Abstract
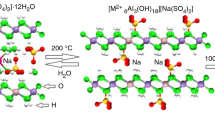
Thermogravimetric analysis (TG) and powder X-ray diffraction (PXRD) were used to study some selected Mg/Al and Zn/Al layered double hydroxides (LDHs) prepared by co-precipitation. A Mg/Al hydrotalcite was investigated before and after reformation in fluoride and nitrate solutions. Little change in the TG or PXRD patterns was observed. It was proposed that successful intercalation of nitrate anions has occurred. However, the absence of any change in the d (003) interlayer spacing suggests that fluoride anions were not intercalated between the LDH layers. Any fluoride anions that were removed from solution are most likely adsorbed onto the outer surfaces of the hydrotalcite. As fluoride removal was not quantified it is not possible to confirm that this has happened without further experimentation. Carbonate is probably intercalated into the interlayer of these hydrotalcites, as well as fluoride or nitrate. The carbonate most likely originates from either incomplete decarbonation during thermal activation or adsorption from the atmosphere or dissolved in the deionised water. Small and large scale co-precipitation syntheses of a Zn/Al LDH were also investigated to determine if there was any change in the product. While the small scale experiment produced a good quality LDH of reasonable purity; the large scale synthesis resulted in several additional phases. Imprecise measurement and difficulty in handling the large quantities of reagents appeared to be sufficient to alter the reaction conditions causing a mixture of phases to be formed.








Similar content being viewed by others
References
Rives V, editor. Layered double hydroxides: present and future. New York: Nova Science Pub Inc; 2001.
Rives V. Characterisation of layered double hydroxides and their decomposition products. Mater Chem Phys. 2002;75:19–25.
Bakon KH, Palmer SJ, Frost RL. Thermal analysis of synthetic reevesite and cobalt substituted reevesite (Ni, Co)6Fe2(OH)16(CO3)·4H2O. J Therm Anal Calorim. 2010;100(1):125–31. doi:10.1007/s10973-009-0145-x.
Bouzaid J, Frost RL. Thermal decomposition of stichtite. J Therm Anal Calorim. 2007;89(1):133–5.
Britto S, Kamath PV. Structure of bayerite-based lithium–aluminum layered double hydroxides (LDHs): observation of monoclinic symmetry. Inorg Chem. 2009;48(24):11646–54. doi:10.1021/ic9016728.
Chitrakar R, Makita Y, Sonoda A, Hirotsu T. Fe–Al layered double hydroxides in bromate reduction: synthesis and reactivity. J Colloid Interface Sci. 2011;354(2):798–803. doi:10.1016/j.jcis.2010.11.010.
Chitrakar R, Tezuka S, Sonoda A, Sakane K, Hirotsu T. A new method for synthesis of Mg–Al, Mg–Fe, and Zn–Al layered double hydroxides and their uptake properties of bromide ion. Ind Eng Chem Res. 2008;47(14):4905–8. doi:10.1021/ie0716417.
Das DP, Das J, Parida K. Physicochemical characterization and adsorption behavior of calcined Zn/Al hydrotalcite-like compound (HTlc) towards removal of fluoride from aqueous solution. J Colloid Interface Sci. 2002;261:213–20.
Frost R, Bouzaid J, Martens W, Kloprogge T. Thermal decomposition of the synthetic hydrotalcite woodallite. J Therm Anal Calorim. 2006;86(2):437–41.
Frost RL, Adebajo MO, Erickson KL. Raman spectroscopy of synthetic and natural iowaite. Spectrochim Acta Part A. 2005;61A(4):613–20. doi:10.1016/j.saa.2004.05.015.
Frost RL, Bakon KH, Palmer SJ. Raman spectroscopic study of synthetic reevesite and cobalt substituted reevesite (Ni, Co)6Fe2(OH)16(CO3)·4H2O. J Raman Spectrosc. 2010;41(1):78–83. doi:10.1002/jrs.2280.
Frost RL, Bouzaid JM, Martens WN. Thermal decomposition of the composite hydrotalcites of iowaite and woodallite. J Therm Anal Calorim. 2007;89(2):511–9.
Frost RL, Bouzaid JM, Musumeci AW, Kloprogge JT, Martens WN. Thermal decomposition of the synthetic hydrotalcite iowaite. J Therm Anal Calorim. 2006;86:437–41.
Frost RL, Erickson KL. Thermal decomposition of natural iowaite. J Therm Anal Calorim. 2004;78(2):367–73.
Frost RL, Erickson KL. Decomposition of the synthetic hydrotalcites mountkeithite and honessite—a high resolution thermogravimetric analysis and infrared emission spectroscopic study. Thermochim Acta. 2004;421(1–2):51–8. doi:10.1016/j.tca.2004.04.008.
Frost RL, Erickson KL. Near-infrared spectroscopy of stitchtite, iowaite, desautelsite and arsenate exchanged takovite and hydrotalcite. Spectrochim Acta Part A. 2004;61A(1–2):51–6. doi:10.1016/j.saa.2004.03.011.
Frost RL, Erickson KL, Kloprogge TJ. Vibrational spectroscopic study of the nitrate containing hydrotalcite mbobomkulite. Spectrochim Acta Part A. 2005;61A(13–14):2919–25. doi:10.1016/j.saa.2004.11.002.
Frost RL, Palmer SJ, Grand L-M. Raman spectroscopy of gallium-based hydrotalcites of formula Mg6Ga2(CO3)(OH)16·4H2O. J Raman Spectrosc. 2010;41(7):791–6. doi:10.1002/jrs.2508.
Frost RL, Palmer SJ, Grand L-M. Synthesis and thermal analysis of indium-based hydrotalcites of formula Mg6In2(CO3)(OH)16·4H2O. J Therm Anal Calorim. 2010;101(3):859–63.
Frost RL, Spratt HJ, Palmer SJ. Infrared and near-infrared spectroscopic study of synthetic hydrotalcites with variable divalent/trivalent cationic ratios. Spectrochim Acta Part A. 2009;72A(5):984–8. doi:10.1016/j.saa.2008.12.018.
Frost RL, Weier ML, Clissold ME, Williams PA. Infrared spectroscopic study of natural hydrotalcites carrboydite and hydrohonessite. Spectrochim Acta Part A. 2003;59A(14):3313–9. doi:10.1016/s1386-1425(03)00160-4.
Grand L-M, Palmer SJ, Frost RL. Synthesis and thermal stability of hydrotalcites containing manganese. J Therm Anal Calorim. 2009;. doi:10.1007/s10973-009-0402-z.
Grand L-M, Palmer SJ, Frost RL. Synthesis and thermal stability of hydrotalcites containing gallium. J Therm Anal Calorim. 2009;. doi:10.1007/s10973-009-0456-y.
Grand L-M, Palmer SJ, Frost RL. Synthesis and thermal stability of hydrotalcites based upon gallium. J Therm Anal Calorim. 2010;101(1):195–8. doi:10.1007/s10973-009-0456-y.
Kloprogge JT, Hickey L, Frost RL. FT-Raman and FT-IR spectroscopic study of synthetic Mg/Zn/Al-hydrotalcites. J Raman Spectrosc. 2004;35(11):967–74. doi:10.1002/jrs.1244.
Kloprogge JT, Hickey L, Trujillano R, Holgado MJ, San Roman MS, Rives V, et al. Characterization of intercalated Ni/Al hydrotalcites prepared by the partial decomposition of urea. Cryst Growth Des. 2006;6(6):1533–6. doi:10.1021/cg0504612.
Palmer SJ, Frost RL, Spratt HJ. Synthesis and Raman spectroscopic study of Mg/Al, Fe hydrotalcites with variable cationic ratios. J Raman Spectrosc. 2009;40(9):1138–43. doi:10.1002/jrs.2198.
Rives V. Layered double hydroxides with the hydrotalcite-type structure containing Cu2+, Ni2+ and Al3+. J Mater Chem. 1999;10:489–95.
Saber O. Preparation and characterization of a new nano layered material, Co–Zr LDH. J Mater Sci. 2007;42(23):9905–12. doi:10.1007/s10853-007-2097-5.
Spratt HJ, Palmer SJ, Frost RL. Thermal decomposition of synthesised layered double hydroxides based upon Mg/(Fe, Cr) and carbonate. Thermochim Acta. 2008;479(1–2):1–6. doi:10.1016/j.tca.2008.08.016.
Theo Kloprogge J, Frost RL. Infrared emission spectroscopic study of the thermal transformation of Mg-, Ni- and Co-hydrotalcite catalysts. Appl Catal A. 1999;184(1):61–71.
Tong DS, Zhou CHC, Li MY, Yu WH, Beltramini J, Lin CX, et al. Structure and catalytic properties of Sn-containing layered double hydroxides synthesized in the presence of dodecylsulfate and dodecylamine. Appl Clay Sci. 2010;48:569–74.
Fawell J, Bailey K, Chilton J, Fewtrell L, Magara Y, editors. Fluoride in drinking-water. London: IWA Publishing; 2006.
Lv L, He J, Wei M, Evans DG, Duan X. Factors influencing the removal of fluoride from aqueous solution by calcined Mg–Al–CO3 layered double hydroxides. J Hazard Mater. 2006;133(1–3):119–28. doi:10.1016/j.jhazmat.2005.10.012.
Liang L, Li L. Adsorption behavior of calcined layered double hydroxides towards removal of iodide contaminants. J Radioanal Nucl Chem. 2006;273(1):221–6.
Theiss FL, Sear-Hall MJ, Palmer SJ, Frost RL. Zinc aluminium layered double hydroxides for the removal of iodine and iodide from aqueous solutions. Desalin Water Treat. 2011;39:166–75. doi:10.1080/19443994.2012.669171.
Frost RL, Musumeci AW. Nitrate absorption through hydrotalcite reformation. J Colloid Interface Sci. 2006;302(1):203–6. doi:10.1016/j.jcis.2006.06.024.
Liu R, Frost RL, Martens WN. Absorption of the selenite anion from aqueous solutions by thermally activated layered double hydroxide. Water Res. 2009;43(5):1323–9. doi:10.1016/j.watres.2008.12.030.
Fetter G, Ramos E, Olguin MT, Bosch P, López T, Bulbulian S. Sorption of 131I− by hydrotalcites. J Radioanal Nucl Chem. 1997;221:63–6.
Kloprogge JT, Weier M, Crespo I, Ulibarri MA, Barriga C, Rives V, et al. Intercalation of iron hexacyano complexes in Zn, Al hydrotalcite. Part 2. A mid-infrared and Raman spectroscopic study. J Solid State Chem. 2004;177(4–5):1382–7. doi:10.1016/j.jssc.2003.11.034.
Kulyukhin SA, Krasavina EP, Rumer IA, Mizina LV, Konovalova NA. Sorption of radioiodine from aqueous solutions on layered double magnesium aluminum hydroxides at 300 K. Radiochemistry. 2007;49(5):499–503. doi:10.1134/S1066362207050098.
Palmer SJ, Frost RL. Use of hydrotalcites for the removal of toxic anions from aqueous solutions. Ind Eng Chem Res. 2010;49(19):8969–76. doi:10.1021/ie101104r.
Theiss F, Palmer S, Ayoko G, Frost R. Sulfate intercalated layered double hydroxides prepared by the reformation effect. J Therm Anal Calorim. 2011:1–6. doi:10.1007/s10973-011-1369-0.
Frost RL, Martens WN, Erickson KL. Thermal decomposition of the hydrotalcite. J Therm Anal Calorim. 2005;82:603–8.
Acknowledgements
The financial and infra-structure support of the Discipline of Nanotechnology and Molecular Science of the Faculty of Science and Engineering, Queensland University of Technology is gratefully acknowledged. The Australian Research Council (ARC) is thanked for funding the instrumentation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Theiss, F.L., Ayoko, G.A. & Frost, R.L. Thermogravimetric analysis of selected layered double hydroxides. J Therm Anal Calorim 112, 649–657 (2013). https://doi.org/10.1007/s10973-012-2584-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2584-z