Abstract
Background:
Angiopoietin-like 4 (ANGPTL4) belongs to the angiopoietin-like protein family and mediates the inhibition of lipoprotein lipase activity. Emerging evidence suggests that ANGPTL4 has pleiotropic functions with anti- and pro-inflammatory properties.
Methods:
A thorough search on PubMed related to ANGPTL4 and inflammation was performed.
Results:
Genetic inactivation of ANGPTL4 can significantly reduce the risk of develo** coronary artery disease and diabetes. However, antibodies against ANGPTL4 result in several undesirable effects in mice or monkeys, such as lymphadenopathy and ascites. Based on the research progress on ANGPTL4, we systematically discussed the dual role of ANGPTL4 in inflammation and inflammatory diseases (lung injury, pancreatitis, heart diseases, gastrointestinal diseases, skin diseases, metabolism, periodontitis, and osteolytic diseases). This may be attributed to several factors, including post-translational modification, cleavage and oligomerization, and subcellular localization.
Conclusion:
Understanding the potential underlying mechanisms of ANGPTL4 in inflammation in different tissues and diseases will aid in drug discovery and treatment development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiopoietin-like 4 (ANGPTL4) is a multifaceted secreted protein. It was discovered simultaneously by three different institutions in 2000 [1,2,3] and later unified by the HUGO Gene Nomenclature Committee as ANGPTL4 [4]. ANGPTL4 is highly expressed in adipose tissues and the liver of humans and mice, and to a lesser extent in the heart, muscle, kidney, skin, and other tissues [2, 5]. Its expression is modulated by the nutritional, metabolic, and inflammatory status of an organism [6]. Initially, ANGPTL4 was found to be induced by fasting, which subsequently inhibited endogenous lipoprotein lipase (LPL), thereby regulating the triglyceride (TG) metabolism. Thus, related studies have focused on lipid metabolism and glucose homeostasis [7,8,9,10]. ANGPTL4-deficient mice exhibit increased plasma LPL activity and TG clearance, and decreased plasma TG levels [11,12,13,14]. Consistent with this finding, human monoclonal antibody against ANGPTL4 reduced the circulating TG levels in mice and monkeys [15]. Human genetic studies have shown that genetic inactivation of ANGPTL4 (E40K variant) can significantly reduce the risk of develo** diabetes and coronary artery disease [8, 10, 15, 16]. Unfortunately, dietary saturated fat induces a pro-inflammatory and ultimately lethal phenotype in mice lacking ANGPTL4, including fibrinopurulent peritonitis, ascites, intestinal fibrosis, and cachexia [13]. Antibodies against ANGPTL4 result in several undesirable effects in mice or monkeys, including lymphadenopathy [15] and ascites [12]. And ANGPTL4−/− mice showed lipid-enriched foamy macrophages in the mesenteric lymph nodes [12]. Carriers of E40K mutation in humans did not have reports of these severe phenotypes suggesting that E40K mutation does not adequately represent ANGPTL4 deletion. These observations indicate that ANGPTL4 functions more than just regulating lipids, which could have confounding effects on other pathological processes [17].
The inflammatory signaling regulation by ANGPTL4 has recently attracted increased attention. ANGPTL4 expression reportedly increases in inflamed brain, adipose, pancreas, colon, and lung tissues, suggesting that ANGPTL4 is an inflammatory mediator [18,19,20,21,22]. In contrast, studies have reported that ANGPTL4 protects against the severe pro-inflammatory effects of saturated fat and increases the number of anti-inflammatory macrophages in peritonitis and myocardial infarction [13, 23]. ANGPTL4’s role in inflammation appears to be bidirectional, and its exact mechanism is not fully understood. Therefore, understanding the role and potential underlying mechanisms of ANGPTL4 in inflammation in different tissues and diseases will aid in drug discovery and treatment development.
Here, we systematically reviewed published studies and identified the functions, possible mechanisms, and therapeutic value of ANGPTL4 in inflammation and inflammatory diseases.
Characteristics of ANGPTL4
ANGPTL4 belongs to the ANGPTL protein family, which consists of eight secreted glycoproteins, known as ANGTPL1–8. Except for ANGPTL5, all ANGPTL proteins have been identified in humans and mice. The human ANGPTL4 gene is located on chromosome 19p13.3, and encodes a glycosylated secreted protein (fANGPTL4, 45–65 kDa). fANGPTL4 is then rapidly post-translationally cleaved into an N-terminal coiled-coil domain (nANGPTL4, ~ 15 kDa) and a C-terminal fibrinogen-like domain (cANGPTL4, ~ 35 kDa) [24]. Alternative splicing results in multiple transcript variants. nANGPTL4 mediates ANGPTL4 oligomerization and binds to LPL to modulate lipoprotein metabolism. cANGPTL4 is involved in energy expenditure and several non-lipid-related processes, including angiogenesis, inflammation, oxidative stress, vascular permeability, and wound healing (Fig. 1) [21, 25,26,27,28]. In mice, ANGPTL4 is expressed in adipose tissue, heart, liver, small intestine, skin, and skeletal muscle. In humans, it is primarily produced in the liver, plasma, small intestine, placenta, heart, and adipose tissue [2].
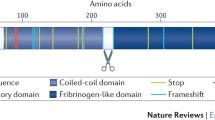
Structure, function, and receptor of ANGPTL4. Angiopoietin-like 4 (ANGPTL4) has three functional domains: the signal peptide, the coiled-coil (N-terminal chain, CCD) and the fibrinogen-like (C-terminal chain, FLD) domains. Each domain is indicated as a different color. Full-length ANGPTL4 (fANGPTL4) is proteolytically cleaved by proprotein convertases (PCs, including PC5/6, PC7, Furin, and PACE4) at their recognition motif (R161RKR164) in the linker region. The cleavage site in ANGPTL4 is indicated by the scissors symbol. Before cleavage, ANGPTL4 forms oligomers mediated by disulphide bond-forming cysteine residues in nANGPTL4. After cleavage, ANGPTL4 is released from the cells where nANGPTL4 remains oligomerized, while cANGPTL4 dissociates into monomers. The ability to bind and inhibit LPL activity is limited to nANGPTL4 and fANGPTL4 by D39E40 sites, whereas, cANGPTL4 is involved in several non-lipid related processes. “ ” denotes cysteine residues in nANGPTL4, and “
” denotes cysteine residues in nANGPTL4, and “ ” denotes potential N-glycosylation site. LPL, lipoprotein lipase; ROS, reactive oxygen species; EGFR, epidermal growth factor receptor; EMT, epithelial–mesenchymal transition
” denotes potential N-glycosylation site. LPL, lipoprotein lipase; ROS, reactive oxygen species; EGFR, epidermal growth factor receptor; EMT, epithelial–mesenchymal transition
ANGPTL protein family is structurally homologous to angiopoietin (ANG), an angiogenic regulator; however, ANGPTLs do not bind to the ANG receptors Tie1 or Tie2 [24, 29]. ANGPTL4 was previously known as an orphan receptor. With research progression, ANGPTL4 receptors were gradually discovered recently. Huang et al. [26] previously reported that cANGPTL4 binds to α5β1, VE-cadherin, and claudin-5, thus modulating vascular junction integrity. In normal and tumor epithelial cells, cANGPTL4 interacts with α5β1 and αvβ5 leading to anoikis resistance [30]. It was also reported that cANGPTL4 modulates keratinocyte migration via integrins β1 and β5 [28]. Using surface plasmon resonance binding and proximity ligation assays, Gomez Perdiguero et al. [31] demonstrated that fANGPTL4 directly binds to αvβ3 integrin in hypoxia-driven VEGF-mediated vascular permeability. Syndecans could mediate ANGPTL4-induced intracellular signaling by binding to nANGPTL4 [32]. And cANGPTL4 binds directly to neuropilins on endothelial cells to induce diabetic macular edema [33]. ANGPTL4 also binds to EGFR and enhanced stress-induced EGFR/JAK1/STAT3 signaling to drive p21 expression in ovarian granulosa cells [34].
Specific agonists and inhibitors regulate ANGPTL4 function. Agonists include peroxisome proliferator-activated receptor (PPAR), glucocorticoid receptor (GR), and protein kinase C (PKC) agonists; inhibitors include angiotensin blockers, leptin, resistin, insulin, and calcineurin inhibitors (cyclosporin A and tacrolimus) [35,36,37,38]. Certain transcription factors directly activate the expression of ANGPTL4, including signal transducer and activator of transcription 3 (STAT3) [20], retinoic acid receptor-related orphan receptor α (RORα) [23], GR [38], forkhead box protein A1 (FOXA1) [38], c-Myc [39], and hypoxia inducible factor-1α (HIF-1α) [40,41,42]. And small mothers against decapentaplegic (Smad) signaling could regulate the expression of human ANGPTL4 [25, 43]. The chromatin immunoprecipitation assay reveals enhanced binding between c-Myc and the promoter region of ANGPTL4 in LN229-vIII cells, which might contribute to angiogenesis induction in gliomas [39]. Real-time PCR analysis of immunoprecipitated chromatin shows that RORα binds to the ANGPTL4 promoter [23]. HIF-1α directly mediates hypoxia-induced ANGPTL4 expression in tumor tissues, cardiomyocytes, and endothelial cells [40,41,42]. Hyperoxia treatment of adipocytes causes downregulation of ANGPTL4 expression, release of reactive oxygen species, and upregulation of pro-inflammatory adipocytokines such as interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) [44]. ANGPTL4 is also regulated by STAT3-[20] and YAP-mediated mechanism [45] and transforming growth factor-β (TGFβ)/Smad signaling pathway [25, 43]. Growth hormone upregulates ANGPTL4 mRNA expression and suppresses LPL activity via fatty acids [46]. Two transcription factors, GR and FOXA1, have been identified as important transcriptional activators of ANGPTL4 by mutational analysis, RNA interference assays, and electrophoretic mobility-shift assays in bovines [38].
In addition to the above, several studies showed that the steps involved in the post-translational modifications, cleavage and oligomerization, subcellular localization, and other aspects deserve further attention.
Post-translational modification
Post-translational modifications, such as glycosylation, phosphorylation, and myristoylation, reportedly play essential roles in ANGPTL4 regulation. The expected molecular weight of the fANGPTL4 protein is 45 kDa, with a predicted glycosylation site at Asparagine-236/242 (in mouse), Asparagine-231/237 (in rat) or Asparagine-177 (in humans). A reduction in the molecular weight of fANGPTL4 after treatment of brown adipose tissue lysates with PNGase-F (an N-glycosidase) demonstrated that ANGPTL4 is an N-glycosylated protein [47]. Treatment of cANGPTL4 with PNGase-F also led to a significant decrease in the molecular mass [48]. These results suggested that cANGPTL4 contains complex oligosaccharide structures. ANGPTL4 has three potential N-glycosylation sites and appears to be sialylated.
Another study found that podocytes secrete two distinct forms of ANGPTL4: (1) a high-isoelectric point (pI) pro-proteinuric form that is hyposialylated and noted only in the glomerulus and urine and (2) a neutral-pI form that is properly sialylated [49]. An in vitro study using cultured glomerular endothelial cells showed that high-pI ANGPTL4 increases and neutral-pI ANGPTL4 reduces endothelial injury in the setting of oxidative stress [49]. Treatment with the sialic acid precursor N-acetyl-d-mannosamine (ManNAc) converts high-pI to neutral-pI glomerular ANGPTL4 in vivo and significantly reduces albuminuria and proteinuria. Supplementation with the sialic acid precursor ManNAc reverses these pathological changes and confers renoprotection in a mouse model of diabetic nephropathy [50].
Sialylation plays a vital role in glycoprotein regulation. Loss of α2-6 sialylation promotes the transformation of synovial fibroblasts into a pro-inflammatory phenotype in arthritis [51]. Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease [52]. Hyposialylated IgG activates the endothelial IgG receptor FcγRIIB to promote obesity-induced insulin resistance [53]. In high-fat diet (HFD)-fed mice, supplementation with the sialic acid precursor, ManNAc, restores IgG sialylation and preserves insulin sensitivity without affecting weight gain [53]. Therefore, the pro- or anti-inflammatory effects of ANGPTL4 are associated with its sialylation.
Cleavage and oligomerization
ANGPTL4 undergoes proteolytic cleavage after secretion, releasing a smaller N-terminal chain (nANGPTL4) and a larger chain containing the fibrinogen C-terminal domain (cANGPTL4). ANGPTL4 N-terminal chain forms disulphide-linked dimers and tetramers. Cysteine-76 and 80 positions of ANGPTL4 are required to form higher-order structures in mice, rats, and humans. Oligomerization results in the increased stability of nANGPTL4 and its ability to inhibit LPL [54]. Future studies are required to assess whether ANGPTL4 oligomerization impacts systemic inflammatory paradigms in vivo.
ANGPTL4 can be proteolytically cleaved by pro-protein convertases (PCs) at its RRKR-consensus cleavage site. The correlation between truncated forms of ANGPTL4 and PC expression or activity remains unclear. Adipocytes express fANGPTL4, which can form dimers and tetramers. In contrast, hepatocytes produce ANGPTL4 with a highly hydrophobic signal peptide, an N-terminal coiled-coil fragment, and a COOH-terminal fibrinogen-like domain. However, the mechanism underlying this tissue-dependent expression remains unknown. During influenza pneumonia, the concomitant increase in furin activity cleaves fANGPTL4 to generate cANGPTL4, corresponding to extensive lung injury characterized by large regions of pulmonary haemorrhage and host immune cell infiltration. cANGPTL4 immunoneutralization significantly reduces tissue leakage and accelerates lung recovery [20]. ANGPTL4 is cleaved in a serum-dependent manner [48]. fANGPTL4 appeared in the medium first, with subsequent increase in the cleavage products during incubation [55]. The changes in fANGPTL4/cANGPTL4 may have different effects at different times. A recent finding has established opposing pro-tumorigenic and anti-tumorigenic functions of ANGPTL4 and cANGPTL4 compared with nANGPTL4. It showed that cANGPTL4 facilitated tumor growth and metastasis while the nANGPTL4 prevented metastasis and enhanced overall survival. Tracing ANGPTL4 and its fragments in tumor patients detected local fANGPTL4 in tumor specimens, whereas nANGPTL4 predominated in systemic circulation, and serum nANGPTL4 levels correlated inversely with disease progression [56].
Subcellular localization
This evolution allows each expressing cell type to independently communicate its physiological status and environmental exposures systemically by secreting ANGPTL4 into the circulation and locally by secreting ANGPTL4 into the extracellular space. ANGPTL4’s role as an inflammatory or anti-inflammatory gene may depend on its subcellular localization in specific cells and tissues. Data from the Human Protein Atlas may assist in this regard. It indicates that ANGPTL4 is localized to the nucleoplasm and vesicles. Recent findings have shown that ANGPTL4 is enriched in exosomes by the markers CD63 and CD9 [57]. Since its discovery, much attention has been paid to the ANGPTL4’s secretory function. ANGPTL4’s role in the nucleoplasm and cytoplasm requires further investigation. ANGPTL4 has a dual role in urothelial carcinoma, either as a tumor suppressor within tumor cells or as an oncogene that is exogenously contributed by the microenvironment [58]. A new tool, Moonlight, successfully identified BCL2, SOX17, and ANGPTL4 as a dual-role gene, which have complex interactions with biological process mediators, oncogenes, and tumor suppressors [59].
The ANGPTL family functions not only as a secreted protein, but also as a cytoplasmic protein. To date, the studies on ANGPTL4 have been limited, its role in cells deserves additional attention. These seemingly contradictory roles of ANGPTL4 in inflammation suggest that the intracellular effects of this protein may differ from its distant hormonal actions on other locations in the body, such as blood vessels.
Other aspects
Other factors may have also been involved. The effect of ANGPTL4 in the early and late stages of stomatitis could reportedly result in different outcomes [60]. Another potential explanation is the varied lipopolysaccharide (LPS) dosages or concentrations used in various experimental models. As outlined previously, 100 ng/ml LPS stimulation can induce ANGPTL4 expression in primary and THP1-derived macrophage cells [21, 61], whereas, ANGPTL4 levels declined in 1 μg/ml LPS-treated Caco-2 cells [62]. ANGPTL4-treated THP-1 macrophages show significant reduction in inflammatory genes in a dose-dependent manner [23]. The different effects of ANGPTL4 on inflammation may depend on the LPS dose administered. Furthermore, ANGPTL4 receptor presence was still rare in inflammation in the current study, the detailed mechanism of ANGPTL4 in inflammatory diseases needs to be explored in future studies.
We noted that ANGPTL4 is silenced by aberrant DNA methylation of CpG islands in human gastric cancers and carcinomas [63, 64]. DNA methylation-mediated downregulation of ANGPTL4 promotes colorectal cancer metastasis by activating the ERK pathway [65]. Whether ANGPTL4 methylation plays a pro- or anti-inflammatory role in inflammatory diseases will require further studies.
ANGPTL4 on inflammatory processes and diseases
Inflammatory response is the primary immunological response of the body to maintain homeostasis after exposure to pathogenic infection, endogenous injury, or tissue stress. Excessive acute inflammatory responses, due to infectious diseases or acute organ injury, and chronic inflammation are thought to be drivers of metabolic syndrome, tumor progression, and autoimmune diseases [66]. In recent studies, inflammatory responses associated with ANGPTL4 include lung injury, pancreatitis, heart diseases, gastrointestinal diseases, skin diseases, metabolism, periodontitis, and osteolytic diseases (Fig. 2).
ANGPTL4 on inflammatory processes and diseases. ANGPTL4 can regulate the inflammation-related gene expression in LPS-induced acute lung injury, influenza infection, and COVID-19 (NF-κB, SIRT1, IL-6, and IL-1β). In acute myocardial infarction and peritonitis, ANGPTL4 mediates anti-inflammatory effects in the pathological microenvironment by modulating the inflammation-related gene expression in macrophages (Arg1, CD206, IL-10, iNOS, IL-6, IL-1β, and MCP-1). In acute pancreatitis, ANGPTL4 enhances macrophage activation and leads to hypercytokinemia (C5a, C5aR, IL-6, TNF-α, and IL-1β). ANGPTL4 reduces leukocyte infiltration in colitis and protects the colon from acute inflammation (CCL2, CCL11, CXCL10, IL-10, IL-1β, and IL-17). ANGPTL4 is critical for inflammation and the increase in NF-κB, IL-6, IL-1β, and TNF-α levels in the early stage of stomatitis. And ANGPTL4 can cause anti-inflammatory effects concomitantly with the increase in PPARα in the late stage of stomatitis. ANGPTL4 modulates the immune cell response to acute skin injury, wound healing, and psoriasis (TNF-α, IL-1β, IL-6, IL-17A, PDGF, TGF-β, VEGF, and IL-10). ANGPTL4 is also associated with chronic low-grade inflammation (atherosclerosis, obesity, diabetes, and complications of these diseases.). ANGPTL4 may play a pivotal role in promoting the expression of MMPs in the periodontitis and osteolytic diseases. VEGF, vascular endothelial growth factor; SIRT1, sirtuin 1; PDGF, platelet derived growth factor; PTGS2, prostaglandin-endoperoxide synthase 2; GDF15, growth differentiation factor-15; CCR2, CC-motif receptor 2; CCL2/11, CC-motif chemokine ligand 2/11; MCP-1, monocyte-chemoattractant protein-1; Arg1, Arginase-1; iNOS, inducible nitric oxide synthase; CREB, cAMP-response element binding protein; PPARα, peroxisome proliferator-activated receptor α; MMPs, matrix metalloproteinases
ANGPTL4 and lung injury
Studies have shown that ANGPTL4 may be involved in the inflammatory response in influenza pneumonia and LPS-induced acute lung injury. Li et al. [20] identified elevated expression of ANGPTL4 in lung biopsy specimens from patients with infectious pneumonia compared to normal lung specimens. Further studies revealed that the influenza infection directly stimulated ANGPTL4 expression through the IL6-STAT3 signaling pathway [20]. The concomitant increase in furin activity cleaves fANGPTL4 to generate cANGPTL4, resulting in extensive lung injury characterized by large regions of pulmonary hemorrhage and infiltration of immune cells. ANGPTL4 deficiency improves pulmonary tissue integrity and accelerates recovery. In another recent study, Li et al. [67] showed that antibody treatment against cANGPTL4 could reduce pulmonary edema and damage in infected mice of secondary bacterial pneumonia. And this effect was also confirmed using ANGPTL4−/− mice.
ANGPTL4 levels are elevated in the lung tissue of an acute injury mouse model and in LPS-induced human alveolar epithelial cells. ANGPTL4 promotes the expression of NF-κBp65 and inhibits the expression of SIRT1 in mouse lung and human alveolar epithelial cells. After silencing ANGPTL4 (siRNA), LPS-induced lung inflammation, including neutrophil infiltration, and TNF-α and IL-6 expression in lung tissues, significantly reduced. This is also seen in human alveolar epithelial cells [18]. LPS induces only a slight increase in ANGPTL4 expression in rat pulmonary microvascular endothelial cells (RPMVECs). Overexpression of ANGPTL4 inhibits the LPS-induced increase in the RPMVECs permeability, which is associated with the depolymerization of central F-actin in RPMVECs. Overexpression of ANGPTL4 exerts protective, anti-inflammatory (TNF-α), and anti-angiogenic effects [68].
In chronic obstructive pulmonary disease patients, circulating ANGPTL4 levels are upregulated and have correlations with pulmonary function and systematic inflammation [69]. Another study reported that bronchoalveolar lavage fluid and serum ANGPTL4 levels are elevated and have prognostic value in patients with acute respiratory distress syndrome [70]. A proteomic analysis of 144 autopsy samples from seven organs in COVID-19 patients showed that ANGPTL4 was significantly upregulated in the liver, renal cortex, and medulla, suggesting it might play a critical role in the COVID-19 infection [71]. In addition, Altmayer et al., demonstrated increased serum ANGPTL4 abundance is associated with COVID-19-related encephalitis, which highlights ANGPTL4 as a potential molecular marker for this disorder [72, 73]. These observations underscore the vital role of ANGPTL4 in lung infection, and may facilitate development of future therapeutic strategies for the treatment of lung injury.
ANGPTL4 and pancreatitis
Genetic variants (LPL, APOA5, APOC3, ANGPTL3, and ANGPTL4) associated with increased plasma TG levels increase the risk of acute pancreatitis [74]. Jung et al. [21] demonstrated that ANGPTL4 accelerates the pathological exacerbation of acute pancreatitis by inducing alveolar cell damage and releasing large amounts of inflammatory cytokines. Microarray analysis of pancreatic tissues from mice with mild and severe acute pancreatitis revealed that ANGPTL4 was one of the most upregulated genes [21]. Clinically, ANGPTL4 expression is also elevated in the serum and pancreatic tissues of patients with pancreatitis. In mice, knockdown of either ANGPTL4 gene or ANGPTL4-neutralizing antibodies attenuated the pancreatitis-related symptoms. In contrast, exogenous ANGPTL4 increases inflammatory factor secretion and apoptosis, thereby exacerbating pancreatic injury. ANGPTL4 enhances macrophage activation and promotes pancreatic infiltration, increasing the complement component 5a (C5a) levels via the PI3K/AKT signaling pathway. Activation of the C5a receptor leads to hypercytokinemia (C5aR, IL-6, TNF-α, and IL-1β), which accelerates alveolar cell damage and induces pancreatitis. C5a-neutralizing antibody reduces the inflammatory response of LPS-activated macrophages and decreases the pancreatitis severity. C5a levels have a significant positive correlation with ANGPTL4 levels in patients with pancreatitis. Jung et al. [21] suggested that targeting ANGPTL4 is a potential treatment strategy for pancreatitis.
The physiological role of ANGPTL4 may be largely contingent on the pathological conditions. It is reported in the LPS-induced peritonitis mouse model, ANGPTL4 was highly induced and released from mesenchymal stem cells (MSCs) to exert the anti-inflammatory effect [23]. Real-time PCR results of peritoneal macrophages showed that the ANGPTL4-treated group had significantly increased anti-inflammatory Arg1, CD206, and IL-10 expression and decreased pro-inflammatory iNOS, IL-6, and IL-1β expression than the vehicle group did. Recombinant ANGPTL4 suppresses the activation of peritoneal and bone marrow-derived macrophages (BMDM).
ANGPTL4 and heart disease
Cho et al. [23] demonstrated that ANGPTL4 secreted by MSCs has potential anti-inflammatory effects in a pathological microenvironment and that ANGPTL4 can regulate the inflammation-related gene expression in macrophages (Arg1, CD206, IL-10, iNOS, IL-6, IL-1β, and MCP-1).
MSCs can inhibit pathological inflammation; however, the underlying mechanisms remain unclear. Under co-culture conditions with macrophages, MSCs increase ANGPTL4 expression to blunt the polarization of macrophages toward the pro-inflammatory phenotype. Myocardial reperfusion injury after acute myocardial infarction (AMI) involves a series of pathological responses, such as hemorrhage, haematoma, and inflammation. During AMI, hypoxia induces ANGPTL4 elevation, which provides secondary myocardial infarction preservation and regulates myocardial infarct size, recurrent flow, and vascular injury. ANGPTL4 mediates cardioprotection during AMI by targeting the no-reflow phenomenon [75]. In addition, plasma ANGPTL4 expression levels at admission in patients with AMI correlate with no-reflow after infarction and can predict disease prognosis [76].
ANGPTL4 secreted by the MSCs in the area of myocardial infarct has potential anti-inflammatory effects in the pathological microenvironment by modulating the inflammation-related gene expression in macrophages [23].
ANGPTL4 and gastrointestinal diseases
Several gastrointestinal diseases exhibit a persistent and exacerbated inflammatory response that can lead to hypercytokinemia and ultimately, extensive tissue damage. ANGPTL4 reduces leukocyte infiltration in colitis and protects the colon from acute inflammation (CCL2, CCL11, CXCL10, IL-10, IL-1β, and IL-17) [22]. ANGPTL4 deficiency (ANGPTL4−/−) exacerbates colonic inflammation caused by dextran sulphate sodium (DSS) or stearic acid. Microarray gene profile analysis of the DSS-treated ANGPTL4−/− mice colon revealed that the ANGPTL4 gene was associated with leukocyte migration, infiltration, and inflammation in ulcerative colitis. Human colonic epithelial cell experiments have shown that ANGPTL4 mediates the colonic inflammatory response through the tristetraprolin (TTP or ZFP36) regulation of chemokine transcriptional stability. ANGPTL4 protects the colon from acute inflammation and its deletion exacerbates the severity of inflammation [22]. Modulation of bile acid-inducible microbial genes in the gut microbiota may be employed to influence ANGPTL4 expression and modulate the intestinal inflammation in inflammatory bowel disease [77].
ANGPTL4 is critical for inflammation and the increase in NF-κB, IL-6, IL-1β, and TNF-α levels in the early stage of stomatitis. The excessive production of ANGPTL4, however, can cause anti-inflammatory effects concomitantly with the increase in PPARα in the late stage of stomatitis [60]. This suggests that the ANGPTL4 role in the early and late stages results in different outcomes.
ANGPTL4 and skin diseases
Normal wound healing requires hemostasis, inflammation, re-epithelialization, and matrix remodeling to re-establish a new epithelial barrier [78]. The inflammatory response plays a bidirectional role in wound healing and determines the time required and the degree of healing. A persistent inflammatory response at the wound site is a major reason for the long-term non-healing of wounds.
Diabetic wounds have low endogenous cANGPTL4 levels and are associated with an elevated F4/80+ macrophage population. F4/80+ macrophage infiltration reduces upon treatment of diabetic wounds with recombinant cANGPTL4 [79]. Recently, Tan et al. [80] identified that ANGPTL4 modulates the immune cell response to acute skin injury. Using flow cytometry and single-cell RNA sequencing, the ratio of immune cells in the wound sections of ANGPTL4+/+ and ANGPTL4−/− mice was examined. They found that ANGPTL4 regulates ifi202b expression, which affects the differentiation of monocytes into macrophages, and in turn wound healing. Furthermore, in patients with diabetes, the function of the gene responsible for re-epithelialization is altered and wound healing is often delayed, which may also be closely linked to the ANGPTL4 gene [81]. ANGPTL4 also promotes keratinocyte proliferation and inflammatory responses (TNF-α, IL-1β, IL-6, and IL-17A) via the ERK1/2 and STAT3-dependent signalling pathways in psoriasis [82].
ANGPTL4 and metabolism
Chronic low-grade inflammation is a non-specific, persistent inflammation characterized by elevated levels of C-reactive protein (CRP), TNF-α, and IL-1. Atherosclerosis, obesity, and diabetes are considered chronic low-grade inflammatory diseases [83]. Recent study has shown that cross-talk between ANGPTL4 gene SNP Rs1044250 and weight management is a risk factor of metabolic syndrome [84]. The role of ANGPTL4 in atherosclerosis has been extensively discussed in a recent review [17] and will not be the focus of this review.
ANGPTL4 can reportedly counteract the severe pro-inflammatory effects of saturated lipids on lipid metabolism [13]. In mesenteric lymph nodes, the ANGPTL4 gene inhibits macrophage LPL, thereby reducing the lipolytic release of fatty acids, macrophage foam cell formation, ER stress, and initiation of a marked inflammatory response (CXCL2, CCR1, PTGS2, and GDF15) [13]. This study provided a novel mechanism for ANGPTL4 regulation of cross-talk between inflammation and metabolism. In addition, ANGPTL4 knockout mice exhibited protection against high-fat diet-induced obesity, which may be associated with the reduced expression of PPAR coactivators [85]. ANGPTL4 silencing in the liver via the antisense oligonucleotides (ASO) reduces plasma TG and glucose levels in mice without causing lymphadenopathy [86]. Hepatocyte-specific suppression of ANGPTL4 improves obesity-associated diabetes and mitigates atherosclerosis in mice [87]. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis [14].
Serum ANGPTL4 is associated with CRP levels in patients with type 2 diabetes, suggesting that ANGPTL4 may be involved in inflammatory progression in the metabolic syndrome [61]. In addition, there is a correlation between vitreous ANGPTL4 expression and inflammatory factor levels in diabetic retinopathy [88]. Knockdown of ANGPTL4 (Si-RNA) suppresses the high glucose-induced cell proliferation, inflammatory response (TNF-α, IL-1β, and IL-6), and ECM accumulation, thus, inhibiting the NF-κB signaling pathway in glomerular mesangial cells [89].
ANGPTL4 partly regulates diabetic retinal inflammation and angiogenesis by activating profilin-1 in vivo and in vitro. The activation of the novel adipocytokine ANGPTL4 was dependent on the overexpression of its upstream mediating factor HIF-1α under high glucose conditions in vivo and in vitro [90].
ANGPTL4 and other diseases
Periodontitis is a major chronic inflammatory disease affecting the oral cavity. Increased expression of ANGPTL4 regulates MMP13 expression in LPS-stimulated gingival fibroblasts and ligature-induced experimental periodontitis [91]. The role of ANGPTL4 in osteolytic diseases, including rheumatoid arthritis, osteoporosis, primary bone tumors, and bone metastatic cancer, has been extensively discussed in a recent review [92]. ANGPTL4 might mediate a component of the hypoxic induction of MMPs and cartilage matrix remodeling [92]. Proposed mechanisms of ANGPTL4 in inflammation above are shown in Fig. 3.
Proposed model of ANGPTL4 in inflammation. In an autocrine/paracrine manner, ANGPTL4 binds to integrins and/or NRP, and subsequently activates FAK, SRC, Rac1, Profilin-1, and RhoA activities, which further activates PI3K/AKT, JAK/STAT3, ERK, and NF-κB signaling pathways. These effects promote inflammation and mediate tissue destruction. On the other hand, the anti-inflammation activities of ANGPTL4 might be attributed to its specific suppression of SRC and ERK pathway. From the current evidence base, the NF-κB signaling pathway is bidirectional in inflammation. ANGPTL4 inhibits LPL activity, controls FA uptake, and regulates circulating TG-rich lipoproteins. Upregulation of ANGPTL4 results in decreased uptake of plasma TG-derived FA, and decreases FA-induced oxidative stress, lipid peroxidation, and inflammation. NRP, neuropilins; FAK, focal adhesion kinase; FFA, fatty acid; LPL, lipoprotein lipase; TG, triglyceride
Summary and outlook
ANGPTL4 is critical for regulating lipid metabolism and may be an attractive therapeutic target for protecting against metabolic disorders. However, antibodies against ANGPTL4 result in several undesirable effects in mice or monkeys, such as lymphadenopathy and ascites. The relationship between inflammation and ANGPTL4 warrants further investigation. By reviewing the literature, we found that ANGPTL4 plays a dual role in inflammatory response. These contradictory results reflect a complex mechanism of ANGPTL4 function and expression across different tissues and cell types, including post-translational modification, cleavage and oligomerization, alternative splicing, subcellular localization, and DNA methylation.
Further studies are needed to elucidate the regulatory mechanisms, including the conditions that increase ANGPTL4 expression in specific diseases, the extracellular or intracellular pathways that mediate ANGPTL4 signaling, and how this regulation affects only the local cellular microenvironment or leads to systemic inflammation. Targeted therapy using alternative approaches, including tissue-specific ASOs targeting ANGPTL4, seem more reasonable.
Availability of data and materials
Not applicable.
References
Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, et al. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J. 2000;346(Pt 3):603–10.
Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000;275:28488–93.
Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, et al. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol. 2000;20:5343–9.
Wain HM, Lush M, Ducluzeau F, Povey S. Genew: the human gene nomenclature database. Nucleic Acids Res. 2002;30:169–71.
Guo L, Li SY, Ji FY, Zhao YF, Zhong Y, Lv XJ, et al. Role of Angptl4 in vascular permeability and inflammation. Inflamm Res. 2014;63:13–22.
Hato T, Tabata M, Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med. 2008;18:6–14.
Liu DJ, Peloso GM, Yu H, Butterworth AS, Wang X, Mahajan A, et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. 2017;49:1758–66.
Gusarova V, O’Dushlaine C, Teslovich TM, Benotti PN, Mirshahi T, Gottesman O, et al. Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nat Commun. 2018;9:2252.
Klarin D, Damrauer SM, Cho K, Sun YV, Teslovich TM, Honerlaw J, et al. Genetics of blood lipids among ~ 300,000 multi-ethnic participants of the Million Veteran Program. Nat Genet. 2018;50:1514–23.
Lotta LA, Stewart ID, Sharp SJ, Day FR, Burgess S, Luan J, et al. Association of genetically enhanced lipoprotein lipase-mediated lipolysis and low-density lipoprotein cholesterol-lowering alleles with risk of coronary disease and type 2 diabetes. JAMA Cardiol. 2018;3:957–66.
Cushing EM, Chi X, Sylvers KL, Shetty SK, Potthoff MJ, Davies BSJ. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol Metab. 2017;6:809–18.
Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, et al. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci U S A. 2007;104:11766–71.
Lichtenstein L, Mattijssen F, de Wit NJ, Georgiadi A, Hooiveld GJ, van der Meer R, et al. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab. 2010;12:580–92.
Aryal B, Singh AK, Zhang X, Varela L, Rotllan N, Goedeke L, et al. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight. 2018;2018:3.
Dewey FE, Gusarova V, O’Dushlaine C, Gottesman O, Trejos J, Hunt C, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med. 2016;374:1123–33.
Stitziel NO, Stirrups KE, Masca NG, Erdmann J, Ferrario PG, König IR, et al. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med. 2016;374:1134–44.
Aryal B, Price NL, Suarez Y, Fernández-Hernando C. ANGPTL4 in metabolic and cardiovascular disease. Trends Mol Med. 2019;25:723–34.
Guo L, Li S, Zhao Y, Qian P, Ji F, Qian L, et al. Silencing angiopoietin-like protein 4 (ANGPTL4) protects against lipopolysaccharide-induced acute lung injury via regulating SIRT1 /NF-kB pathway. J Cell Physiol. 2015;230:2390–402.
Brown R, Imran SA, Wilkinson M. Lipopolysaccharide (LPS) stimulates adipokine and socs3 gene expression in mouse brain and pituitary gland in vivo, and in N-1 hypothalamic neurons in vitro. J Neuroimmunol. 2009;209:96–103.
Li L, Chong HC, Ng SY, Kwok KW, Teo Z, Tan EHP, et al. Angiopoietin-like 4 increases pulmonary tissue leakiness and damage during influenza pneumonia. Cell Rep. 2015;10:654–63.
Jung KH, Son MK, Yan HH, Fang Z, Kim J, Kim SJ, et al. ANGPTL4 exacerbates pancreatitis by augmenting acinar cell injury through upregulation of C5a. EMBO Mol Med. 2020;12: e11222.
Phua T, Sng MK, Tan EH, Chee DS, Li Y, Wee JW, et al. Angiopoietin-like 4 mediates colonic inflammation by regulating chemokine transcript stability via tristetraprolin. Sci Rep. 2017;7:44351.
Cho DI, Kang HJ, Jeon JH, Eom GH, Cho HH, Kim MR, et al. Antiinflammatory activity of ANGPTL4 facilitates macrophage polarization to induce cardiac repair. JCI Insight. 2019;2019:4.
Zhu P, Goh YY, Chin HF, Kersten S, Tan NS. Angiopoietin-like 4: a decade of research. Biosci Rep. 2012;32:211–9.
Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77.
Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ, Tan CK, et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood. 2011;118:3990–4002.
Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, et al. Angiopoietin-like 4 interacts with integrins beta1 and beta5 to modulate keratinocyte migration. Am J Pathol. 2010;177:2791–803.
Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, et al. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J Biol Chem. 2010;285:32999–3009.
Grootaert C, Van de Wiele T, Verstraete W, Bracke M, Vanhoecke B. Angiopoietin-like protein 4: health effects, modulating agents and structure-function relationships. Expert Rev Proteomics. 2012;9:181–99.
Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC, Pal M, et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2(-):H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell. 2011;19:401–15.
Gomez Perdiguero E, Liabotis-Fontugne A, Durand M, Faye C, Ricard-Blum S, Simonutti M, et al. ANGPTL4-αvβ3 interaction counteracts hypoxia-induced vascular permeability by modulating Src signalling downstream of vascular endothelial growth factor receptor 2. J Pathol. 2016;240:461–71.
Kirsch N, Chang LS, Koch S, Glinka A, Dolde C, Colozza G, et al. Angiopoietin-like 4 Is a Wnt signaling antagonist that promotes LRP6 turnover. Dev Cell. 2017;43:71-82.e6.
Sodhi A, Ma T, Menon D, Deshpande M, Jee K, Dinabandhu A, et al. Angiopoietin-like 4 binds neuropilins and cooperates with VEGF to induce diabetic macular edema. J Clin Invest. 2019;129:4593–608.
Jiang Q, Miao R, Wang Y, Wang W, Zhao D, Niu Y, et al. ANGPTL4 inhibits granulosa cell proliferation in polycystic ovary syndrome by EGFR/JAK1/STAT3-mediated induction of p21. FASEB J. 2023;37: e22693.
Wiesner G, Morash BA, Ur E, Wilkinson M. Food restriction regulates adipose-specific cytokines in pituitary gland but not in hypothalamus. J Endocrinol. 2004;180:R1-6.
Koliwad SK, Kuo T, Shipp LE, Gray NE, Backhed F, So AY, et al. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J Biol Chem. 2009;284:25593–601.
Shen X, Zhang Y, Lin C, Weng C, Wang Y, Feng S, et al. Calcineurin inhibitors ameliorate PAN-induced podocyte injury through the NFAT-Angptl4 pathway. J Pathol. 2020;252:227–38.
Mi X, Ning Y, Wang X, Junjvlieke Z, Wang L, Wang S, et al. GR and Foxa1 promote the transcription of ANGPTL4 in bovine adipocytes. Mol Cell Probes. 2019;48: 101443.
Katanasaka Y, Kodera Y, Kitamura Y, Morimoto T, Tamura T, Koizumi F. Epidermal growth factor receptor variant type III markedly accelerates angiogenesis and tumor growth via inducing c-myc mediated angiopoietin-like 4 expression in malignant glioma. Mol Cancer. 2013;12:31.
Li H, Ge C, Zhao F, Yan M, Hu C, Jia D, et al. Hypoxia-inducible factor 1 alpha-activated angiopoietin-like protein 4 contributes to tumor metastasis via vascular cell adhesion molecule-1/integrin β1 signaling in human hepatocellular carcinoma. Hepatology. 2011;54:910–9.
Inoue T, Kohro T, Tanaka T, Kanki Y, Li G, Poh HM, et al. Cross-enhancement of ANGPTL4 transcription by HIF1 alpha and PPAR beta/delta is the result of the conformational proximity of two response elements. Genome Biol. 2014;15:R63.
Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–69.
Gong X, Hou Z, Endsley MP, Gronseth EI, Rarick KR, Jorns JM, et al. Interaction of tumor cells and astrocytes promotes breast cancer brain metastases through TGF-β2/ANGPTL4 axes. NPJ Precis Oncol. 2019;3:24.
Quintero P, González-Muniesa P, García-Díaz DF, Martínez JA. Effects of hyperoxia exposure on metabolic markers and gene expression in 3T3-L1 adipocytes. J Physiol Biochem. 2012;68:663–9.
Cheng JC, Fang L, Li Y, Thakur A, Hoodless PA, Guo Y, et al. G protein-coupled estrogen receptor stimulates human trophoblast cell invasion via YAP-mediated ANGPTL4 expression. Commun Biol. 2021;4:1285.
Hjelholt AJ, Søndergaard E, Pedersen SB, Møller N, Jessen N, Jørgensen JOL. Growth hormone upregulates ANGPTL4 mRNA and suppresses lipoprotein lipase via fatty acids: Randomized experiments in human individuals. Metabolism. 2020;105: 154188.
Dijk W, Heine M, Vergnes L, Boon MR, Schaart G, Hesselink MK, et al. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. Elife. 2015;2015:4.
Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK, Zhang J, et al. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008;28:835–40.
Clement LC, Macé C, Avila-Casado C, Joles JA, Kersten S, Chugh SS. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37–46.
Guo K, Pan P, Wu M, Ma Y, Lu J, Chen H. Hyposialylated angiopoietin-like-4 induces apoptosis of podocytes via β1 Integrin/FAK signaling in diabetic nephropathy. Mol Cell Endocrinol. 2020;505: 110730.
Wang Y, Khan A, Antonopoulos A, Bouché L, Buckley CD, Filer A, et al. Loss of α2-6 sialylation promotes the transformation of synovial fibroblasts into a pro-inflammatory phenotype in arthritis. Nat Commun. 2021;12:2343.
Pagan JD, Kitaoka M, Anthony RM. Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell. 2018;172:564-77.e13.
Tanigaki K, Sacharidou A, Peng J, Chambliss KL, Yuhanna IS, Ghosh D, et al. Hyposialylated IgG activates endothelial IgG receptor FcγRIIB to promote obesity-induced insulin resistance. J Clin Invest. 2018;128:309–22.
Ge H, Yang G, Yu X, Pourbahrami T, Li C. Oligomerization state-dependent hyperlipidemic effect of angiopoietin-like protein 4. J Lipid Res. 2004;45:2071–9.
Lei X, Shi F, Basu D, Huq A, Routhier S, Day R, et al. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J Biol Chem. 2011;286:15747–56.
Hübers C, Abdul-Pari AA, Grieshober D, Petkov M, Schmidt A, Messmer T, et al. Primary tumor-derived systemic nANGPTL4 inhibits metastasis. J Exp Med. 2023;2023:220.
Zhang Y, Liu X, Zeng L, Zhao X, Chen Q, Pan Y, et al. Exosomal protein angiopoietin-like 4 mediated radioresistance of lung cancer by inhibiting ferroptosis under hypoxic microenvironment. Br J Cancer. 2022;127:1260.
Hsieh HY, Jou YC, Tung CL, Tsai YS, Wang YH, Chi CL, et al. Epigenetic silencing of the dual-role signal mediator, ANGPTL4 in tumor tissues and its overexpression in the urothelial carcinoma microenvironment. Oncogene. 2018;37:673–86.
Colaprico A, Olsen C, Bailey MH, Odom GJ, Terkelsen T, Silva TC, et al. Interpreting pathways to discover cancer driver genes with Moonlight. Nat Commun. 2020;11:69.
Tian MM, Wang YS, **ao HB. Dual roles of ANGPTL4 in multiple inflammatory responses in stomatitis mice. Mol Biol Rep. 2022;49:9195–204.
Tjeerdema N, Georgiadi A, Jonker JT, van Glabbeek M, Alizadeh Dehnavi R, Tamsma JT, et al. Inflammation increases plasma angiopoietin-like protein 4 in patients with the metabolic syndrome and type 2 diabetes. BMJ Open Diabetes Res Care. 2014;2: e000034.
Li K, Yang J, Lei XF, Li SL, Yang HL, Xu CQ, et al. EZH2 inhibition promotes ANGPTL4/CREB1 to suppress the progression of ulcerative colitis. Life Sci. 2020;250: 117553.
Kaneda A, Kaminishi M, Yanagihara K, Sugimura T, Ushijima T. Identification of silencing of nine genes in human gastric cancers. Cancer Res. 2002;62:6645–50.
Hattori N, Okochi-Takada E, Kikuyama M, Wakabayashi M, Yamashita S, Ushijima T. Methylation silencing of angiopoietin-like 4 in rat and human mammary carcinomas. Cancer Sci. 2011;102:1337–43.
Zhang K, Zhai Z, Yu S, Tao Y. DNA methylation mediated down-regulation of ANGPTL4 promotes colorectal cancer metastasis by activating the ERK pathway. J Cancer. 2021;12:5473–85.
Yang J, Song QY, Niu SX, Chen HJ, Petersen RB, Zhang Y, et al. Emerging roles of angiopoietin-like proteins in inflammation: mechanisms and potential as pharmacological targets. J Cell Physiol. 2021;237:98–117.
Li L, Foo BJW, Kwok KW, Sakamoto N, Mukae H, Izumikawa K, et al. Antibody treatment against angiopoietin-like 4 reduces pulmonary edema and injury in secondary pneumococcal pneumonia. MBio. 2019;2019:10.
Wang Y, Chen H, Li H, Zhang J, Gao Y. Effect of angiopoietin-like protein 4 on rat pulmonary microvascular endothelial cells exposed to LPS. Int J Mol Med. 2013;32:568–76.
Wu YQ, Shen YC, Wang H, Zhang JL, Li DD, Zhang X, et al. Serum angiopoietin-like 4 is over-expressed in COPD patients: association with pulmonary function and inflammation. Eur Rev Med Pharmacol Sci. 2016;20:44–53.
Hu J, Liu L, Zeng X, Wang K, Wang H, Zeng Z, et al. Prognostic value of angiopoietin-like 4 in patients with acute respiratory distress syndrome. Shock. 2021;56:403–11.
Nie X, Qian L, Sun R, Huang B, Dong X, **ao Q, et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184:775-91.e14.
Altmayer V, Ziveri J, Frère C, Salem JE, Weiss N, Cao A, et al. Endothelial cell biomarkers in critically ill COVID-19 patients with encephalitis. J Neurochem. 2022;161:492–505.
Bhatraju PK, Morrell ED, Stanaway IB, Sathe NA, Srivastava A, Postelnicu R, et al. Angiopoietin-Like4 is a novel marker of COVID-19 severity. Crit Care Explor. 2023;5: e0827.
Hansen SEJ, Madsen CM, Varbo A, Tybjærg-Hansen A, Nordestgaard BG. Genetic variants associated with increased plasma levels of triglycerides, via effects on the lipoprotein lipase pathway, increase risk of acute pancreatitis. Clin Gastroenterol Hepatol. 2021;19:1652-60.e6.
Galaup A, Gomez E, Souktani R, Durand M, Cazes A, Monnot C, et al. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation. 2012;125:140–9.
Bouleti C, Mathivet T, Serfaty JM, Vignolles N, Berland E, Monnot C, et al. Angiopoietin-like 4 serum levels on admission for acute myocardial infarction are associated with no-reflow. Int J Cardiol. 2015;187:511–6.
Hernández-Rocha C, Borowski K, Turpin W, Filice M, Nayeri S, Raygoza Garay JA, et al. Integrative analysis of colonic biopsies from inflammatory bowel disease patients identifies an interaction between microbial bile acid-inducible gene abundance and human angiopoietin-like 4 gene expression. J Crohns Colitis. 2021;15:2078–87.
Cañedo-Dorantes L, Cañedo-Ayala M. Skin acute wound healing: a comprehensive review. Int J Inflamm. 2019;2019:3706315.
Chong HC, Chan JS, Goh CQ, Gounko NV, Luo B, Wang X, et al. Angiopoietin-like 4 stimulates STAT3-mediated iNOS expression and enhances angiogenesis to accelerate wound healing in diabetic mice. Mol Ther. 2014;22:1593–604.
Wee WKJ, Low ZS, Ooi CK, Henategala BP, Lim ZGR, Yip YS, et al. Single-cell analysis of skin immune cells reveals an Angptl4-ifi20b axis that regulates monocyte differentiation during wound healing. Cell Death Dis. 2022;13:180.
Arya AK, Tripathi K, Das P. Promising role of ANGPTL4 gene in diabetic wound healing. Int J Low Extrem Wounds. 2014;13:58–63.
Zuo Y, Dai L, Li L, Huang Y, Liu X, Liu X, et al. ANGPTL4 regulates psoriasis via modulating hyperproliferation and inflammation of keratinocytes. Front Pharmacol. 2022;13: 850967.
Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–80.
Tong Z, Peng J, Lan H, Sai W, Li Y, **e J, et al. Cross-talk between ANGPTL4 gene SNP Rs1044250 and weight management is a risk factor of metabolic syndrome. J Transl Med. 2021;19:72.
Quintero P, Gonzalez-Muniesa P, Martinez JA. Influence of different oxygen supply on metabolic markers and gene response in murine adipocytes. J Biol Regul Homeost Agents. 2012;26:379–88.
Deng M, Kutrolli E, Sadewasser A, Michel S, Joibari MM, Jaschinski F, et al. ANGPTL4 silencing via antisense oligonucleotides reduces plasma triglycerides and glucose in mice without causing lymphadenopathy. J Lipid Res. 2022;63: 100237.
Singh AK, Chaube B, Zhang X, Sun J, Citrin KM, Canfrán-Duque A, et al. Hepatocyte-specific suppression of ANGPTL4 improves obesity-associated diabetes and mitigates atherosclerosis in mice. J Clin Invest. 2021;2021:131.
Wu G, Liu B, Wu Q, Tang C, Du Z, Fang Y, et al. Correlations between different angiogenic and inflammatory factors in vitreous fluid of eyes with proliferative diabetic retinopathy. Front Med (Lausanne). 2021;8: 727407.
Qin L, Zhang R, Yang S, Chen F, Shi J. Knockdown of ANGPTL-4 inhibits inflammatory response and extracellular matrix accumulation in glomerular mesangial cells cultured under high glucose condition. Artif Cells Nanomed Biotechnol. 2019;47:3368–73.
Lu Q, Lu P, Chen W, Lu L, Zheng Z. ANGPTL-4 induces diabetic retinal inflammation by activating Profilin-1. Exp Eye Res. 2018;166:140–50.
Kondo S, Kojima K, Nakamura N, Miyabe M, Kikuchi T, Ohno T, et al. Increased expression of angiopoietin-like protein 4 regulates matrix metalloproteinase-13 expression in Porphyromonas gingivalis lipopolysaccharides-stimulated gingival fibroblasts and ligature-induced experimental periodontitis. J Periodontal Res. 2023;58:43–52.
Knowles HJ. Multiple roles of angiopoietin-Like 4 in osteolytic disease. Front Endocrinol (Lausanne). 2017;8:80.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
YZ, ZH, and YC drafted the manuscript and prepared the figures. LD designed and critically revised the final version of the manuscript. All authors have contributed to the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuo, Y., He, Z., Chen, Y. et al. Dual role of ANGPTL4 in inflammation. Inflamm. Res. 72, 1303–1313 (2023). https://doi.org/10.1007/s00011-023-01753-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01753-9