-linked ubiquitin from CaMKIIδ at residue K227. Recently, Erik’s group has reported that JOSD2 directly controls a metabolic enzyme complex as a positive regulator of cancer cells [23]. We silenced JOSD2 in NRVMs and detected the gene levels of Hexokinase (HK), phosphofructokinase (PFK), and pyruvate kinase (PK), which are the key enzymes of the glycolytic pathway. Our data showed that ISO treatment did not affect these gene levels in the glycolytic pathway, while JOSD2 knockdown indeed decreased the basal pfk and hk mRNA levels in NRVMs (Supplementary Figure S8). These results, consistent with Erik’s study, indicate that JOSD2 knockdown has inhibitory effects on the glycolytic pathway in cardiomyocytes. In addition, recent reports found that an augmented glycolytic pathway can potentially contribute to cardiomyocyte hypertrophy [51, 52]. Therefore, the contribution of the glycolytic pathway in JOSD2-mediated cardiomyocyte hypertrophy warrants further investigation.
In conclusion, as summarized in the Graphical Abstract, JOSD2 mediates the cardiac damage induced by ISO/MI via deubiquitinating CaMKIIδ in cardiomyocytes. JOSD2 could directly remove K63-linked ubiquitin from CaMKIIδ at residue K227 and affect the phosphorylation of CaMKIIδ to induce calcium mishandling. In summary, our findings suggest that targeting JOSD2 may be a promising treatment strategy for hypertrophic, fibrotic and heart failure.
Availability of data and materials
All other data and materials are included within the article or Supplementary Information or available from the authors on request.
Abbreviations
- ANP:
-
Atrial natriuretic peptide
- CAMKII:
-
Ca/calmodulin-dependent protein kinases or CaM kinases
- COL-I:
-
Collagen I
- cTnT:
-
Cardiac troponin T
- DUB:
-
Deubiquitinate
- EF:
-
Ejection fraction
- FS:
-
Fractional shortening
- HK:
-
Hexokinase
- HR:
-
Heart rate
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- ISO:
-
Isoprenaline
- JOSD2:
-
Josephin domain-containing protein 2
- LV:
-
Left ventricular
- MJDs:
-
Machado-Joseph domain-containing proteases
- MPMs:
-
Primary mouse peritoneal macrophages
- MI:
-
Myocardial infarction
- NRVMS:
-
Neonatal rat ventricular myocytes
- MyHC:
-
Myosin heavy chain
- PF:
-
Primary fibroblasts
- PFK:
-
Phosphofructokinase
- PK:
-
Pyruvate kinase
- SR:
-
Sarcoplasmic reticulum
- TGF-β:
-
Transforming growth factor-beta
- TTC:
-
Triphenyl tetrazolium chloride
- UB:
-
Ubiquitin
- WGA:
-
Wheat germ agglutinin
References
Nakamura M, Sadoshima J (2018) Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 15:387–407. https://doi.org/10.1038/s41569-018-0007-y
Charkoudian N, Wallin BGS (2014) ympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr Physiol 4:825–850. https://doi.org/10.1002/cphy.c130038
Dunser MW, Hasibeder WR (2009) Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensiv Care Med 24:293–316. https://doi.org/10.1177/0885066609340519
El-Armouche A, Eschenhagen T (2009) Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev 14:225–241. https://doi.org/10.1007/s10741-008-9132-8
Lymperopoulos A, Rengo G, Koch WJ (2013) Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res 113:739–753. https://doi.org/10.1161/CIRCRESAHA.113.300308
Bers DM (2008) Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70:23–49. https://doi.org/10.1146/annurev.physiol.70.113006.100455
Laurent S (2017) Antihypertensive drugs. Pharmacol Res 124:116–125. https://doi.org/10.1016/j.phrs.2017.07.026
Harrigan JA, Jacq X, Martin NM, Jackson SP (2018) Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat Rev Drug Discov 17:57–78. https://doi.org/10.1038/nrd.2017.152
Mevissen TET, Komander D (2017) Mechanisms of deubiquitinase specificity and regulation. Annu Rev Biochem 86:159–192. https://doi.org/10.1146/annurev-biochem-061516-044916
Gupta I, Varshney NK, Khan S (2018) Emergence of members of TRAF and DUB of ubiquitin proteasome system in the regulation of hypertrophic cardiomyopathy. Front Genet 9:336. https://doi.org/10.3389/fgene.2018.00336
Bi HL, Zhang XL, Zhang YL, **e X, **a YL, Du J, Li HH (2020) The deubiquitinase UCHL1 regulates cardiac hypertrophy by stabilizing epidermal growth factor receptor. Sci Adv 6:eaax4826. https://doi.org/10.1126/sciadv.aax4826
Bi HL, Zhang YL, Yang J, Shu Q, Yang XL, Yan X, Chen C, Li Z, Li HH (2020) Inhibition of UCHL1 by LDN-57444 attenuates Ang II-Induced atrial fibrillation in mice. Hypertens Res 43:168–177. https://doi.org/10.1038/s41440-019-0354-z
Ye B, Zhou H, Chen Y, Luo W, Lin W, Zhao Y, Han J, Han X, Huang W, Wu G, Wang X, Liang G (2023) USP25 ameliorates pathological cardiac hypertrophy by stabilizing SERCA2a in cardiomyocytes. Circ Res 132:465–480. https://doi.org/10.1161/CIRCRESAHA.122.321849
Tang LJ, Zhou YJ, **ong XM, Li NS, Zhang JJ, Luo XJ, Peng J (2021) Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic Biol Med 162:339–352. https://doi.org/10.1016/j.freeradbiomed.2020.10.307
Qi L, Zang H, Wu W, Nagarkatti P, Nagarkatti M, Liu Q, Robbins J, Wang X, Cui T (2020) CYLD exaggerates pressure overload-induced cardiomyopathy via suppressing autolysosome efflux in cardiomyocytes. J Mol Cell Cardiol 145:59–73. https://doi.org/10.1016/j.yjmcc.2020.06.004
Lei H, Yang L, Wang Y, Zou Z, Liu M, Xu H, Wu Y (2022) JOSD2 regulates PKM2 nuclear translocation and reduces acute myeloid leukemia progression. Exp Hematol Oncol 11:42. https://doi.org/10.1186/s40164-022-00295-w
Qian M, Yan F, Wang W, Du J, Yuan T, Wu R, Zhao C, Wang J, Lu J, Zhang B, Lin N, Dong X, Dai X, Dong X, Yang B, Zhu H, He Q (2021) Deubiquitinase JOSD2 stabilizes YAP/TAZ to promote cholangiocarcinoma progression. Acta Pharm Sin B 11:4008–4019. https://doi.org/10.1016/j.apsb.2021.04.003
Zhang B, Zheng A, Hydbring P, Ambroise G, Ouchida AT, Goiny M, Vakifahmetoglu-Norberg H, Norberg E (2017) PHGDH defines a metabolic subtype in lung adenocarcinomas with poor prognosis. Cell Rep 19:2289–2303. https://doi.org/10.1016/j.celrep.2017.05.067
Huang Y, Zeng J, Liu T, Xu Q, Song X, Zeng J (2022) Deubiquitinating enzyme JOSD2 promotes hepatocellular carcinoma progression through interacting with and inhibiting CTNNB1 degradation. Cell Biol Int 46:1089–1097. https://doi.org/10.1002/cbin.11812
Wang Y, Li ZX, Wang JG, Li LH, Shen WL, Dang XW (2023) Deubiquitinating enzyme Josephin-2 stabilizes PHGDH to promote a cancer stem cell phenotype in hepatocellular carcinoma. Genes Genomics 45:215–224. https://doi.org/10.1007/s13258-022-01356-4
Zhou L, Chen G, Liu T, Liu X, Yang C, Jiang J (2022) MJDs family members: potential prognostic targets and immune-associated biomarkers in hepatocellular carcinoma. Front Genet 13:965805. https://doi.org/10.3389/fgene.2022.965805
Zhou L, Liu T, Huang B, Luo M, Chen Z, Zhao Z, Wang J, Leung D, Yang X, Chan KW, Liu Y, **ong L, Chen P, Wang H, Ye L, Liang H, Masters SL, Lew AM, Gong S, Bai F, Yang J, Pui-Wah Lee P, Yang W, Zhang Y, Lau YL, Geng L, Zhang Y, Cui J (2021) Excessive deubiquitination of NLRP3-R779C variant contributes to very-early-onset inflammatory bowel disease development. J Allergy Clin Immunol 147:267–279. https://doi.org/10.1016/j.jaci.2020.09.003
Krassikova L, Zhang B, Nagarajan D, Queiroz AL, Kacal M, Samakidis E, Vakifahmetoglu-Norberg H, Norberg E (2021) The deubiquitinase JOSD2 is a positive regulator of glucose metabolism. Cell Death Differ 28:1091–1109. https://doi.org/10.1038/s41418-020-00639-1
Orgil BO, Xu F, Munkhsaikhan U, Alberson NR, Bajpai AK, Johnson JN, Sun Y, Towbin JA, Lu L, Purevjav E (2023) Echocardiography phenoty** in murine genetic reference population of BXD strains reveals significant QTLs associated with cardiac function and morphology. Physiol Genom 55:51–66. https://doi.org/10.1152/physiolgenomics.00120.2022
Chang SC, Ren S, Rau CD, Wang JJ (2018) Isoproterenol-induced heart failure mouse model using osmotic pump implantation. Methods Mol Biol 1816:207–220. https://doi.org/10.1007/978-1-4939-8597-5_16
Ye B, Chen X, Dai S, Han J, Liang X, Lin S, Cai X, Huang Z, Huang W (2019) Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Devel Ther 13:975–990. https://doi.org/10.2147/DDDT.S195412
Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME (2005) Calmodulin kinase II inhibition protects against structural heart disease. Nat Med 11:409–417. https://doi.org/10.1038/nm1215
Wang P, Xu S, Xu J, **n Y, Lu Y, Zhang H, Zhou B, Xu H, Sheu SS, Tian R, Wang W (2022) Elevated MCU expression by CaMKIIdeltaB limits pathological cardiac remodeling. Circulation 145:1067–1083. https://doi.org/10.1161/CIRCULATIONAHA.121.055841
Odiase E, Zhang X, Chang Y, Nelson M, Balaji U, Gu J, Zhang Q, Pan Z, Spechler SJ, Souza RF (2021) In esophageal squamous cells from eosinophilic esophagitis patients, Th2 cytokines increase eotaxin-3 secretion through effects on intracellular calcium and a non-gastric proton pump. Gastroenterology 160(2072–2088):e2076. https://doi.org/10.1053/j.gastro.2021.02.016
Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patterson C (2010) Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ Res 106:463–478. https://doi.org/10.1161/CIRCRESAHA.109.208801
Swatek KN, Komander D (2016) Ubiquitin modifications. Cell Res 26:399–422. https://doi.org/10.1038/cr.2016.39
Wang B, Tang X, Yao L, Wang Y, Chen Z, Li M, Wu N, Wu D, Dai X, Jiang H, Ai D (2022) Disruption of USP9X in macrophages promotes foam cell formation and atherosclerosis. J Clin Invest. https://doi.org/10.1172/JCI154217
An W, Luong LA, Bowden NP, Yang M, Wu W, Zhou X, Liu C, Niu K, Luo J, Zhang C, Sun X, Poston R, Zhang L, Evans PC, **ao Q (2022) Cezanne is a critical regulator of pathological arterial remodelling by targeting beta-catenin signalling. Cardiovasc Res 118:638–653. https://doi.org/10.1093/cvr/cvab056
Luo M, Anderson ME (2013) Mechanisms of altered Ca(2)(+) handling in heart failure. Circ Res 113:690–708. https://doi.org/10.1161/CIRCRESAHA.113.301651
Giese KP (2021) The role of CaMKII autophosphorylation for NMDA receptor-dependent synaptic potentiation. Neuropharmacology 193:108616. https://doi.org/10.1016/j.neuropharm.2021.108616
Hudmon A, Schulman H (2002) Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem 71:473–510. https://doi.org/10.1146/annurev.biochem.71.110601.135410
Beckendorf J, van den Hoogenhof MMG, Backs J (2018) Physiological and unappreciated roles of CaMKII in the heart. Basic Res Cardiol 113:29. https://doi.org/10.1007/s00395-018-0688-8
Zhu W, Woo AY, Yang D, Cheng H, Crow MT, **ao RP (2007) Activation of CaMKIIdeltaC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J Biol Chem 282:10833–10839. https://doi.org/10.1074/jbc.M611507200
Bers DM (2006) Altered cardiac myocyte Ca regulation in heart failure. Physiology 21:380–387. https://doi.org/10.1152/physiol.00019.2006
Maier LS, Bers DM (2007) Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res 73:631–640. https://doi.org/10.1016/j.cardiores.2006.11.005
Mattiazzi A, Kranias EG (2014) The role of CaMKII regulation of phospholamban activity in heart disease. Front Pharmacol 5:5. https://doi.org/10.3389/fphar.2014.00005
Hegyi B, Polonen RP, Hellgren KT, Ko CY, Ginsburg KS, Bossuyt J, Mercola M, Bers DM (2021) Cardiomyocyte Na(+) and Ca(2+) mishandling drives vicious cycle involving CaMKII, ROS, and ryanodine receptors. Basic Res Cardiol 116:58. https://doi.org/10.1007/s00395-021-00900-9
Helmstadter KG, Ljubojevic-Holzer S, Wood BM, Taheri KD, Sedej S, Erickson JR, Bossuyt J, Bers DM (2021) CaMKII and PKA-dependent phosphorylation co-regulate nuclear localization of HDAC4 in adult cardiomyocytes. Basic Res Cardiol 116:11. https://doi.org/10.1007/s00395-021-00850-2
He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, Anderson ME (2011) Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med 17:1610–1618. https://doi.org/10.1038/nm.2506
Thiel WH, Chen B, Hund TJ, Koval OM, Purohit A, Song LS, Mohler PJ, Anderson ME (2008) Proarrhythmic defects in Timothy syndrome require calmodulin kinase II. Circulation 118:2225–2234. https://doi.org/10.1161/CIRCULATIONAHA.108.788067
Kreusser MM, Lehmann LH, Keranov S, Hoting MO, Oehl U, Kohlhaas M, Reil JC, Neumann K, Schneider MD, Hill JA, Dobrev D, Maack C, Maier LS, Grone HJ, Katus HA, Olson EN, Backs J (2014) Cardiac CaM Kinase II genes delta and gamma contribute to adverse remodeling but redundantly inhibit calcineurin-induced myocardial hypertrophy. Circulation 130:1262–1273. https://doi.org/10.1161/CIRCULATIONAHA.114.006185
Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM (2013) Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 502:372–376. https://doi.org/10.1038/nature12537
Erickson JR, Nichols CB, Uchinoumi H, Stein ML, Bossuyt J, Bers DM (2015) S-nitrosylation induces both autonomous activation and inhibition of calcium/calmodulin-dependent protein kinase II delta. J Biol Chem 290:25646–25656. https://doi.org/10.1074/jbc.M115.650234
Colbran RJ (1993) Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J Biol Chem 268:7163–7170
Liu M, Yan M, Lv H, Wang B, Lv X, Zhang H, **ang S, Du J, Liu T, Tian Y, Zhang X, Zhou F, Cheng T, Zhu Y, Jiang H, Cao Y, Ai D (2020) Macrophage K63-linked ubiquitination of YAP promotes its nuclear localization and exacerbates atherosclerosis. Cell Rep 32:107990. https://doi.org/10.1016/j.celrep.2020.107990
Kashihara T, Mukai R, Oka SI, Zhai P, Nakada Y, Yang Z, Mizushima W, Nakahara T, Warren JS, Abdellatif M, Sadoshima J (2022) YAP mediates compensatory cardiac hypertrophy through aerobic glycolysis in response to pressure overload. J Clin Invest. https://doi.org/10.1172/JCI150595
Ritterhoff J, Young S, Villet O, Shao D, Neto FC, Bettcher LF, Hsu YA, Kolwicz SC Jr, Raftery D, Tian R (2020) Metabolic remodeling promotes cardiac hypertrophy by directing glucose to aspartate biosynthesis. Circ Res 126:182–196. https://doi.org/10.1161/CIRCRESAHA.119.315483
Acknowledgements
We would like to thank Lingli Hou, Tongliang Huang and Yanni Dong from the Scientific Research Center of Wenzhou Medical University for their help in echocardiography. We also appreciate Jiansong Lin and Mengxin Zhang from the Scientific Research Center of Wenzhou Medical University for their help in calcium handling experiment.
Funding
This work was supported by the National Natural Science Foundation of China (21961142009), and the Natural Science Foundation of Zhejiang Province (LY19H020004).
Author information
Authors and Affiliations
Contributions
GL and HZ contributed to the literature search and study design. JX and WL participated in the drafting of the article. JX, SL, QW, QZ, JQ, MW, TY and SL carried out the experiments. GL and WL revised the manuscript. WL contributed to data collection and analysis.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval and consent to participate
All animal experiments were reviewed and approved by the Ethics Examination and Approval Committee of the Animal Experimental Center of The First Affiliated Hospital of Wenzhou Medical University (wydw-2021-347).
Consent for publication
Authors are responsible for correctness of the statements provided in the manuscript. The authors declare agreement for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, J., Liang, S., Wang, Q. et al. JOSD2 mediates isoprenaline-induced heart failure by deubiquitinating CaMKIIδ in cardiomyocytes. Cell. Mol. Life Sci. 81, 18 (2024). https://doi.org/10.1007/s00018-023-05037-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-05037-7