Abstract
Neural progenitor cell (NPC) transplantation represents a promising treatment strategy for spinal cord injury (SCI); however, the underlying therapeutic mechanisms remain incompletely understood. We demonstrate that severe spinal contusion in adult rats causes transcriptional dysregulation, which persists from early subacute to chronic stages of SCI and affects nearly 20,000 genes in total tissue extracts. Functional analysis of this dysregulated transcriptome reveals the significant downregulation of cAMP signalling components immediately after SCI, involving genes such as EPAC2 (exchange protein directly activated by cAMP), PKA, BDNF, and CAMKK2. The ectopic transplantation of spinal cord-derived NPCs at acute or subacute stages of SCI induces a significant transcriptional impact in spinal tissue, as evidenced by the normalized expression of a large proportion of SCI-affected genes. The transcriptional modulation pattern driven by NPC transplantation includes the rescued expression of cAMP signalling genes, including EPAC2. We also explore how the sustained in vivo inhibition of EPAC2 downstream signalling via the intrathecal administration of ESI-05 for 1 week impacts therapeutic mechanisms involved in the NPC-mediated treatment of SCI. NPC transplantation in SCI rats in the presence and absence of ESI-05 administration prompts increased rostral cAMP levels; however, NPC and ESI-05 treated animals exhibit a significant reduction in EPAC2 mRNA levels compared to animals receiving only NPCs treatment. Compared with transplanted animals, NPCs + ESI-05 treatment increases the scar area (as shown by GFAP staining), polarizes microglia into an inflammatory phenotype, and increases the magnitude of the gap between NeuN + cells across the lesion. Overall, our results indicate that the NPC-associated therapeutic mechanisms in the context of SCI involve the cAMP pathway, which reduces inflammation and provides a more neuropermissive environment through an EPAC2-dependent mechanism.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) occurs when a traumatic insult disrupts the communication of impulses through the central nervous system (CNS), resulting in the loss of motor and sensory function below the injured area. Even given recent advances, we still lack effective strategies that restore spinal cord functionality after injury [1]. The search for SCI treatments remains challenging, given the highly complex physiopathological events induced by the initial injury and the involvement of multiple cell types and thousands of transcriptional events [2]. Our incomplete understanding of the molecular mechanisms underlying the dynamic pathophysiology of SCI represents a significant impediment to the development of efficient therapeutic strategies and makes the selection of rationalized therapies for acute, sub-acute, and chronic disease states a challenging task.
Cell-based therapies [3] are an attractive alternative to conventional neuronal preservation and regeneration approaches for acute or chronic SCI [4]. Neural progenitor cell (NPC) transplantation represents a promising therapeutic approach, as these multipotent cells can re-establish and replace damaged neuronal circuits (reviewed by Lane, Lepore, and Fischer [5]). The transplantation of adult-derived NPCs obtained from naïve [6] or injured [7] spinal cord tissue has been extensively explored in SCI models; however, the limited availability of NPCs in human adult tissues has hampered clinical translation and prompted the widespread application of NPCs prepared from earlier developmental stages (i.e., from neonatal or foetal tissues) [8].
NPCs differentiate into three different lineages (including neurons and glia) after transplantation into the injured spinal cord. Differentiated neurons can synaptically integrate into the disrupted circuitry and create novel neuronal relays across the injury [9], while glia [10] (astrocytes and oligodendrocytes) provide support to both host and NPC-derived neurons, attenuate glial scar formation [11], and enhance the remyelination of injured axons [12]. NPC transplantation simultaneously provides multiple therapeutic activities and, as such, represents an intrinsically multifactorial therapy. NPCs can (i) serve as a source of new cells to restore the structural integrity of the injured spinal cord [13], (ii) provide neurotrophic support through the secretion of specific factors (such as nerve growth factor, brain-derived neurotrophic factor [BDNF], and glial cell-derived neurotrophic factor) into the injured microenvironment [14, 15], (iii) modulate inflammatory responses [16], and (iv) protect against glutamate-mediated excitotoxicity [17, 18]. Of note, the precise molecular mechanisms driving the NPC-induced pleiotropic spectrum of effects remain minimally defined.
Elevating cyclic adenosine monophosphate (cAMP) levels also represents a promising means of promoting neural regeneration after SCI, as studies have revealed that high levels of cAMP promote axonal growth in a range of species [19, 20]. Throughout development, endogenous levels of cAMP decrease in dorsal root ganglia neurons in parallel with the loss of axon-growing capacity; thus, the decay in basal cAMP levels associates with a switch from the regenerative state of embryonic neurons to the non-regenerative state of the adult CNS [21].
Unfortunately, cAMP levels decay by around four-fold after SCI, thus generating a non-conducive environment for CNS regeneration [22]. Pearse et al. reported that cAMP levels decayed by 64.3% in the spinal cord rostral to the injury, 68.1% in the brainstem, and 69.7% in the sensorimotor cortex for at least 2 weeks after SCI [20]; however, the mechanism driving cAMP decay and whether decay occurs in all spinal cord cell types or a specific subpopulation remains unclear. Overall, findings from a range of related studies have provided insight into the importance of cAMP signalling during CNS regeneration; therefore, a more profound study of this pathway may provide therapeutic targets for CNS pathologies in general and, more specifically, SCI.
Reports have suggested that the cAMP signalling cascade signals solely through protein kinase A (PKA) activation; however, recent research has demonstrated that cAMP binding activates a guanine nucleotide exchange factor—Rap guanine nucleotide exchange factor (GEF) 4 (RAPGEF4), also known as exchange protein directly activated by cAMP 2 (EPAC2) [23]. Guijarro-Belmar et al. demonstrated that an EPAC2 activator (S-220) delivered to ex vivo spinal cord slices modulated the lesion environment, prompted axonal outgrowth by reducing astrocyte/microglial activation, and resulted in significantly better locomotor performance for up to 4 weeks after treatment when applied in vivo [24].
To date, cAMP-targeted SCI treatments have focused on the application of general modulators of the pathway, such as adenylate cyclase (AC) stimulators, phosphodiesterase (PDE) inhibitors [25, 26], or cAMP analogues [Full size image
Principal component analysis (PCA) of the samples comprising this data set provided evidence of the clear segregation of the uninjured and injured animals and amongst all time points after SCI (Fig. 1B). While we detected small intra-group dispersion between the animals of the same experimental condition, we observed higher dispersion during earlier stages when compared to later stages (Fig. 1B).
Transcriptional analysis at each time point after SCI provided evidence for robust transcriptional dysregulation compared to the uninjured group (T0), which affected 19,675 differentially expressed genes (DEGs) during SCI progression (representing 55.48% of the rat transcriptome—Online Resource Fig. 1). We observed evident transcriptional alterations from the first week after injury (up to 15,655 genes) that persisted over time, as evidenced by the similar numbers of DEGs observed at 2-, 4-, and 8-weeks post-injury compared to uninjured spinal cords (14,178, 13,641, and 14,097 DEGs, respectively) (Fig. 1C, upper panel). All comparisons provided more downregulated genes than upregulated genes (Fig. 1C); Online Resource Table 1 lists all DEGs for each comparison.
We next performed a differential expression analysis of each stage versus subsequent time points to explore the evolution of SCI, which revealed that the most significant transcriptional differences took place at the earliest stages—between the first and second weeks after injury (522 DEGs)—and a lower number of DEGs between the second to fourth (27 DEGs) and fourth to eighth weeks after injury (32 DEGs) (Fig. 1C, lower panel). These findings suggest that an initial burst of transcriptional activity stabilizes 2 weeks after injury. Furthermore, we discovered that most DEGs (9993 genes) appeared for every time point after SCI when compared to the uninjured spinal cord, as represented by the high number of transcripts at the central intersections of the Venn diagram shown in Fig. 1D. Online Resource Table 2 lists the genes comprising each Venn diagram intersection.
Functional pathway analysis clusters altered biological functions according to their temporal regulation pattern
We functionally analysed the temporal evolution of SCI-induced transcriptomic alterations using the Hipathia algorithm for pathway activity analysis and computed the activation level of each altered function [34]. The functional analysis represented in the heatmap (Fig. 2A, Online Resource Table 3, and interactive heat map in Online Resource File 1) demonstrated the aggregation of altered biological functions into eight different clusters according to their temporal regulation pattern. Functions included in clusters 1–4 become upregulated after SCI, while functions included in clusters 5–8 become downregulated after SCI (Fig. 2B). Figure 2B depicts individual representations of selected functions in clusters 3 and 7 (which display opposite profiles), while Online Resource Fig. 2 depicts selected functions for clusters 1, 2, 4, 5,6, and 8. Online Resource Table 3 describes additional information on cluster composition.
Functional analysis during SCI evolution. A Heatmap representing the activation level computed by Hipathia algorithm of differentially activated GO functions for the comparisons on weeks 1, 2, 4, and 8 after SCI relative to the uninjured spinal cord (T0). Red, upregulation; blue, downregulation; v1–v4 indicates replicates for each condition. B Graphical representation of the mean activation levels and associated standard deviation for selected functions encountered in clusters 3 and 7 (*, adjusted p-value < 0.05). C Dot plot depicting significantly altered cAMP-related GO terms after SCI. Colour intensity represents the corresponding adjusted p-value, while dot size indicates the logFC
Cluster 3 (Fig. 2A, B) comprises a group of strongly upregulated functions at early stages after SCI (1–4 weeks post-injury) that resolve at later chronic stages (8 weeks after SCI). These functions include the "Adenosine Receptor signalling pathway" (GO:0,060,167) and "G-protein coupled glutamate receptor signalling" (GO:0,007,216). The alteration of these functions may result from the deleteriously high concentrations of ATP [35] and glutamate [36] associated with SCI, which ultimately derive from cell disruption. The upregulation of these functions suggests that these molecules can trigger secondary signalling pathways within the post-injury tissue to induce excitotoxicity by binding to their respective receptors [37].
In contrast to Cluster 3, Cluster 7 (Fig. 2A, B) comprises functions that become downregulated at early stages (from 1 to 4 weeks) after injury but return to pre-injury levels during chronic stages (8 weeks) and includes terms such as "Cellular response to cAMP" (GO:0,071,320). The close temporal coincidence with the upregulated functions in Cluster 3 may indicate that these functions induce the downregulation of Cluster 7 functions. Interestingly, we detected a recurrent representation of cAMP signalling during the exploration of altered functions. We also encountered cAMP-related GO terms among the differentially activated functions (listed in Fig. 2C—indicating the corresponding adjusted p-value and logFC at each temporal comparison). The common overrepresentation of cAMP functions discovered during transcriptional comparisons suggests that SCI profoundly impacts cAMP-related processes.
Cluster 1 comprises a set of functions that became highly upregulated 1 week after injury and then exhibited a slow but progressive downregulation while remaining significantly elevated compared to the uninjured spinal cord. Among these sets of functions, we encountered "Actin cytoskeleton reorganization" (GO:0,031,532) and "positive regulation of JAK/STAT cascade" (GO:0,046,427), with the latter triggering the proliferation and differentiation of multipotent and self-renewing adult stem cells [7] (Sox2/Sox3 + ependymal cells) in response to SCI [38], promoting glial scar formation (preventing secondary damage) [39], and enabling successful axonal regeneration [40]. Previous studies reported that SCI regeneration requires the precise temporal control of JAK/STAT activity, with an early and transient upregulation observed in regenerative species but a slightly delayed and sustained upregulation (at least 30 days) in non-regenerative species [41]. Thus, this temporal profile of JAK/STAT functions may relate to a non-regenerative response.
Cluster 2 includes persistently upregulated functions at all time points after SCI and displays an enrichment in functions related to immunological processes, including "Positive regulation of interleukin-8 production" (GO:0,032,637), "Neutrophil chemotaxis" (GO:0,030,593), "Positive regulation of leukocyte migration" (GO:0,002,687), and "NK T cell proliferation" (GO:0,001,866). The presence of these functions suggests that immunological processes induced by primary injury remain unresolved with time, leading to a chronic inflammatory state (as previously described by Schwab et al. [42]).
Cluster 4 comprises functions that display progressive upregulation over time after SCI and includes terms such as "Cholesterol metabolic process" (GO: 0,008,203), which has been previously linked to SCI and other CNS disorders [43]. Similarly, we encountered additional cholesterol-related processes such as "Cholesterol transport" (GO:0,030,301), which displayed an upward tendency over time (as found in Cluster 6), and "Cholesterol biosynthetic process" (GO:0,006,695), which displayed a downward tendency over time (as found in Cluster 8) [44] among the significantly altered biological functions. Importantly, studies have shown that glutamate excitotoxicity mediates cholesterol-related alterations in CNS injury and disease [45].
SCI severely alters cAMP signalling
We next undertook a deeper evaluation of SCI-induced alterations to cAMP signalling components at the transcriptional level. Figure 3A depicts a schema describing the most relevant components of cAMP signalling and their roles. cAMP signalling pathway initiation occurs after the activation of adenylate cyclases (ADCY), a family of enzymes devoted to cAMP production. cAMP molecules bind and activate the PKA and EPAC effector proteins, which then activate the mitogen-activated protein kinase (MAPK) cascade via Ras-related protein 1 (RAP1) by PKA-mediated protein phosphorylation or the EPAC2-mediated conversion of guanosine diphosphate/guanosine triphosphate [46]. The MAPK cascade culminates with cAMP response element-binding protein (CREB) phosphorylation, which acts as a transcriptional factor to induce the expression of regenerative-associated genes [47]. Such genes include BDNF, an essential axon regeneration modulator that promotes neural plasticity and neurogenesis, exerts pro-nociceptive perception in the uninjured spinal cord, and elicits neuroprotective effects after injury to promote functional recovery [48]. BDNF mediates its effects by binding to tyrosine receptor kinase B (TRKB), which activates the Ras/MAPK cascade through a feedback mechanism [49]. PKA also phosphorylates dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32—a complex integrator of dopaminergic signalling) at Thr34 to inhibit the activity of protein phosphatase 1 (PP1) on CREB; however, DARPP32 also acts as a PKA inhibitor when phosphorylated at Thr75 [50]. In myocytes, EPAC2 evokes intracellular Ca2+ spikes via calmodulin kinase II (CAMKII) activation and ryanodine receptor (RyR) modulation, thereby linking the cAMP pathway to Ca2+ signalling [51].
qPCR-mediated Confirmation of transcriptional alterations to the cAMP signalling pathway after SCI. A Schematic representation of the cAMP pathway depicting relevant components. The clock icon next to each component represents the evolution of gene expression alterations (from 1 week [T1] to 8 weeks [T8]) as indicated in the legend. B Heatmap of cAMP signalling pathway genes showing logFC for each gene over time. Red, upregulation; Blue, downregulation. C Microarray expression data validation by qPCR-mediated assessment of gene expression of cAMP-related genes for evolution (from top to bottom) and tissue localization (from left to right) in uninjured spinal cord/control (Black) versus SCI (Green). Data shown as mean ± SEM. Each gene independently assessed for normality using the Shapiro–Wilk test and then analysed using a one-tailed unpaired t-student; *p < 0.05, **p < 0.01, ***p < 0.001
For all evaluated time points after SCI, we confirmed the significant dysregulation of multiple critical genes involved in the cAMP signalling pathway (as represented in Fig. 3A and shown in an associated heatmap in Fig. 3B [interactive version in Online Resource File 1]) compared to the uninjured spinal cord (T0) when using a false discovery rate (FDR) of < 0.05. We discovered the significant downregulation of ADCY2, 3, 5, and 8 and upregulation of ADCY1, 4, and 6, which agrees with previous observations made in dorsal root ganglia neurons after SCI [52]. We also observed the downregulated expression of the direct cAMP targets PKA and EPAC2 after SCI; however, EPAC2 levels suffered a more robust downregulation than PKA at all evaluated time points, with fold changes ranging from – 2.04 to – 2.89 for EPAC2 and – 0.66 to – 0.88 for PKA in comparison with the uninjured spinal cord (T0). Similarly, we encountered the repression of TRKB2, CAMKK2, and BDNF in all groups after SCI (Fig. 3B, lower section), the upregulation of RAP1B, PPP1CA, and CREB, and the maintained expression of MEK, ERK, and other cAMP-related genes (Fig. 3B). A closer analysis of DARPP32 expression revealed a biphasic behaviour, with downregulated expression occurring at early stages after injury followed by subsequent upregulated expression at chronic stages (Fig. 3B).
We further validated EPAC2, BDNF, PKA, CAMKK2, and CREB transcriptional changes by qPCR analysis of independent samples in rostral (thoracic vertebrae 5–7), injury-affected (thoracic vertebrae 8–9), and caudal (thoracic vertebrae 10–11) segments (1.5 cm long each) (Fig. 3C). The segmental analysis of EPAC2 expression demonstrated significant downregulation at the epicentre of the injury at one (T1), two (T2), and eight (T8) weeks after injury. At week 1, we also encountered significantly downregulated EPAC2 expression within rostral segments (Fig. 3C). BDNF mRNA expression levels significantly diminished caudal to the injury at every time point after SCI compared to the uninjured spinal cord (T0) (Fig. 3C), which agrees with the independent microarray dataset. At chronic stages (T8), BDNF expression became significantly reduced within the injury zone, suggesting an extended lack of neurotrophic factors within more rostral zones. The slight downregulation detected in PKA by the microarray could not be reproduced by qPCR; this discrepancy may arise from the difference in the relative sensitivities of the associated techniques. We found a significant downregulation of CAMKK2 expression at the injury site 1 and 4 weeks after SCI, which partially replicates previous results from the microarray data set. In agreement with microarray data, qPCR analysis replicated the bi-phasic behaviour of DARPP32 expression with an early downregulation at 1 week (rostral and caudally to the lesion site) that became upregulated 8 weeks after SCI (within rostral and injury segments). Meanwhile, we observed upregulated CREB expression 1 week after SCI in the injury site and the rostral region 2 weeks after SCI. Hence, qPCR analysis faithfully replicated most of the transcriptional changes observed in the whole rat spinal cord transcriptional analysis in separate sets of samples. The results provide more evidence for the significant alteration of genes related to cAMP signalling after SCI.
NPC transplantation rescues SCI-induced alterations to the cAMP signalling pathway
The functional evaluation of rats receiving NPC transplantation after contusive injury demonstrated significant improvements starting from the second week after therapy (Fig. 4A). Thus, we aimed to transcriptionally evaluate the early impact of NPC therapy on SCI to reveal those mechanisms eliciting later functional recovery. Therefore, we compared the transcriptional profile of animals 1 week after receiving NPC transplants at acute (right after injury) or subacute (1 week after injury) stages after SCI with time-matching non-transplanted animals (Fig. 4B).
Transcriptional profile analysis after acute/subacute NPC transplantation and analysis of transcriptional changes in cAMP-related genes. A Functional locomotor evaluation by open-field BBB scale over 8 weeks post-SCI and after acute or sub-acute NPC transplantation (parallel animals to those used for the microarray analysis). Data expressed as mean ± S.E.M., determined by two-way mixed model ANOVA with Tukey's multiple comparison test (#p < 0.05 T1-NPC versus SCI; &p < 0.05 T2-NPC versus SCI) (n = 6 animals). B Schema describing the in vivo experimental design. C Principal component analysis (PCA) of our data set, showing clear segregation between the different experimental groups used to analyse the impact of NPC transplantation on SCI-induced transcriptional alterations; v1–v4 indicates replicates for each condition. D Bar-graph representing logFC regarding cellular responses to cAMP for indicated comparisons, showing that NPC transplantation reverses the SCI-induced downregulation in this biological function. E Heatmap representing differentially-activated GO functions computed by the Hipathia algorithm, which provided significant results between uninjured (dark grey), injured at week 1 (T1—purple) or week 2 (T2—orange), acute (pink), and subacute (green) NPC transplantation; v1-v4 indicates replicates for each condition (interactive version at Online Resource File 1) F qPCR analysis of cAMP-related genes after acute (upper row) and subacute (bottom row) NPC transplantation. Data shown as mean ± SEM, evaluated with the Shapiro–Wilk test to assess normality and one-tailed unpaired t-student or Mann–Whitney test to compare groups: *p < 0,05, **p < 0.01, ***p < 0.001
PCA analysis showed clear segregation of the different experimental groups, demonstrating consistency between the experimental replicates (Fig. 4C). The differential expression analysis of transplanted versus non-transplanted injured animals revealed that acute NPC transplantation (T1NPC) transcriptionally modulated a total of 4227 genes (1933 upregulated and 2294 downregulated), impacting 1032 SCI-related genes (846 early subacute-SCI DEGs and 186 subacute-SCI DEGs) (Online Resource Fig. 3). The subacute transplantation of NPCs (T2NPC) modulated a lower number of genes than acute transplantation (3364 genes; 1639 up- and 1725 down-regulated) but had a more significant impact (1281 genes) on SCI-related genes (1019 early subacute-SCI DEGs and 262 late subacute-SCI DEGs). These findings suggest that NPC transplantation at acute and subacute stage SCI significantly impacts transcription in spinal cord tissue; however, subacute NPC transplantation provided a more specific effect regarding the modulation of SCI-induced alterations (Online Resource Fig. 3).
We next functionally analysed the mechanism underlying NPC therapy by applying the Hipathia algorithm to calculate the activation levels of GO biological functions (Fig. 4E). Online Resource Fig. 4 and Online Resource Table 4 depict some functions that reported differential activation levels in injured non-transplanted versus injured NPC transplanted animals. Among the differentially modulated functions, we encountered GO terms related to immunomodulation, such as "Interferon-gamma mediated signalling" (GO:0,060,333), "Positive regulation of interleukin-1 beta secretion" (GO:0,032,731), and "Positive regulation of T-cell proliferation" (GO:0,042,102). We also observed the modulation of neural functions such as "Regulation of short-term neuronal synaptic plasticity" (GO:0,048,172), "Neuron projection morphogenesis" (GO:0,048,812), or "Regulation of cell communication by electrical coupling" (GO:0,010,649) after NPC transplantation. NPC-modulated GO terms also included "Removal of superoxide radicals" (GO:0,019,430), "Ion transmembrane transport" (GO:0,034,220), and "Regulation of generation of L-type calcium current" (GO:1,902,514), suggesting the scavenger function and ionic modulatory capacity of NPCs. Furthermore, NPCs modulated functions related to cell survival ("Negative regulation of mitochondrial membrane permeability involved in apoptotic process," GO:1,902,109) and cell proliferation and differentiation ("Astrocyte differentiation," GO:0,048,708; "Endothelial cell morphogenesis," GO:0,001,886). NPC transplantation also regulated molecular pathways such as "Notch signalling" (GO:0,007,219), "Neurotrophin TRK receptor signalling" (GO:0,048,011), "Cellular response to cAMP" (GO:0,071,320) and "Inositol-phosphate mediated signalling" (GO:0,048,016) (Online Resource Fig. 4).
While focusing on cAMP functions, we discovered that NPC transplantation during acute stages after SCI significantly reversed the downregulation of cAMP cellular responses compared to time-matched injured non-transplanted animals (Fig. 4D). We detected the same trend for subacute transplantation, although this failed to reach significance. We assessed gene expression of cAMP-related targets by qPCR (Fig. 4F) to further characterize the impact of NPC therapy on cAMP signalling. We reproduced the experiment using neonatal NPCs, as a more clinically relevant model, to validate previous high content gene transcriptional results (which employed adult NPC transplantation). We found that acute neonatal NPC transplantation rescued SCI-induced reductions in EPAC2 expression in rostral and caudal segments, while subacute NPC transplantation prompted increased EPAC2 expression compared to the non-transplanted group rostral to the injury. Additionally, NPC transplantation increased BDNF expression in the caudal region when applied at acute or subacute stages compared to injured non-transplanted animals. Furthermore, we found a significant increase in BDNF levels in the injured site for the subacute transplantation group. After sub-acute transplantation, we observed a significant induction in PKA and CAMKK2 mRNA levels throughout the spinal cord in rostral, injury, and caudal regions. While we observed a similar trend for acute transplantation, these results failed to reach significance. Finally, NPC transplantation reduced the upregulated levels of CREB encountered after SCI to control levels. Overall, our results suggest that both adult and neonatal NPC transplantation can correct most SCI-driven alterations in the expression of cAMP signalling components.
NPC transplantation after SCI increases rostral axonal levels of EPAC2
To evaluate the influence of EPAC2 on therapeutic outcomes after NPC transplantation at the sub-acute stage after SCI, we administered a specific EPAC2 inhibitor (ESI-05) alongside NPC transplantation (schema shown in Fig. 5A). The quantification of cAMP concentrations in rostral and caudal spinal cord homogenates (0.5 cm distal from the injury epicentre; Fig. 5B) by ELISA immunoassay revealed that animals with SCI exhibited significantly lower cAMP levels in rostral segments compared to the corresponding caudal segment, which indicates an area-dependent depletion of cAMP induced by SCI (Fig. 5B). Interestingly, NPC transplantation (in the absence and presence of ESI-05) prompted a significant increase in rostral cAMP levels compared to control group, indicating that NPC transplantation inhibits the SCI-induced rostral depletion of cAMP levels. In agreement, anti-cAMP staining in the injured segment revealed a higher percentage of total immunolabeled area in injured animals receiving NPC transplantation (SCI + NPCs) and injured animals receiving NPC transplantation and ESI-05 (SCI + NPCs + ESI-05) compared to untreated injured animals (SCI) (Fig. 5C).
In vivo transplantation of NPCs with the EPAC2 inhibitor ESI-05. A Experimental design for the in vivo administration of ESI-05 or vehicle (in SCI and SCI + NPCs groups). Experimental groups: SCI, SCI + NPCs, and SCI + NPCs + ESI-05 (n = 3/group). B Determination of cAMP concentrations by ELISA immunoassay of the regions rostral and caudal to the injury epicentre relative to injured non-transplanted (SCI) animals. C cAMP immunostaining (left panel) and quantification (right panel) in the injured region D qPCR analysis of EPAC2 expression in the rostral spinal segment (one-way ANOVA: *p < 0.05; **p < 0.01). E Histological visualization of EPAC2 immunostaining (orange) overlap** with B-III-Tubulin + tracts (green) (left panel). Representative images (middle panel) of EPAC2 staining in rostral white matter areas for each experimental condition and its quantification (right panel) (one-way ANOVA: *p < 0.05)
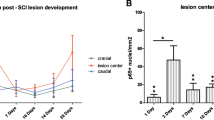
Next, we evaluated the expression of the direct cAMP target EPAC2 in our experimental groups (SCI, SCI + NPCs, and SCI + NPCs + ESI-05). qPCR analysis revealed a significant increase in rostral EPAC2 mRNA levels following NPC transplantation (SCI + NPCs) compared to injured non-transplanted rats (SCI) (Fig. 5D), which agrees with our previous findings (Fig. 4F); however, the addition of ESI-05 (SCI + NPCs + ESI-05) inhibited the NPC-mediated increase in EPAC2 mRNA levels, suggesting that EPAC2 may participate in a positive feedback loop regulating its expression.
Protein immunoreactivity analysis in spinal cord tissues revealed EPAC2 staining within the cytoplasm or plasma membrane of B-III-Tubulin + neuronal bodies in the grey matter and B-III-Tubulin + axonal tracts of the white matter (Online Resource Fig. 5 and Fig. 5E, respectively). We failed to encounter significant differences in the proportion of cytoplasmic or plasma membrane-resident EPAC2 + cells after normalizing data to the total number of EPAC2 + neuronal bodies; however, we observed a significant accumulation of EPAC2 protein in the axonal tracts 0.5 cm rostral to the injury in NPC-transplanted animals (SCI + NPCs) compared to injured non-transplanted animals (SCI) (in agreement with our qPCR data), as supported by a previous study showing elevated levels of EPAC2 only in neurites and growth cones [53]. In contrast, NPC transplantation combined with ESI-05 treatment (SCI + NPCs + ESI-05) reduced the amount of EPAC2 encountered in rostral axonal tracts (Fig. 5E). This result agrees with previous observations showing that EPAC2 inhibition leads to a reduction in the number of vesicles expressing EPAC2 after prolonged synaptic activity, given that EPAC2 activity remains essential for the maintenance of the readily-releasable vesicle pool [54]. Although we failed to detect EPAC2 staining in non-neuronal cells, previous data support the expression of EPAC2 in many other cell types, including astrocytes, ependymal cells, and NPCs [55, 56].
EPAC2 inhibition reverses NPC-mediated effects resulting in enlarged injury-affected areas
We next assessed the influence of NPC transplantation in the presence/absence of ESI-05 during the early stages of scar resolution (2 weeks after injury) by measuring the extent of the area delimited by the astrocytic barrier (the GFAP- area). Analysis of the GFAP- area throughout the central slices of the injured spinal cord revealed that NPC-treated animals (SCI + NPCs) displayed a significantly reduced scar area compared to injured non-transplanted animals (SCI); however, co-treatment with ESI-05 (SCI + NPCs + ESI-05) abolished the effect of NPCs on scar resolution, resulting in the development of a scar area similar to injured non-transplanted animals (Fig. 6A, B). Overall, our results describe a critical role for EPAC2 during the early stages of glial scarring in vivo.
Effects of dual NPC and ESI-05 treatment on scar area, microglial phenotype, and NeuN + cell gap. A Quantification of the scar area (GFAP-) throughout longitudinal slices in the mid-region of the injured spinal cord. Two-way ANOVA: ****p < 0.0001. B Representative images of GFAP immunostaining and traced GFAP- area (white line). C Morphological visualization of microglial cells by Iba-1 staining and determination of the percentage of ramified microglial cells. D Pie charts representing the microglial population segregated in ramified or circular cells for each experimental group. E Representative images of P2X4 immunostaining and its quantification. One-way ANOVA: *p < 0.05. F Representative images (left panel) of the sparsity of NeuN + cells through the injury and quantification of the gap distance (right panel). G Heat map showing the mean number of NeuN + cells per experimental group in each spinal segment of 2 mm
ESI-05 treatment evokes a phenotypical microglial shift towards amoeboid-like cells reversing the ramified phenotype induced by NPC transplantation
We next evaluated microglial morphology by Iba-1 staining in terms of circularity [57]. Microglia are morphologically dynamic cells that change from a multi-branched "ramified" state under homeostatic conditions to an amoeboid-like shape under pathological conditions [58]. Circular microglia generally display a proinflammatory M1 profile, while ramified microglia have been associated with an anti-inflammatory and neuroprotective M2 state [59]. To focus on the microglial cell population with greater relevance to neuroprotection, we specifically studied the morphology of microglia in white matter regions rich in NeuN + neurons. Furthermore, the study of Iba-1 staining in those specific regions allows the exclusion of macrophages from our analysis since they localize to peri-injury areas. We discovered that NPC transplantation (SCI + NPCs) increased the percentage of ramified microglia (Fig. 6C) up to 54.0 ± 2.6% in comparison to untreated injured animals (SCI) in these areas, thereby creating a larger anti-inflammatory microglial population (Fig. 6D). Overall, these findings suggest that NPC transplantation prompts a shift in microglial morphology in the NeuN + cell microenvironment within the injured spinal cord. Strikingly, dual treatment with NPCs and ESI-05 (SCI + NPCs + ESI-05) partially reversed the effect of NPCs on microglial status (resulting in a population of 41.9 ± 5.4% ramified-like cells), which suggests that EPAC2 mediates the NPC-induced alterations in the microglial population (Fig. 6C, D). Accordingly, animals receiving NPC transplantation (SCI + NPCs) displayed a reduction in the area positive for P2X4 receptor expression (Fig. 6E), which becomes upregulated in pathological conditions in activated microglia/macrophages [60], when compared to injured untreated animals (SCI) or animals receiving NPC transplantation and ESI-05 treatment (NPCs + ESI-05).
EPAC2 mediates NPC transplantation-related neuroprotection after SCI
Finally, we measured the gap between rostral and caudal NeuN + cells across the injury to evaluate the effects of various treatments on neuronal sparsity caused by SCI. Interestingly, NPC transplantation (SCI + NPCs) significantly reduced the gap between NeuN + cells (Fig. 6F) and increased the density of NeuN + cells encountered in the areas surrounding the injury epicentre (Fig. 6G) compared to injured untreated animals (SCI). Of note, animals receiving NPC transplantation and ESI-05 treatment (NPCs + ESI-05) exhibited a similar gap distance and NeuN density to injured non-transplanted animals (SCI), thereby demonstrating the involvement of EPAC2 in NPC-mediated neuroprotection (Fig. 6F, G).