Abstract
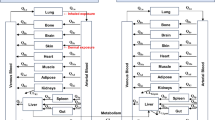
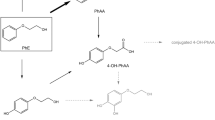
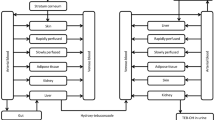
Assessment of the risks posed by the residential use of methyl parathion requires an understanding of its pharmacokinetics after different routes of exposure. Thus, studies were performed using adult female rats to define the pharmacokinetic parameters for methyl parathion after intravenous injection and to apply the described model to an examination of its pharmacokinetics after single oral or dermal exposure. The pharmacokinetics of methyl parathion after intravenous administration (1.5 mg/kg) were best described by a three-compartment model; the apparent volume of the central compartment was 1.45 liters/kg, clearance was 1.85 liters/h/kg and the terminal half-life was 6.6 h with an elimination constant of 0.50 h−1. The apparent oral absorption coefficient for methyl parathion (1.5 mg/kg) was 1.24 h−1, and its oral bioavailability was approximately 20%. The latter likely includes a significant first pass effect. Concentrations of methyl parathion increased during the initial 10–60 min and then declined during the next 15–36 h. After dermal administration (6.25–25 mg/kg), methyl parathion concentrations peaked within 12–26 h and then declined dose dependently. The apparent dermal absorption coefficient was approximately 0.41 h−1, and only two pharmacokinetic compartments could be distinguished. In conclusion, the pharmacokinetics of methyl parathion are complex and route dependent. Also, dermal exposure, because of sustained methyl parathion concentrations, may pose the greatest risk.
Similar content being viewed by others
References
Agency for Toxic Substances and Disease Registry. Illegal Use of Methyl Parathion Insecticide (http://www.atsdr.cdc.gov/alerts/961213.html), 1996.
Bledsoe FH, Seymour EQ. Acute pulmonary edema associated with parathion poisoning. Radiology 103:53–56;1972.
Braeckman RA, Audenaert F, Willems JL, Belpaire FM, Bogaert MG. Toxicokinetics of methyl parathion and parathion in the dog after intravenous and oral administration. Arch Toxicol 54:71–82;1983.
Braeckman RA, Godefroot MG, Blondeel GM, Belpaire FM, Willems JL. Kinetic analysis of methyl parathion in the dog. Arch Toxicol 43:263–271;1980.
Carver MP, Riviere JE. Percutaneous absorption and excretion of xenobiotics after topical and intravenous administration to pigs. Fundam Appl Toxicol 13:714–722;1989.
Chadwick RW, Copeland MF, Froehlich R, Cooke N, Whitehouse DA. Interaction between γ-hexachlorocyclohexane and the gastrointestinal microflora and their effects on the absorption, biotransformation and excretion of parathion by the rat. J Agric Food Chem 32:755–759;1984.
Chambers JE, Chambers HW, Snawder JE. Target site bioactivation of the neurotoxic organophosphorus insecticide parathion in partially hepatectomized rats. Life Sci 48:1023–1029;1991.
Chang RR, Jarman WM, Hennings JA. Sample cleanup by solid-phase extraction for the ultratrace determination of polychlorinated dibenzo-p-dioxins and dibenzofurans in biological samples. Anal Chem 65:2420–2427;1993.
Chang SK, Williams PL, Dauterman WC, Riviere JE. Percutaneous absorption, dermatopharmacokinetics and related bio-transformation studies of carbaryl, lindane, malathion, and parathion in isolated perfused porcine skin. Toxicology 91:269–280;1994.
Costa LG. Basic toxicology of pesticides. Occup Med 12:251–268;1997.
De Bleecker J, Willems J, Van Den Neucker K, De Reuck J, Vogelaers D. Prolonged toxicity with intermediate syndrome after combined parathion and methyl parathion poisoning. J Toxicol Clin Toxicol 30:333–349;1992.
De Potter M, Muller R, Willems J. A method for the determination of some organophosphorus insecticides in human serum. Chromatographia 11:220–222;1978.
De Schryver E, De Reu L, Belpaire F, Willems J. Toxicokinetics of methyl paraoxon in the dog. Arch Toxicol 59:319–322;1987.
Dulaney MD Jr, Rockhold RW, Porter AC, Hoskins B, Ho IK. Changes in drinking activity, urine volume and urinary electrolytes after intracerebroventricular administration of diisopropylfluorophosphate. J Pharmacol Exp Ther 250:202–209;1989.
Durham WF, Wolfe HR, Elliot JW. Absorption and excretion of parathion by spraymen. Arch Environ Health 24:381–387;1972.
Eigenberg DA, Pazdernik TL, Doull J. Hemoperfusion and pharmacokinetic studies with parathion and paraoxon in the rat and dog. Drug Metab Dispos 11:366–370;1983.
Esteban E, Rubin C, Hill R, Olson D, Pearce K. Association between indoor residential contamination with methyl parathion and urinary para-nitrophenol. J Expo Anal Environ Epidemiol 6:375–387;1996.
Gaines TB. The acute toxicology of pesticides to rats. Toxicol Appl Pharmacol 2:88–99;1960.
Garcia-Repetto R, Martinez D, Repetto M. Biodisposition study of the organophosphorus pesticide, methyl-parathion. Bull Environ Contam Toxicol 59:901–908;1997.
George J, Andrade C, Thangam J. Delayed effects of acute oral and chronic inhalational exposure to methylparathion on learning and memory in rats. Indian J Exp Biol 30:819–822;1992.
Grissom RE Jr, Brownie C, Guthrie FE. In vivo and in vitro dermal penetration of lipophilic and hydrophilic pesticides in mice. Bull Environ Contam Toxicol 38:917–924;1987.
Hayes WJ Jr, Funckes AJ, Hartwell WV. Dermal exposure of human volunteers to parathion. Arch Environ Health 8:829–833;1964.
Hollingworth RM, Metcalf RL, Fukuto TR. The selectivity of sumithion compared with methylparathion. Metabolism in the white mouse. J Agric Food Chem 15:242–249;1967.
Hussain M, Yoshida K, Atiemo M, Johnston D. Occupational exposure of grain farmers to carbofuran. Arch Environ Contam Toxicol 19:197–204;1990.
Kao J, Carver MP. Cutaneous metabolism of xenobiotics. Drug Metab Rev 22:363–410;1990.
Kramer RE, Wellman SE, Zhu H, Rockhold RW, Baker RC. A comparison of cholinesterase activity after intravenous, oral or dermal administration of methyl parathion. J Biomed Sci 9:140–148;2002.
Lacorte S, Barcelo D. Determination of parts per trillion levels of organophosphorus pesticides in groundwater by automated on-line liquid-solid extraction followed by liquid chromatography/atmospheric pressure chemical ionization mass spectrometry using positive and negative ion modes of operation. Anal Chem 68:2464–2470;1996.
Miyamoto J, Sato Y, Kadota T, Fu**ami A, Endo M. Studies on the mode of action of organophosphorus compounds. 1. Metabolic fate of P32 labeled sumithion and methylparathion in guinea pig and white rat. Agric Biol Chem 27:381–389;1963.
National Technical Information Service. PB277-077. Springfield, National Technical Information Service.
Neal RA. Studies on the metabolism of diethyl 4-nitrophenyl phosphorothionate (parathion) in vitro. Biochem J 103:183–191;1967.
Okonek S, Kilbinger H. Determination of acetylcholine, nitrostigmine and acetylcholinesterase activity in four patients with severe nitrostigmine (E 605 forte) intoxication. Arch Toxicol 32:97–108;1974.
Pena-Egido MJ, Rivas-Gonzalo JC, Marino-Hernandez EL. Toxicokinetics of parathion in the rabbit. Arch Toxicol 61:196–200;1988.
Qiao GL, Riviere JE. Significant effects of application site and occlusion on the pharmacokinetics of cutaneous penetration and biotransformation of parathion in vivo in swine. J Pharm Sci 84:425–432;1995.
Qiao GL, Williams PL, Riviere JE. Percutaneous absorption, biotransformation and systemic disposition of parathion in vivo in swine. 1. Comprehensive pharmacokinetic model. Drug Metab Dispos 22:459–471;1994.
Reifenrath WG, Chellquist EM, Shipwash EA, Jederberg WW, Krueger GG. Percutaneous penetration in the hairless dog, weanling pig and grafted athymic mouse: Evaluation of models for predicting skin penetration in man. Br J Dermatol 111(suppl 27):123–135;1984.
Senanayake N, Karalliedde L. Neurotoxic effects of organophosphorus insecticides: An intermediate syndrome. N Engl J Med 316:761–763;1987.
Spear RC, Popendorf WJ, Leffingwell JT, Milby TH, Davies JE, Spencer WF. Fieldworkers' response to weathered residues of parathion. J Occup Med 19:406–410;1977.
Tsachalinas D, Logaras G, Paradelis A. Observations on 246 cases of acute poisoning with parathion in Greece. Eur J Toxicol 4:1–8;1971.
United States Environmental Protection Agency, Office of Pesticide Programs. Illegal Indoor Use of Methyl Parathion (http://www.epa.gov/pesticides/citizens/methyl.htm), 2000.
Ware GW, Morgan DP, Estesen BJ, Cahill WP. Establishment of reentry intervals for organophosphate-treated cotton fields based on human data. 3. 12–72 hours post-treatment exposure to monocrotophos, ethyl- and methyl-parathion. Arch Environ Contam Toxicol 3:289–306;1975.
Wester RC, Quan D, Maibach HI. In vitro percutaneous absorption of model compounds glyphosate and malathion from cotton fabric into and through human skin. Food Chem Toxicol 34:731–735;1996.
Wolfe HR, Armstrong JF, Staiff DC, Comer SW, Durham WF. Exposure of apple thinners to parathion residues. Arch Environ Contam Toxicol 3:257–267;1975.
Wolff MS, McConnell R, Cedillo L, Rivera M. Dermal levels of methyl-parathion, organochlorine pesticides and acetylcholinesterase among formulators. Bull Environ Contam Toxicol 48:671–678;1992.
Zhang HX, Sultatos LG. Biotransformation of the organophosphorus insecticides parathion and methyl parathion in male and female rat livers perfused in situ. Drug Metab Dispos 19:473–477;1991.
Zhu H, Rockhold RW, Baker RC, Kramer RE, Ho IK. Effects of single or repeated dermal exposure to methyl parathion on behavior and blood cholinesterase activity in rats. J Biomed Sci 8:467–474;2001.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kramer, R.E., Wellman, S.E., Rockhold, R.W. et al. Pharmacokinetics of methyl parathion: A comparison following single intravenous, oral or dermal administration. J Biomed Sci 9, 311–320 (2002). https://doi.org/10.1007/BF02256586
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02256586